Preferred Treatment Patterns of Retinopathy of Prematurity: An International Survey
Abstract
1. Introduction
2. Materials and Methods
3. Results
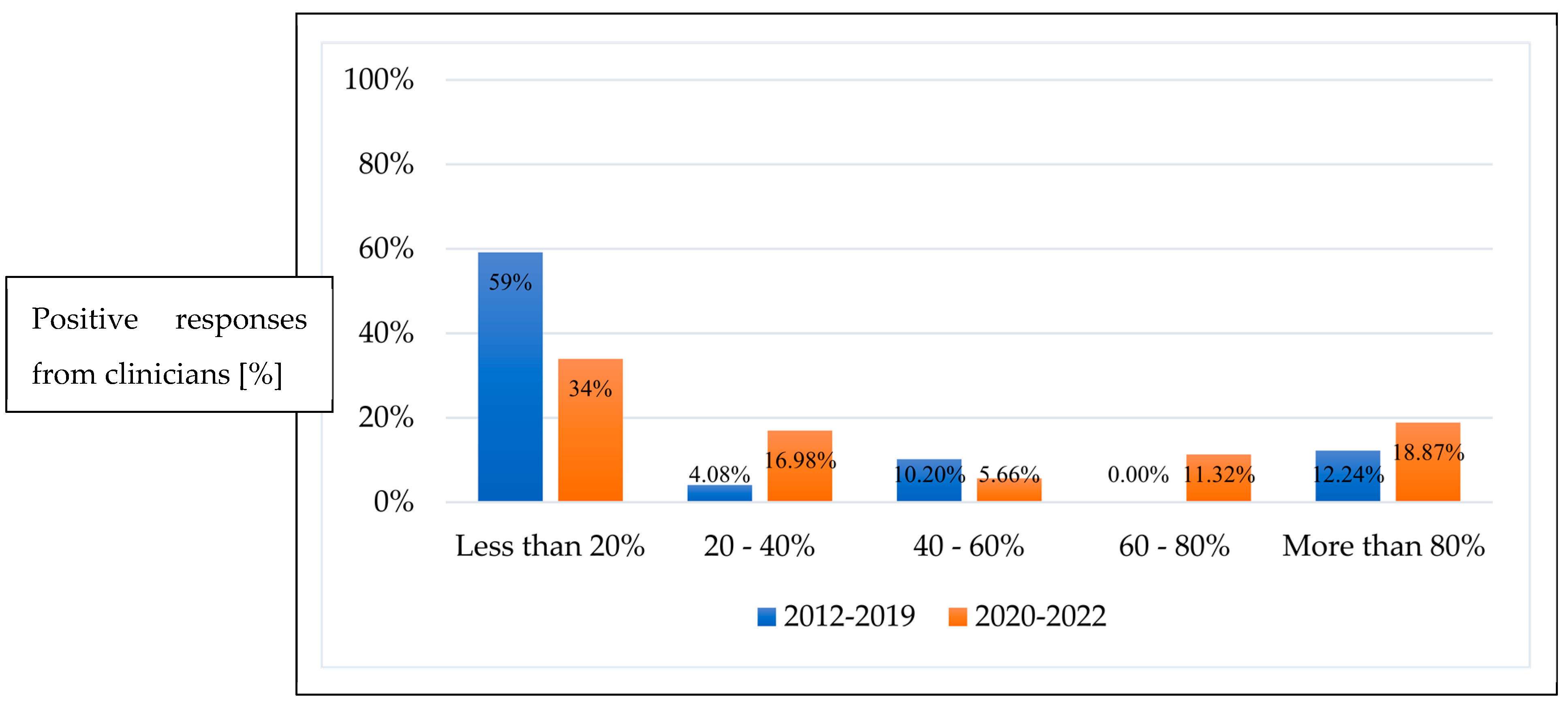
3.1. First-Line Treatment
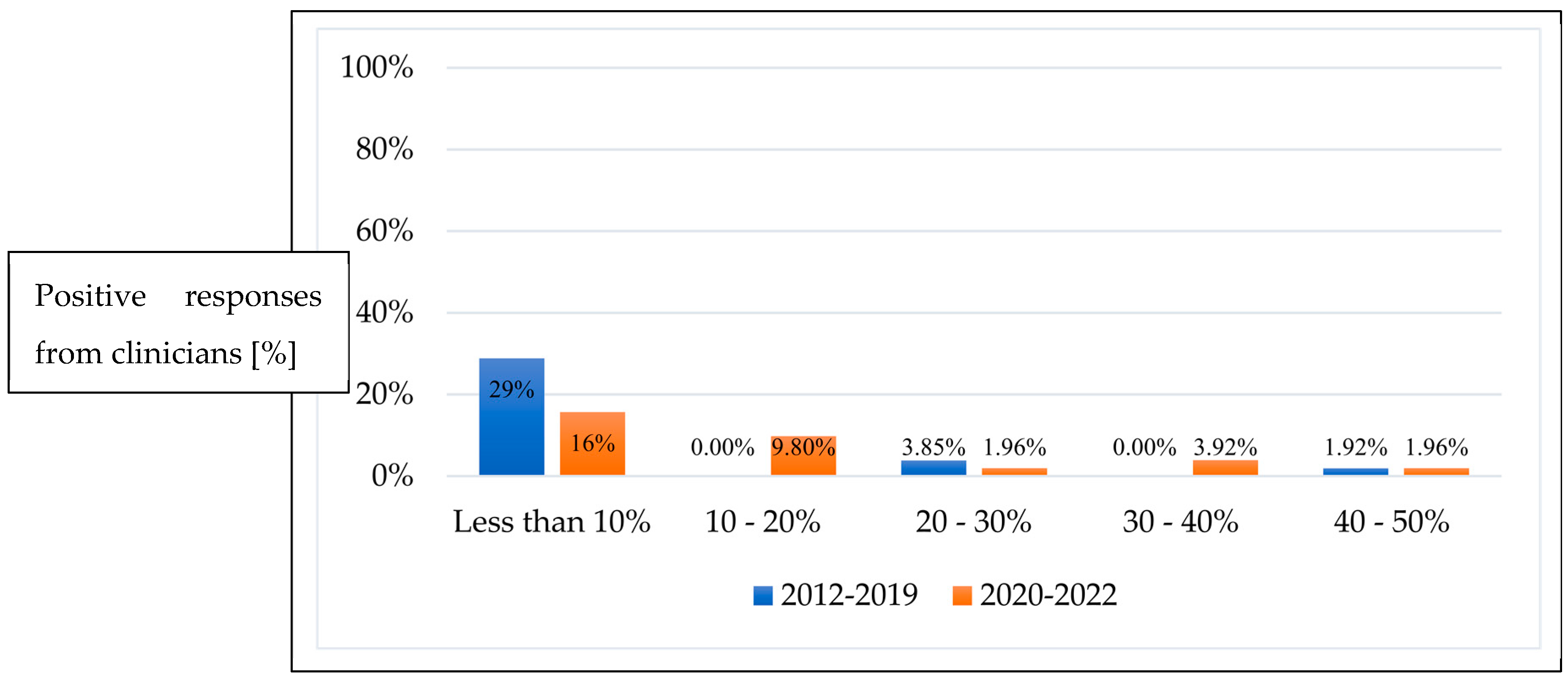
3.2. Repeat Treatment Post Initial Anti-VEGF Monotherapy
3.3. Preferred Anti-VEGF Agents
3.4. Follow-Up Timeframe
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Chan, R.P.; Berrocal, A.; Binenbaum, G.; Blair, M.; Campbell, J.P.; Capone, A., Jr.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef] [PubMed]
- Sabri, K.; Ells, A.L.; Lee, E.Y.; Dutta, S.; Vinekar, A. Retinopathy of Prematurity: A Global Perspective and Recent Developments. Pediatrics 2022, 150, e2021053924. [Google Scholar] [CrossRef]
- Strube, Y.N.J.; Wright, K.W. Pathophysiology of retinopathy of prematurity. Saudi J. Ophthalmol. 2022, 36, 239–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sankar, M.J.; Sankar, J.; Chandra, P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst. Rev. 2018, 8, Cd009734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: Preliminary Results. Arch. Ophthalmol. 1988, 106, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Crouch, E.R.; Kraker, R.T.; Wallace, D.K.; Holmes, J.M.; Repka, M.X.; Collinge, J.E.; Bremer, D.L.; Gray, M.E.; Smith, H.A.; Steinkuller, P.G.; et al. Secondary 12-Month Ocular Outcomes of a Phase 1 Dosing Study of Bevacizumab for Retinopathy of Prematurity. JAMA Ophthalmol. 2020, 138, 14–20. [Google Scholar] [CrossRef]
- Good, W.V. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 2004, 102, 233–248, discussion 48–50. [Google Scholar] [PubMed] [PubMed Central]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 17, 603–615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stahl, A.; Lepore, D.; Fielder, A.; Fleck, B.; Reynolds, J.D.; Chiang, M.F.; Li, J.; Liew, M.; Maier, R.; Zhu, Q.; et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): An open-label randomised controlled trial. Lancet 2019, 394, 1551–1559. [Google Scholar] [CrossRef]
- Stahl, A.; Krohne, T.U.; Eter, N.; Oberacher-Velten, I.; Guthoff, R.; Meltendorf, S.; Ehrt, O.; Aisenbrey, S.; Roider, J.; Gerding, H.; et al. Comparing Alternative Ranibizumab Dosages for Safety and Efficacy in Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2018, 172, 278–286. [Google Scholar] [CrossRef]
- Stahl, A.; Sukgen, E.A.; Wu, W.C.; Lepore, D.; Nakanishi, H.; Mazela, J.; Moshfeghi, D.M.; Vitti, R.; Athanikar, A.; Chu, K.; et al. Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial. JAMA 2022, 328, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Dogra, M.R.; Vinekar, A. Role of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) in the Treatment of Retinopathy of Prematurity: A Narrative Review in the Context of Middle-Income Countries. Pediatr. Health Med. Ther. 2023, 14, 59–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerd, H.; Ann, H.; Peter, J.; Pia, L.; Kristina, T.; Agneta, W. Five years of treatment for retinopathy of prematurity in Sweden: Results from SWEDROP, a national quality register. Br. J. Ophthalmol. 2016, 100, 1656. [Google Scholar]
- Winter, K.; Pfeil, J.M.; Engmann, H.; Aisenbrey, S.; Lorenz, B.; Hufendiek, K.; Breuss, H.; Khattab, M.; Süsskind, D.; Kakkassery, V.; et al. Comparability of input parameters in the German Retina.net ROP registry and the EU-ROP registry—An exemplary comparison between 2011 and 2021. Acta Ophthalmol. 2024, 102, e314–e321. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Zhang, G.M. Laser therapy versus intravitreal injection of anti-VEGF agents in monotherapy of ROP: A Meta-analysis. Int. J. Ophthalmol. 2020, 13, 806–815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Süren, E.; Özkaya, D.; Çetinkaya, E.; Kalaycı, M.; Yiğit, K.; Kücük, M.F.; Erol, M.K. Comparison of bevacizumab, ranibizumab and aflibercept in retinopathy of prematurity treatment. Int. Ophthalmol. 2022, 42, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.E.; Bagheri, M.; Niyousha, M.R.; Rezaei, L.; Hazeri, S.; Safarpoor, S.; Abdollahi, M. Comparison of Clinical Outcomes of Intravitreal Bevacizumab Aflibercept in Type 1 Prethreshold Retinopathy of Prematurity in Posterior Zone II. J. Curr. Ophthalmol. 2022, 34, 87–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasin, A.; Sinha, S.; Smith, R.; Jain, S.F.; Hejkal, T.; Rychwalski, P. Reactivation of retinopathy of prematurity six years after intravitreal injection of bevacizumab. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2023, 27, 236–239. [Google Scholar] [CrossRef]
- Valikodath, N.G.; Chiang, M.F.; Chan, R.V.P. Description and management of retinopathy of prematurity reactivation after intravitreal antivascular endothelial growth factor therapy. Curr. Opin. Ophthalmol. 2021, 32, 468–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valikodath, N.; Cole, E.; Chiang, M.F.; Campbell, J.P.; Chan, R.V.P. Imaging in Retinopathy of Prematurity. Asia Pac. J. Ophthalmol. 2019, 8, 178–186. [Google Scholar] [PubMed] [PubMed Central]
- Wu, A.-L.; Wu, W.-C. Anti-VEGF for ROP and Pediatric Retinal Diseases. Asia Pac. J. Ophthalmol. 2018, 7, 145–151. [Google Scholar] [CrossRef]
| Survey Questions | Number of Respondents (n) |
|---|---|
| 1. In which country do you practice? | 54 |
| 2. How many premature babies do you screen (per annum)? | 52 |
| 3. How many ROP babies have you treated between 1 January 2012 to 31 March 2022? | 51 |
| 4. Among all ROP babies treated with anti-VEGF between 1 January 2012 to 31 March 2022, how many were treated with anti-VEGF as first-line therapy? | 52 |
| 5. Between the period of 1 January 2012 to 31 December 2019, what percentage of ROP babies were treated with anti-VEGF as first-line therapy? | 53 |
| 6. Between the period of 1 January 2020 to 31 March 2022, what percentage of ROP babies were treated with anti-VEGF as first-line therapy? | 53 |
| 7. What is your first-line treatment option for: AROP (APROP), Type 1 ROP in Zone 1, Type 1 ROP in posterior Zone 2, Type 1 ROP in anterior Zone 2, Type 1 ROP in Zone 3 | 53 |
| 8. When an anti-VEGF treatment is administered, which agent is used? | 50 |
| 9. What dose of intravitreal anti-VEGF is administered? | 50 |
| 10. During the period of 1 January 2012 to 31 December 2019, what percentage of ROP babies required repeat anti-VEGF treatment? | 52 |
| 11. During the period of 1 January 2020 to 31 March 2022, what percentage of ROP babies required repeat anti-VEGF treatment? | 52 |
| 12. For ROP babies requiring repeat treatment, which treatment modality was used? A-ROP (APROP), Type 1 ROP in Zone 1, Type 1 ROP in posterior Zone 2, Type 1 ROP in anterior Zone 2, Type 1 ROP in Zone 3. | 27 |
| 13. How long were ROP babies followed up post anti-VEGF treatment (in weeks post-menstrual age)? * Prior to discharge from active screening. | 50 |
| 14. What is your follow-up interval (in the outpatient setting) for ROP babies who underwent anti-VEGF treatment? * After discharge from active screening. | 50 |
| (a) Developed Countries (number of respondents (n) = 34) | |||||
| A-ROP n (%) | Type 1 ROP in Zone 1 n (%) | Type 1 ROP in posterior Zone 2 n (%) | Type 1 ROP in anterior Zone 2 n (%) | Type 1 ROP in Zone 3 n (%) | |
| Anti-VEGF | 26 (76.4%) | 27 (79.4%) | 23 (67.6%) | 6 (17.6%) | 1 (2.9%) |
| Combination (Laser & Anti-VEGF) | 5 (14.7%) | 5 (14.7%) | 6 (17.6%) | 2 (5.8%) | 1 (2.9%) |
| Laser | 3 (8.8%) | 2 (5.8%) | 5 (14.7%) | 26 (76.4%) | 28 (82.3%) |
| Other (Cryotherapy) | 0 | 0 | 0 | 0 | 4 (11.7%) |
| (b) Developing Countries (number of respondents (n) = 19) | |||||
| A-ROP n (%) | Type 1 ROP in Zone 1 n (%) | Type 1 ROP in posterior Zone 2 n (%) | Type 1 ROP in anterior Zone 2 n (%) | Type 1 ROP in Zone 3 n (%) | |
| Anti-VEGF | 8 (42.1%) | 11 (57.8%) | 7 (36.8%) | 3 (15.7%) | 1 (5.2%) |
| Combination (Laser & Anti-VEGF) | 4 (21.0%) | 1 (5.2%) | 0 | 1 (5.2%) | 0 |
| Laser | 7 (36.8%) | 4 (21.0%) | 9 (47.3%) | 13 (68.4%) | 14 (73.6%) |
| Other (Cryotherapy) | 0 | 3 (15.7%) | 3 (15.7%) | 2 (10.5%) | 4 (21.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.T.; Dai, S. Preferred Treatment Patterns of Retinopathy of Prematurity: An International Survey. Pediatr. Rep. 2024, 16, 816-822. https://doi.org/10.3390/pediatric16030069
Wang AT, Dai S. Preferred Treatment Patterns of Retinopathy of Prematurity: An International Survey. Pediatric Reports. 2024; 16(3):816-822. https://doi.org/10.3390/pediatric16030069
Chicago/Turabian StyleWang, Amy T., and Shuan Dai. 2024. "Preferred Treatment Patterns of Retinopathy of Prematurity: An International Survey" Pediatric Reports 16, no. 3: 816-822. https://doi.org/10.3390/pediatric16030069
APA StyleWang, A. T., & Dai, S. (2024). Preferred Treatment Patterns of Retinopathy of Prematurity: An International Survey. Pediatric Reports, 16(3), 816-822. https://doi.org/10.3390/pediatric16030069






