Is It Safe to Operate without Frozen Section Biopsies in Short-Segment Hirschsprung’s Disease? An Overview of 60 Cases
Abstract
1. Introduction
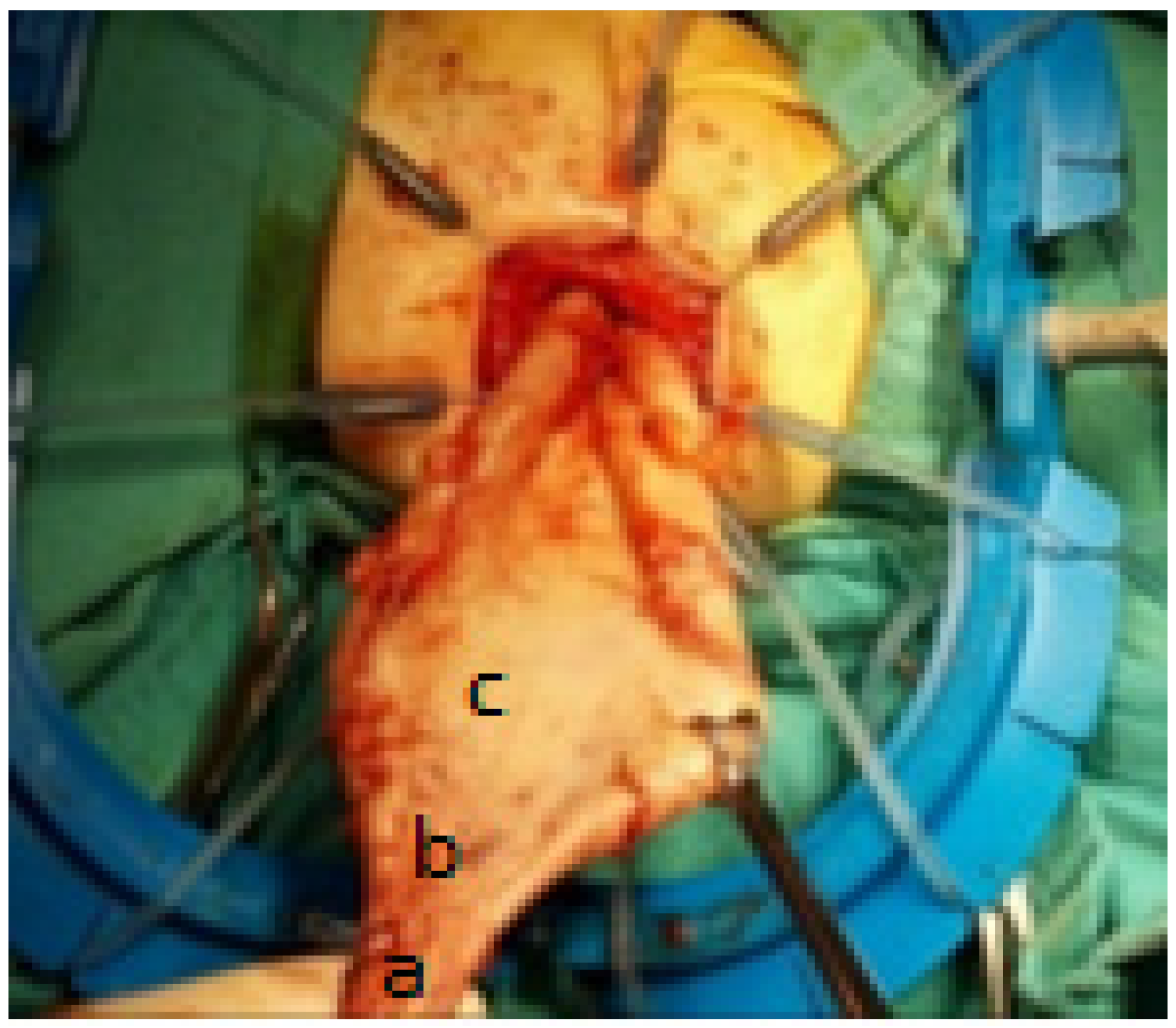
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGS | Aganglionic segment |
| FN | False negatives |
| FP | False positives |
| FSB | Frozen-section biopsy |
| FTB | Full-thickness biopsy |
| HE | Hematoxylin and eosin |
| HSCR | Hirschsprung’s disease |
| HAEC | Hirschsprung-associated enterocolitis |
| IHC | Immunohistochemistry |
| NGS | Normoganglionic segment |
| Ss-HSCR | Short-segment Hirschsprung’s disease |
| TCA | Total colon aganglionosis |
| TP | True positives |
| TN | True negatives |
| TZ | Transition zone |
| TZPT | Transition zone pull-through |
| TEPT | Trans anal endorectal pull-through |
References
- Jamieson, D.H.; Dundas, S.E.; Al Belushi, S.; Cooper, M.; Blair, G.K. Does the transition zone reliably delineate aganglionic bowel in Hirschsprung’s disease? Pediatr. Radiol. 2004, 34, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.P.; Kennedy, A.J. Transitional zone pull through: Surgical pathology considerations. Semin. Pediatr. Surg. 2012, 21, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Frongia, G.; Günther, P.; Schenk, J.P.; Strube, K.; Kessler, M.; Mehrabi, A.; Romero, P. Contrast Enema for Hirschsprung Disease Investigation: Diagnostic Accuracy and Validity for Subsequent Diagnostic and Surgical Planning. Eur. J. Pediatr. Surg. 2016, 26, 207–214. [Google Scholar] [PubMed]
- Smith, C.; Ambartsumyan, L.; Kapur, R.P. Surgery, Surgical Pathology, and Postoperative Management of Patients with Hirschsprung Disease. Pediatr. Dev. Pathol. 2020, 23, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.M. The reliability of frozen-section diagnosis in the pathologic evaluation of Hirschsprung’s disease. Am. J. Surg Pathol. 2000, 24, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.P.; Arnold, M.A.; Conces, M.R.; Ambartsumyan, L.; Avansino, J.; Levitt, M.; Wood, R.; Mast, K.J. Remodeling of Rectal Innervation After Pullthrough Surgery for Hirschsprung Disease: Relevance to Criteria for the Determination of Retained Transition Zone. Pediatr. Dev. Pathol. 2019, 22, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Teeraratkul, S. Transanal one-stage endorectal pull-through for Hirschsprung’s disease in infants and children. J. Pediatr. Surg. 2003, 38, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Shehata, S. Avoiding Complications in Hirschsprung’s Disease, by Prof Sameh Shehata, Hosted by Ankara University—YouTube. Available online: https://www.youtube.com/watch?v=iH5-G9OCUqw (accessed on 28 December 2023).
- Negash, S.; Getachew, H.; Tamirat, D.; Mammo, T.N. Hirschsprung disease managed with one-stage transanal endorectal pullthrough in a low-resource setting without frozen section. BMC Surg. 2022, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.M.; Jafor, A.; Ahmed, S. Transanal Endorectal Pullthrough for Hirschsprung’s Disease Without Frozen-Section Biopsy Facility. BIRDEM Med. J. 2014, 4, 84–87. [Google Scholar] [CrossRef]
- Hall, N.J.; Rahman, A.; Morini, F.; Prato, A.P.; Friedmacher, F.; Koivusalo, A.; van Heurn, E.; Pierro, A.; Zani, A. European Paediatric Surgeons’ Association Survey on the Management of Pediatric Appendicitis. Eur. J. Pediatr. Surg. 2019, 29, 053–061. [Google Scholar] [CrossRef]
- Proctor, M.; Traubici, J.; Langer, J.; Gibbs, D.; Ein, S.; Daneman, A.; Kim, P. Correlation between radiographic transition zone and level of aganglionosis in Hirschsprung’s disease: Implications for surgical approach. J. Pediatr. Surg. 2003, 38, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Stranzinger, E.; DiPietro, M.A.; Teitelbaum, D.H.; Strouse, P.J. Imaging of total colonic Hirschsprung disease. Pediatr. Radiol. 2008, 38, 1162–1170. [Google Scholar] [CrossRef]
- Schäppi, M.; Staiano, A.; Milla, P.; Smith, V.; Dias, J.; Heuschkel, R.; Husby, S.; Mearin, M.; Papadopoulou, A.; Ruemmele, F.; et al. A Practical Guide for the Diagnosis of Primary Enteric Nervous System Disorders. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.C.; Durrant, A.C.; de la Torre, L.; Teitelbaum, D.H.; Minkes, R.K.; Caty, M.G.; Barbara, E.W.; Jose, J.S.; Shinjiro, H.; Craig, A.; et al. One-Stage Transanal Soave Pullthrough for Hirschsprung Disease: A Multicenter Experience with 141 Children. Trans. Meet Am. Surg Assoc. 2003, 121, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.P.; Kennedy, A.J. Histopathologic Delineation of the Transition Zone in Short-Segment Hirschsprung Disease. Pediatr. Dev. Pathol. 2013, 16, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Tomuschat, C.; Mietzsch, S.; Dwertmann-Rico, S.; Clauditz, T.; Schaefer, H.; Reinshagen, K. The Length of the Transition Zone in Patients with Rectosigmoid Hirschsprung Disease. Children 2022, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.A.; Marc, L.A. Hirschsprung Disease. In Fundam Pediatr Surgery, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–935. Available online: https://link.springer.com/chapter/10.1007/978-3-319-27443-0_62 (accessed on 3 December 2023).
- Thakkar, H.S.; Blackburn, S.; Curry, J.; De Coppi, P.; Giuliani, S.; Sebire, N.; Cross, K. Variability of the transition zone length in Hirschsprung disease. J. Pediatr. Surg. 2020, 55, 63–66. [Google Scholar] [CrossRef]
- Beltman, L.; Shirinskiy, I.; Donner, N.; Backes, M.; Benninga, M.; Roelofs, J.; van der Voorn, P.; van Schuppen, J.; Oosterlaan, J.; van Heurn, E.; et al. Determining the Correct Resection Level in Patients with Hirschsprung Disease Using Contrast Enema and Full Thickness Biopsies: Can the Diagnostic Accuracy be Improved by Examining Submucosal Nerve Fiber Thickness? J. Pediatr. Surg. 2023, 58, 1463–1470. [Google Scholar] [CrossRef]
- Saad, S.A.; Elseed, M.M.G.; AbouZeid, A.A.; Ibrahim, E.A.; Radwan, A.B.; Hay, S.A.; El-Behery, M.M. Histopathological perspective of the pulled-through colon in disease: On clinical outcome. J. Pediatr. Surg. 2020, 55, 1829–1833. [Google Scholar] [CrossRef]
- White, F.V.; Langer, J.C. Circumferential Distribution of Ganglion Cells in the Transition Zone of Children with Hirschsprung Disease. Pediatr. Dev. Pathol. 2000, 3, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; O’Donnell, A.M.; Tomuschat, C.; Gillick, J.; Puri, P. The Extent of the Transition Zone in Hirschsprung Disease. J. Pediatr. Surg. 2019, 54, 2318–2324. [Google Scholar] [CrossRef]
- Kapur, R.P. Histology of the Transition Zone in Hirschsprung Disease. Am. J. Surg. Pathol. 2016, 40, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Georgeson, K.E. Laparoscopic-Assisted Pull-Through for Hirschsprung’s Disease. Semin. Pediatr. Surg. 2002, 11, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.C.; On behalf of the American Pediatric Surgical Association Hirschsprung Disease Interest Group; Rollins, M.D.; Levitt, M.; Gosain, A.; de la Torre, L.; Kapur, R.P.; Cowles, R.A.; Horton, J.; Rothstein, D.H.; et al. Guidelines for the management of postoperative obstructive symptoms in children with Hirschsprung disease. Pediatr. Surg. Int. 2017, 33, 523–526. [Google Scholar] [CrossRef]
- Levitt, M.A.; Dickie, B.; Peña, A. Evaluation and treatment of the patient with Hirschsprung disease who is not doing well after a pull-through procedure. Semin. Pediatr. Surg. 2010, 19, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, L.A.; Reppucci, M.L.; Stevens, J.; Arnold, M.; Lovell, M.; Zornoza, M.; Bischoff, A.; De la Torre, L. Should we perform a Hirschsprung redo pull-through on patients with retained transition zone? J. Pediatr. Surg. Open 2023, 3, 100058. [Google Scholar] [CrossRef]
- Earlam, R.J. A vascular cause for aganglionic bowel. Am. J. Dig. Dis. 1972, 17, 255–261. [Google Scholar] [CrossRef]
- Taguchi, T.; Suita, S.; Hirata, Y.; Hirose, R.; Yamada, T.; Toyohara, T. Abnormally shaped arteries in the intestine of Children with Hirschsprung’s disease: Etiological Considerations Relating to Ischemic Theory. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 200–204. [Google Scholar] [CrossRef]
- O’Donovan, A.N.; Habra, G.; Somers, S.; Malone, D.E.; Rees, A.; Winthrop, A.L.; O’Donovan, G.H.A.N.; Martin, L.C.; Merkle, E.M.; Thompson, W.M.; et al. Diagnosis of Hirschsprung’s disease. Am. J. Roentgenol. 1996, 167, 517–520. [Google Scholar] [CrossRef]
- Sahu, R.K.; Kothari, S.; Rahaman, S.R.; Chattopadhyay, A.; Dasgupta, S.; Sen, S. Evaluation of suspicious Hirschsprung disease in children using radiologic investigation method: A prospective observational study. Int. Surg. J. 2017, 4, 1525. [Google Scholar] [CrossRef][Green Version]
| Length of Normoganglionic Segment in Resections | Number of Cases |
|---|---|
| 5 cm | 7 |
| 7 cm | 18 |
| 10 cm | 23 |
| 15 cm | 12 |
| Evaluation | Macroscopic Evaluation: Normal | Macroscopic Evaluation: Abnormal |
|---|---|---|
| Microscopic evaluation: normal | True Positives—60 | False positives—0 |
| Microscopic evaluation: abnormal | False negatives—0 | True negatives—0 |
| Evaluation | True Positive | Accuracy |
|---|---|---|
| Macroscopic | 17 | 28.33% |
| Microscopic | 60 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ademaj, I.; Hyseni, N.; Gjonbalaj, N. Is It Safe to Operate without Frozen Section Biopsies in Short-Segment Hirschsprung’s Disease? An Overview of 60 Cases. Pediatr. Rep. 2024, 16, 542-550. https://doi.org/10.3390/pediatric16030045
Ademaj I, Hyseni N, Gjonbalaj N. Is It Safe to Operate without Frozen Section Biopsies in Short-Segment Hirschsprung’s Disease? An Overview of 60 Cases. Pediatric Reports. 2024; 16(3):542-550. https://doi.org/10.3390/pediatric16030045
Chicago/Turabian StyleAdemaj, Isber, Nexhmi Hyseni, and Naser Gjonbalaj. 2024. "Is It Safe to Operate without Frozen Section Biopsies in Short-Segment Hirschsprung’s Disease? An Overview of 60 Cases" Pediatric Reports 16, no. 3: 542-550. https://doi.org/10.3390/pediatric16030045
APA StyleAdemaj, I., Hyseni, N., & Gjonbalaj, N. (2024). Is It Safe to Operate without Frozen Section Biopsies in Short-Segment Hirschsprung’s Disease? An Overview of 60 Cases. Pediatric Reports, 16(3), 542-550. https://doi.org/10.3390/pediatric16030045






