Clinical Study and Microbiological Analysis of Periodontopathogenic Microflora Analyzed among Children and Adolescents with Cardiovascular Diseases Compared to Group with Good General Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
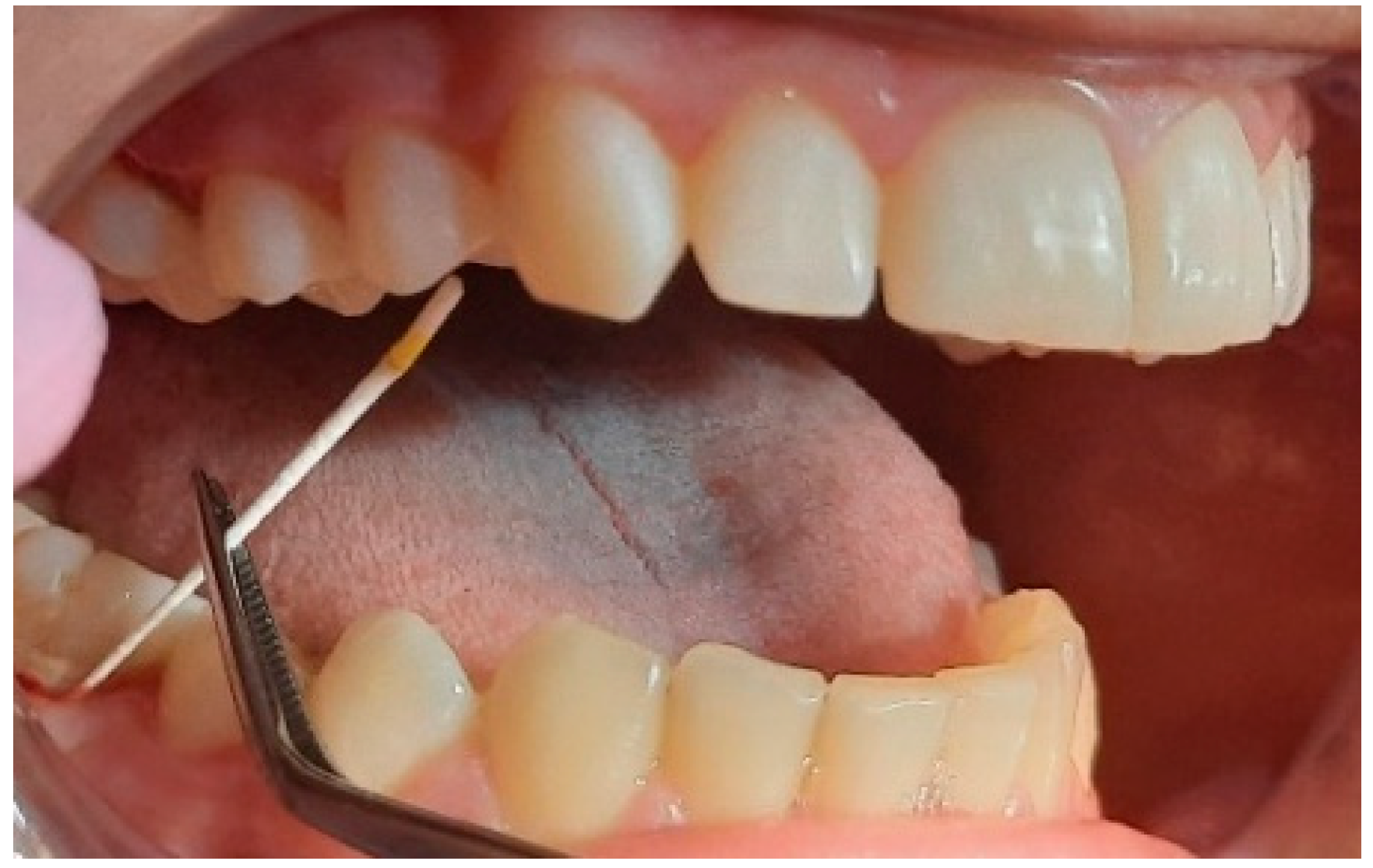
2.2. Clinical Examinations
2.3. Sampling for PCR
2.4. Extraction of DNA
2.5. Specialist Treatment
2.6. Statistical Data Analysis
3. Results
3.1. Analysis of Demographic Data
3.2. Analysis of Clinical Data
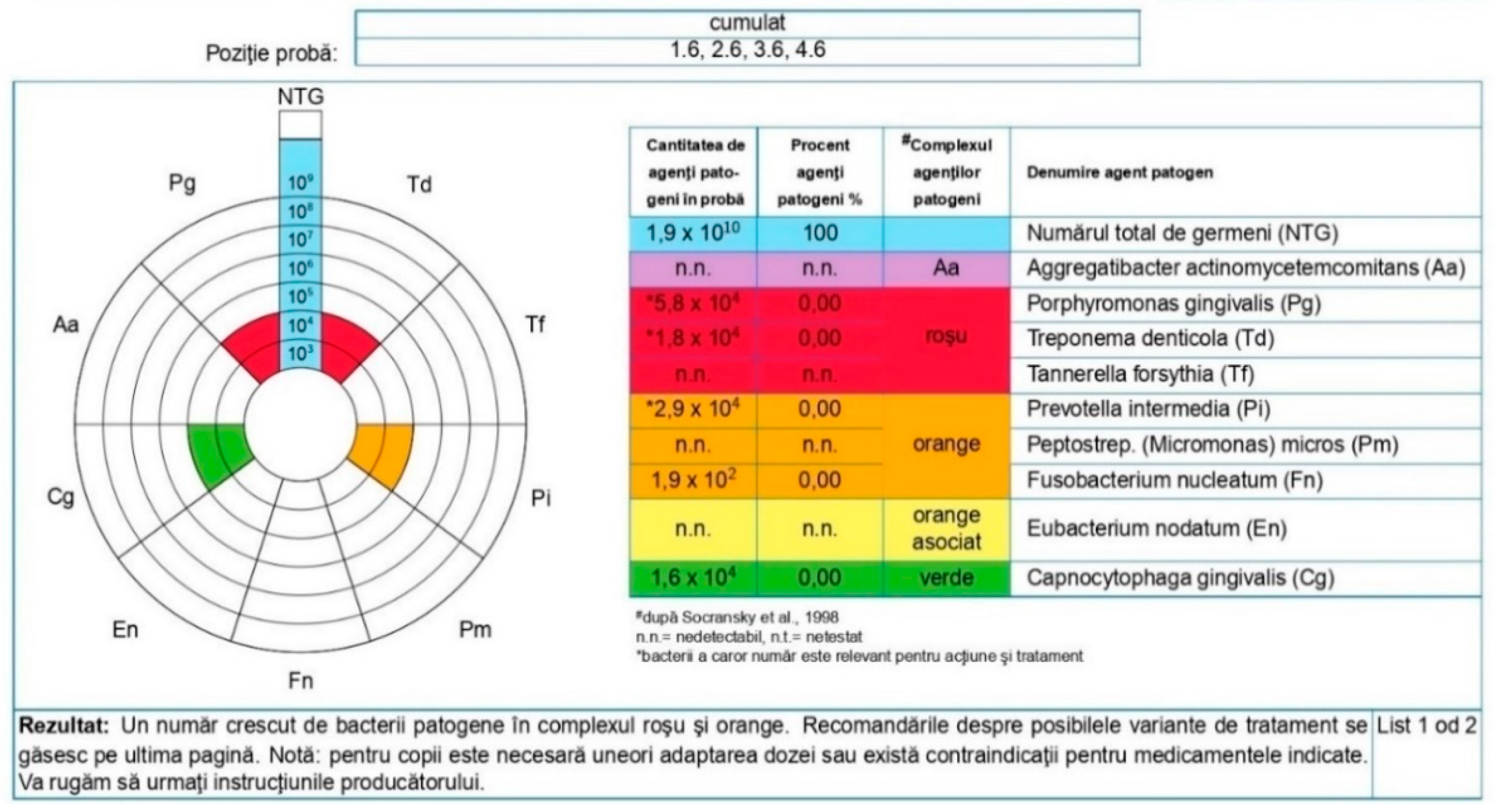
3.3. Results of Microbiological Determinations
| Signif. Codes | p Value | Interpretation |
| **** | aproximately 0 | Extremely strong evidence against the null hypothesis |
| *** | 0 < p < 0.001 | Rather strong evidence against the null hypothesis |
| ** | 0.001 ≤ p < 0.01 | Strong evidence against the null hypothesis |
| * | 0.01 ≤ p < 0.05 | Moderate evidence against the null hypothesis |
| . | 0.05 ≤ p < 0.1 | Weak evidence against the null hypothesis |
| ns | 0.1 ≤ p | Insignificant, with a lack of evidence against the null hypothesis (the data are consistent with the null hypothesis) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology: Newman and Carranza’s Clinical Periodontology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Valm, A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Inquimbert, C.; Bourgeois, D.; Bravo, M.; Viennot, S.; Tramini, P.; Llodra, J.C.; Molinari, N.; Dussart, C.; Giraudeau, N.; Carrouel, F. The oral bacterial microbiome of interdental surfaces in adolescents according to carious risk. Microorganisms 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Roman, A. Parodontologie vol. 1, Noțiuni de bază, Ed Med Univ Iuliu Hatieganuu 2017.
- Olanié, F. Les Tests Biologiques en Parodontologie. Doctoral Dissertation. 2008. Available online: https://paroconseil.ca/wp-content/uploads/2020/11/Les-tests-biologiques-en-parodontologie.pdf (accessed on 15 April 2024).
- Socransky, S. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1988, 25, 134–144. [Google Scholar]
- Mitova, N.; Rashkova, M. Depth of Gingival Sulcus in Healthy Children with Erupting Permanent Teeth. Folia Medica 2020, 62, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Vodiță, C.; Ghergic, D.L.; Vodiță, E.A.; Comăneanu, R.M.; Mihai, L.L. Detection of bacterial species associated with periodontal disease in a group of patients. Rom. J. Stomatol. 2021, 67, 128. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol. 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.S.; Chauhan, R.S.; Devkar, N.; Vibhute, A.; More, S. Gingival and periodontal diseases in children and adolescents. J. Dent. Allied Sci. 2012, 1, 26–29. [Google Scholar] [CrossRef]
- Vagdouti, T.; Tsilingaridis, G. Periodontal diseases in children and adolescents affected by systemic disorders—A literature review. Int. J. Oral. Dent. Health 2018, 4, 1–10. [Google Scholar]
- Meyle, J.; Gonzales, J.R. Influences of systemic diseases on periodontitis in children and adolescents. Periodontol. 2000 2001, 26, 92–112. [Google Scholar] [CrossRef]
- Khocht, A.; Albandar, J.M. Aggressive forms of periodontitis secondary to systemic disorders. Periodontol. 2000 2014, 65, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S44–S67. [Google Scholar] [CrossRef]
- FitzGerald, K.; Fleming, P.; Franklin, O. Dental health and management for children with congenital heart disease. Prim. Dent. Care 2010, os17, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, E.; Eckert, G.J.; Kowolik, M.J.; Ho, J.G.; Schamberger, M.S.; Kowolik, J.E. Gingival evaluation of the pediatric cardiac patient. Pediatr. Dent. 2013, 35, 456–462. [Google Scholar] [PubMed]
- Inqu Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 2013, 84, S51–S69. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.; Ray-Chaudhuri, A.; Vaidyanathan, M.; Johnson, J.; Sood, S. Simplified basic periodontal examination (BPE) in children and adolescents: A guide for general dental practitioners. Dent. Update 2014, 41, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Masamatti, S.S.; Kumar, A.; Virdi, M.S. Periodontal diseases in children and adolescents: A clinician’s perspective part 1. Dent. Update 2012, 39, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Masamatti, S.S.; Virdi, M.S. Periodontal diseases in children and adolescents: A clinician’s perspective part 2. Dent. Update 2012, 39, 639–652. [Google Scholar] [CrossRef]
- Mitova, N.; Rashkova, M.; Popova, C. Quantity, diversity and complexity of subgingival microorganisms in children with plaque-induced gingivitis. Biotechnol. Biotechnol. Equip. 2019, 33, 620–626. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Li, J.; Aprecio, R.M.; Zhang, W.; Li, Y. Real-time PCR quantification of six periodontal pathogens in saliva samples from healthy young adults. Clin. Oral Investig. 2015, 19, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Asociaţia Societatea Română. Ghid de Practică în Prevenţia Oro-Dentară; Asociaţia Societatea Română de Ergonomie Dentară: Bucureşti, Romania, 2013; 104p. [Google Scholar]
- Shimpi, N.; Dart, R.; Umukoro, P.; Acharya, A. Interdisciplinary care model: Cardiovascular diseases and oral health. In Integration of Medical and Dental Care and Patient Data; Springer: Cham, Switzerland, 2019; pp. 71–85. [Google Scholar]
- Shetty, D.; Dua, M.; Kumar, K.; Dhanpal, R.; Astekar, M.; Shetty, D.C. Oral hygiene status of individuals with cardiovascular diseases and associated risk factors. Clin. Pract. 2012, 2, e86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.; Kim, H.J.; Jeon, J.; Song, T.J. Association between oral health and cardiovascular outcomes in patients with hypertension: A nationwide cohort study. J. Hypertens. 2022, 40, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kozarov, E.; Sweier, D.; Shelburne, C.; Progulske-Fox, A.; Lopatin, D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006, 8, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Igboin, C.O.; Griffen, A.L.; Leys, E.J. Porphyromonas gingivalis strain diversity. J. Clin. Microbiol. 2009, 47, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Huang, Y.F.; Chou, M.Y. Occurrence of Porphyromonas gingivalis and Tannerella forsythensis in periodontally diseased and healthy subjects. J. Periodontol. 2004, 75, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Del Pinto, R.; Pietropaoli, D.; Ferri, C. Oral health as a modifiable risk factor for cardiovascular diseases. Trends Cardiovasc. Med. 2023, 34, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hase, S.; Hofstad, T.; Rietschel, E.T. Chemical structure of the lipid A component of lipopolysaccharides from Fusobacterium nucleatum. J. Bacteriol. 1977, 129, 9–14. [Google Scholar] [CrossRef]
- Hofstad, T.; Skaug, N.; Bjørnland, T. O-antigenic cross-reactivity in Fusobacterium nucleatum. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. 1979, 87, 371–374. [Google Scholar] [CrossRef]
- Bachrach, G.; Rosen, G.; Bellalou, M.; Naor, R.; Sela, M.N. Identification of a Fusobacterium nucleatum 65 kDa serine protease. Oral. Microbiol. Immunol. 2004, 19, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.; Silva-Boghossian, C.M.; do Souto, R.M.; Colombo, A.P.V. Subgingival microbial profiles of generalized aggressive and chronic periodontal diseases. Arch. Oral Biol. 2012, 57, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Antezack, A.; Etchecopar-Etchart, D.; La Scola, B.; Monnet-Corti, V. New putative periodontopathogens and periodontal health-associated species: A systematic review and meta-analysis. J. Periodontal Res. 2023, 58, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Sabella, F.M.; de Feiria, S.N.B.; Ribeiro, A.D.A.; Theodoro, L.H.; Höfling, J.F.; Parisotto, T.M.; Duque, C. Exploring the interplay between oral diseases, microbiome, and chronic diseases driven by metabolic dysfunction in childhood. Front. Dent. Med. 2021, 2, 718441. [Google Scholar] [CrossRef]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]




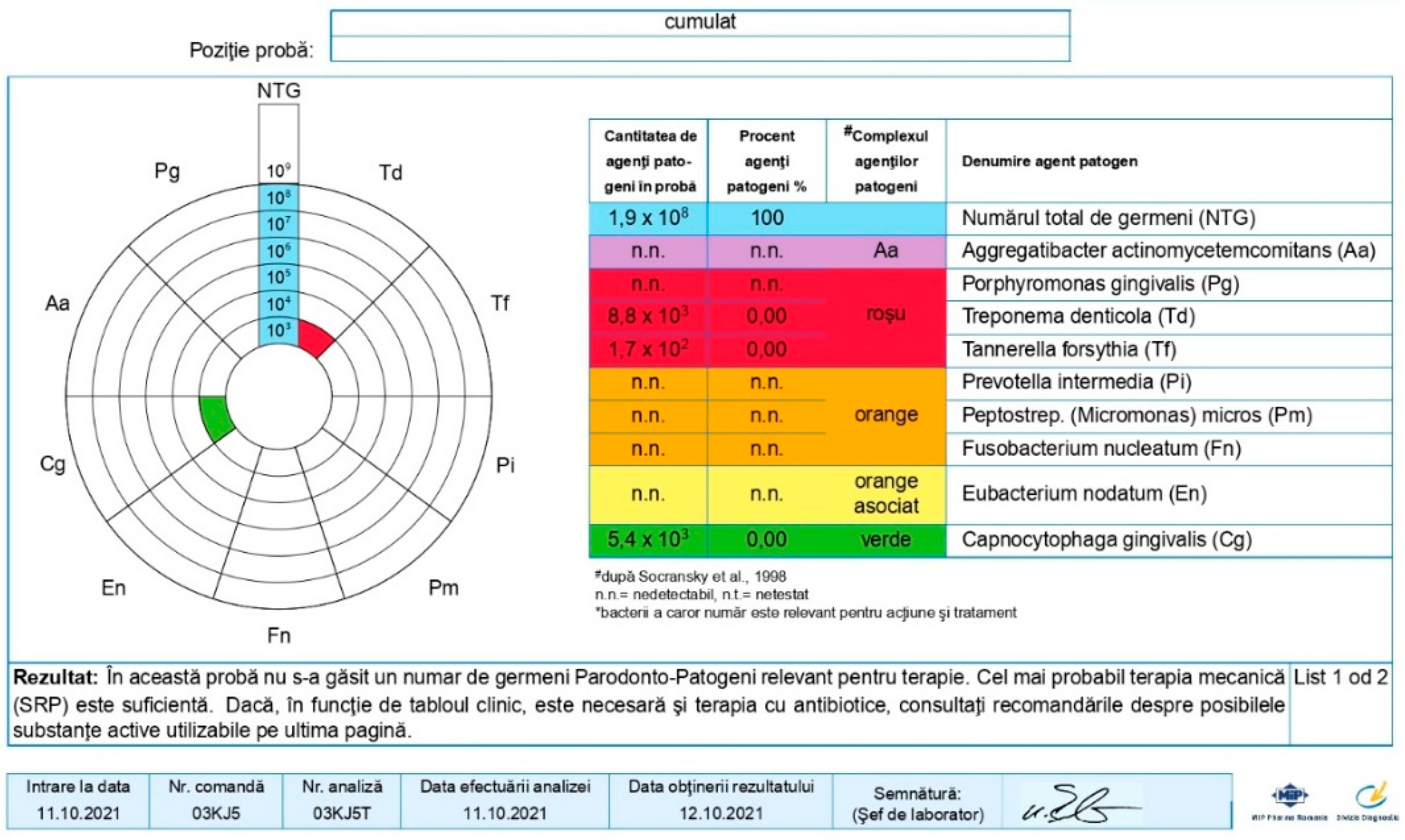

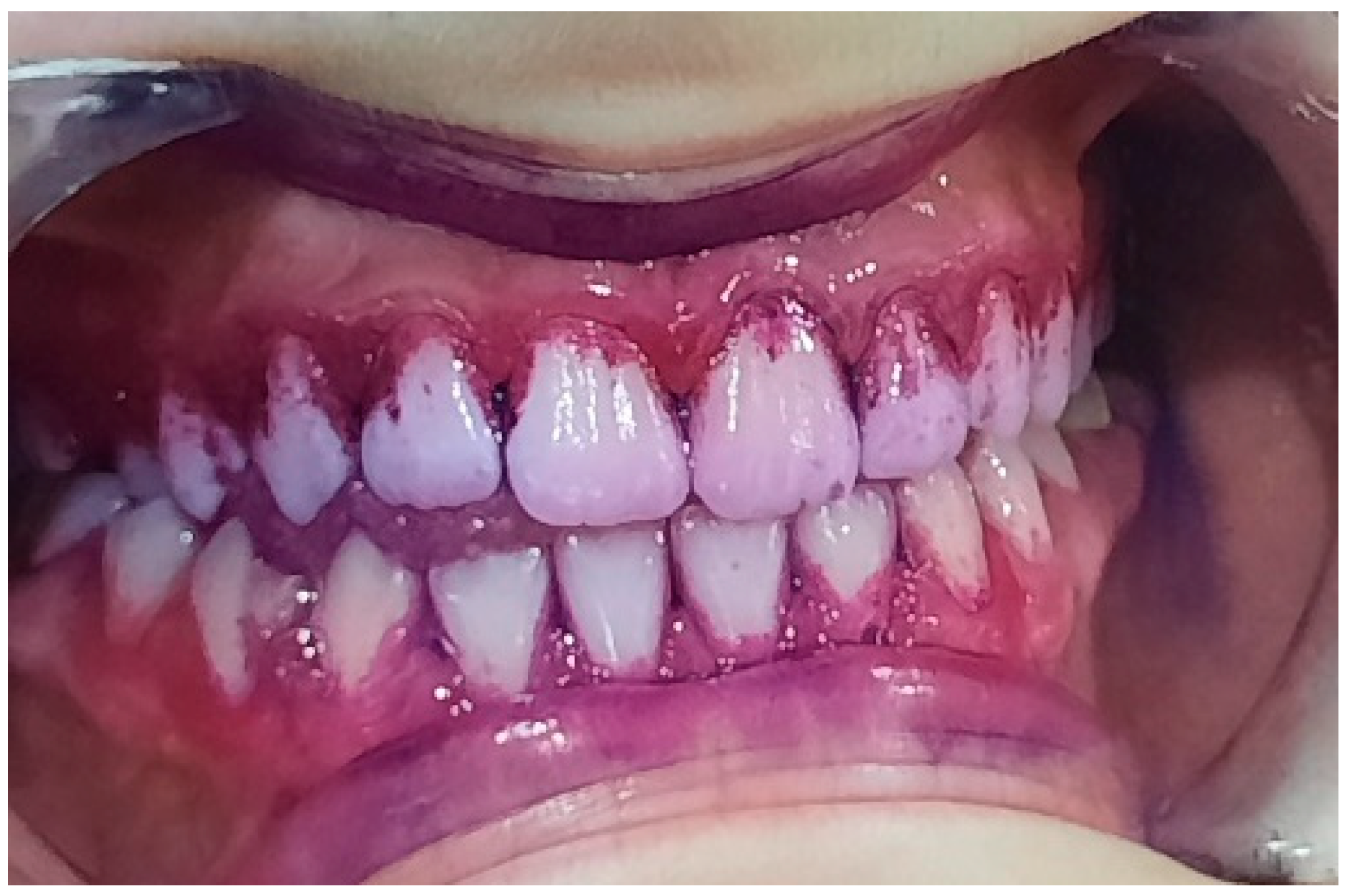

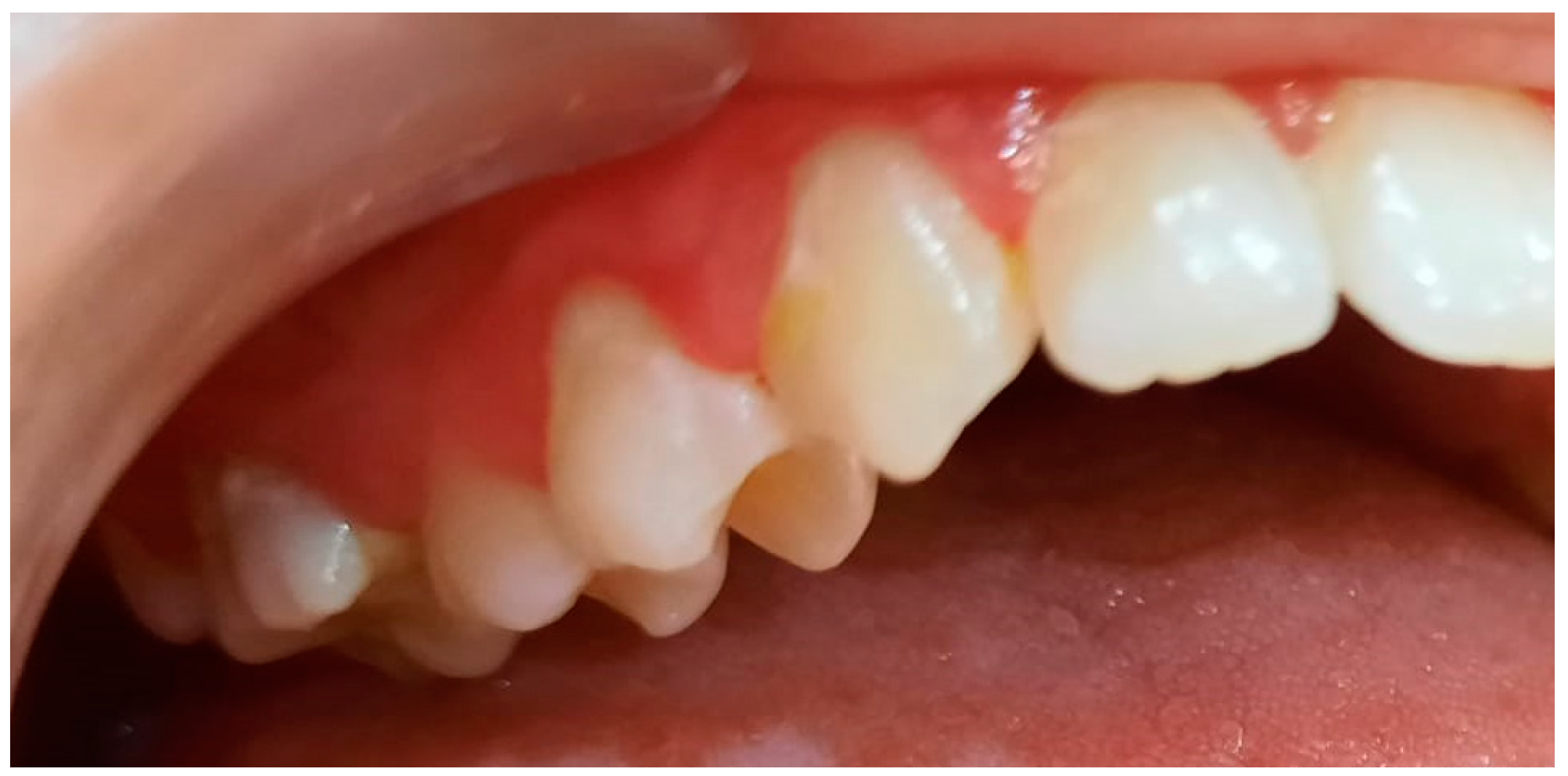
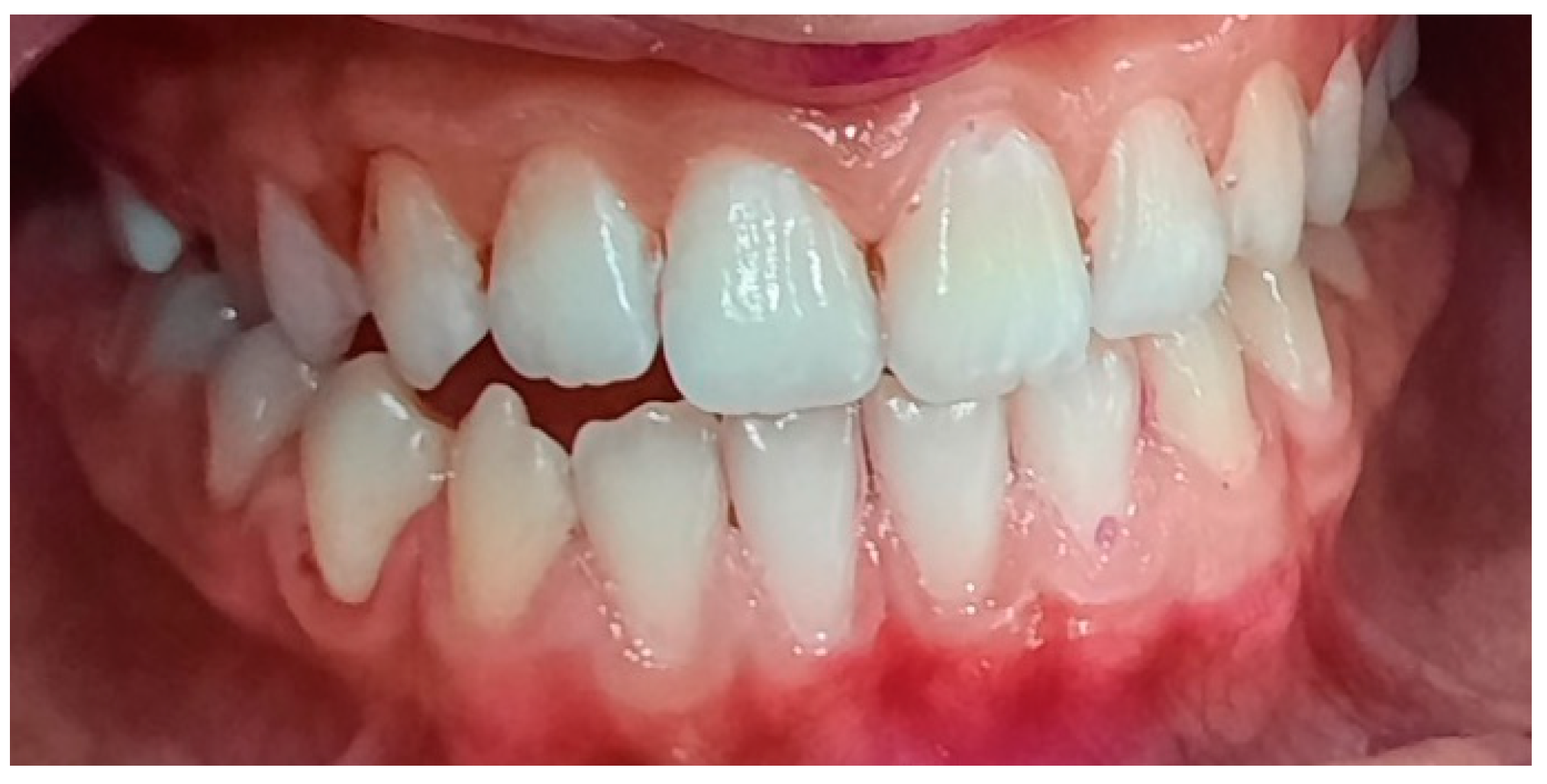

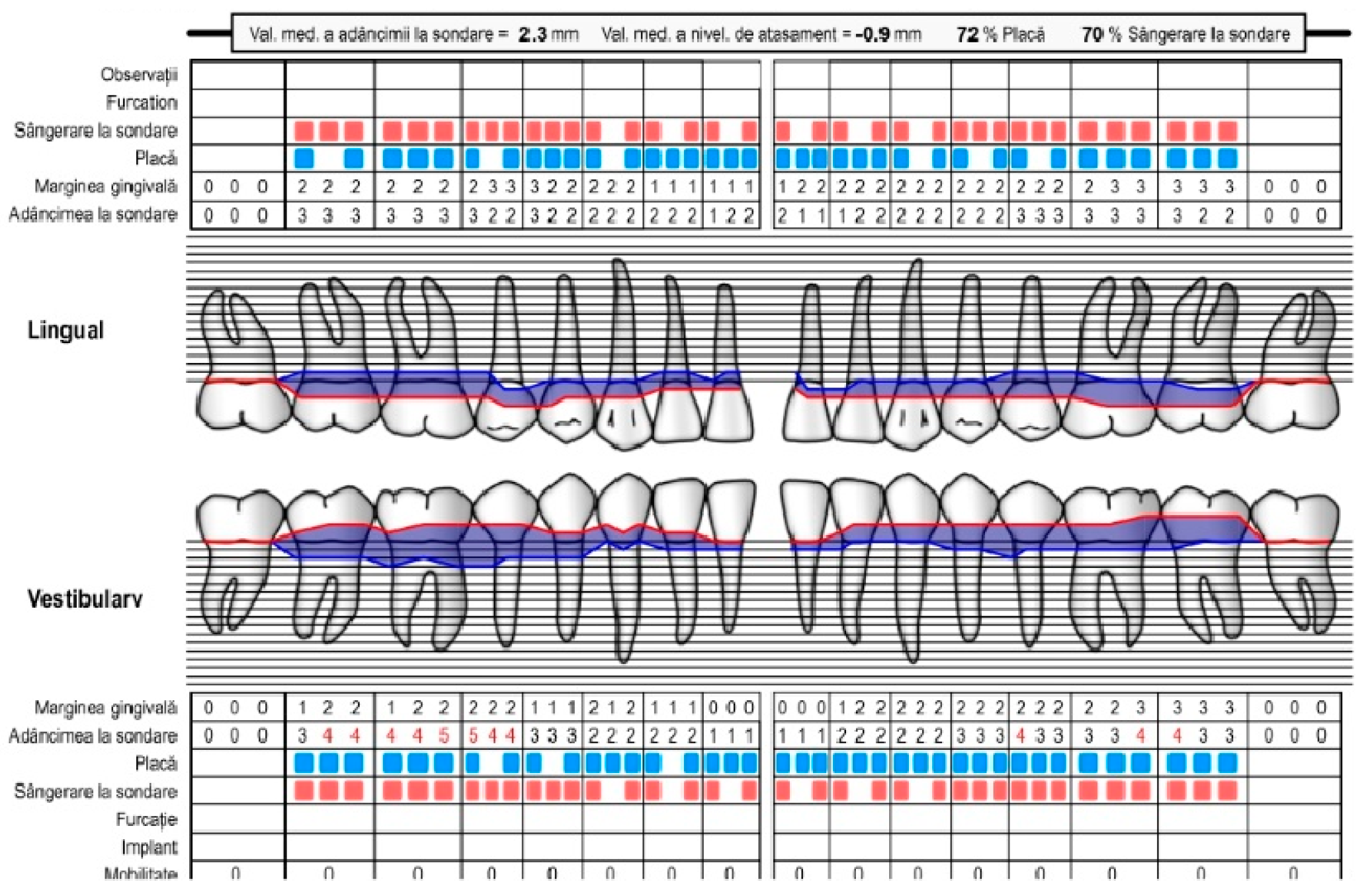
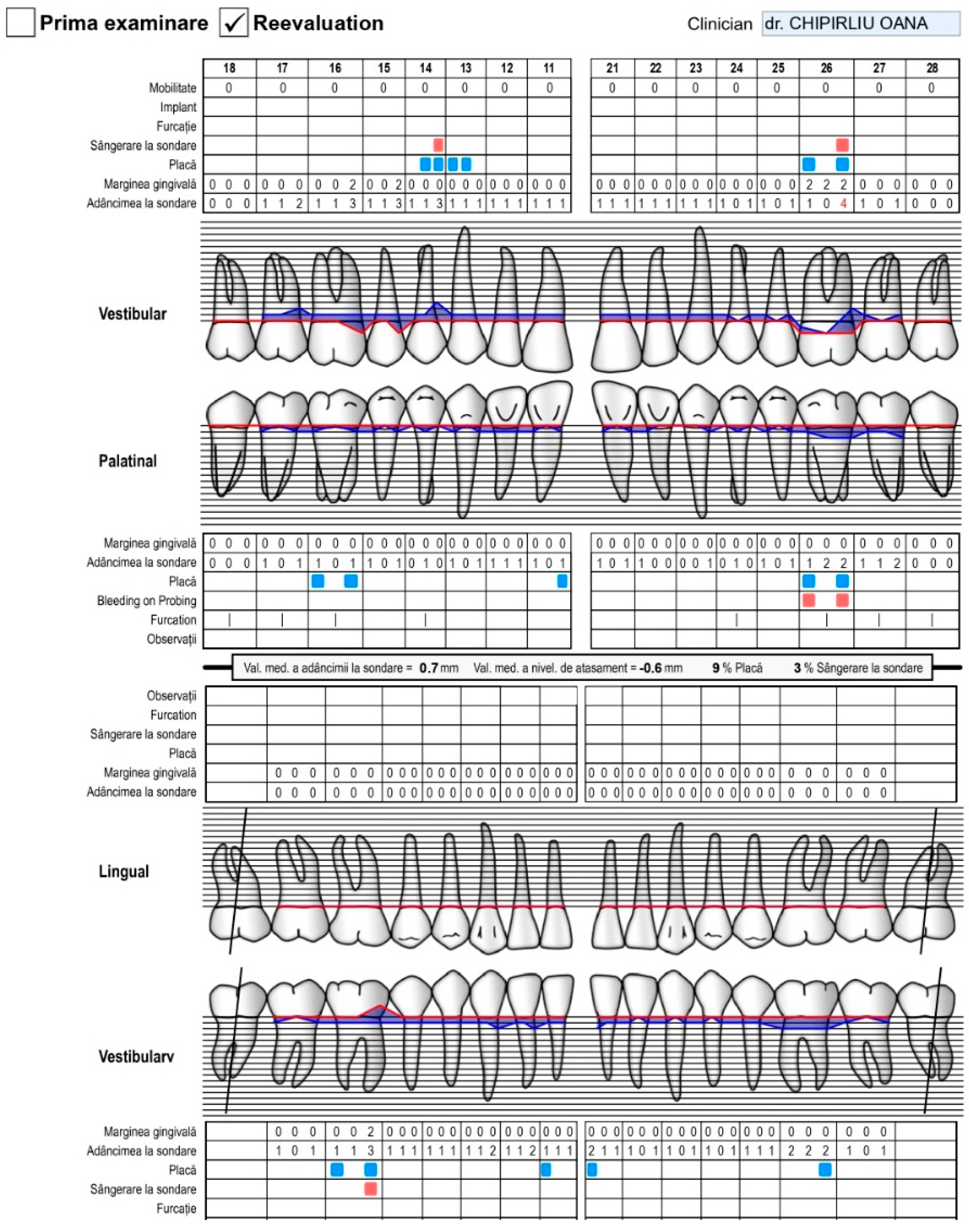
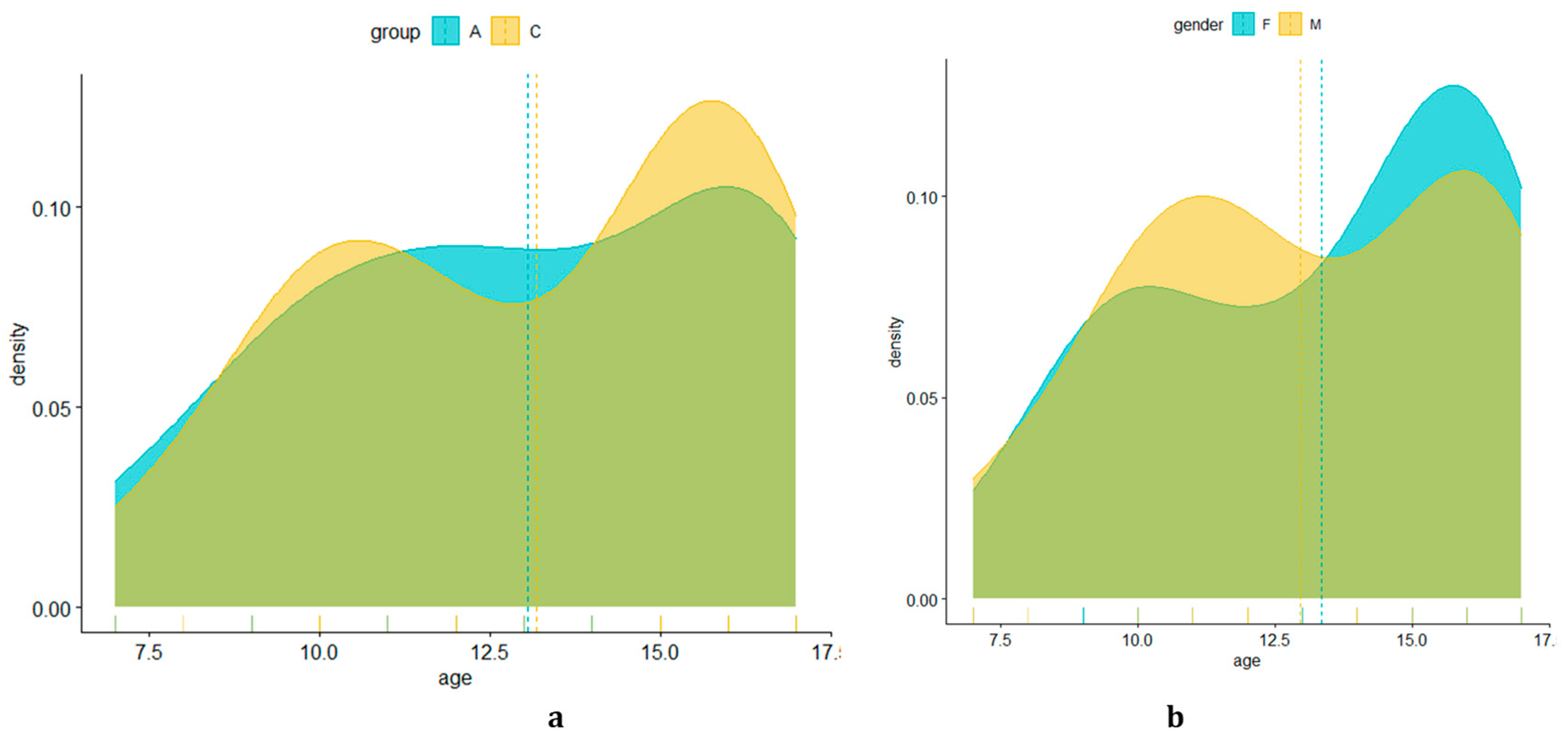
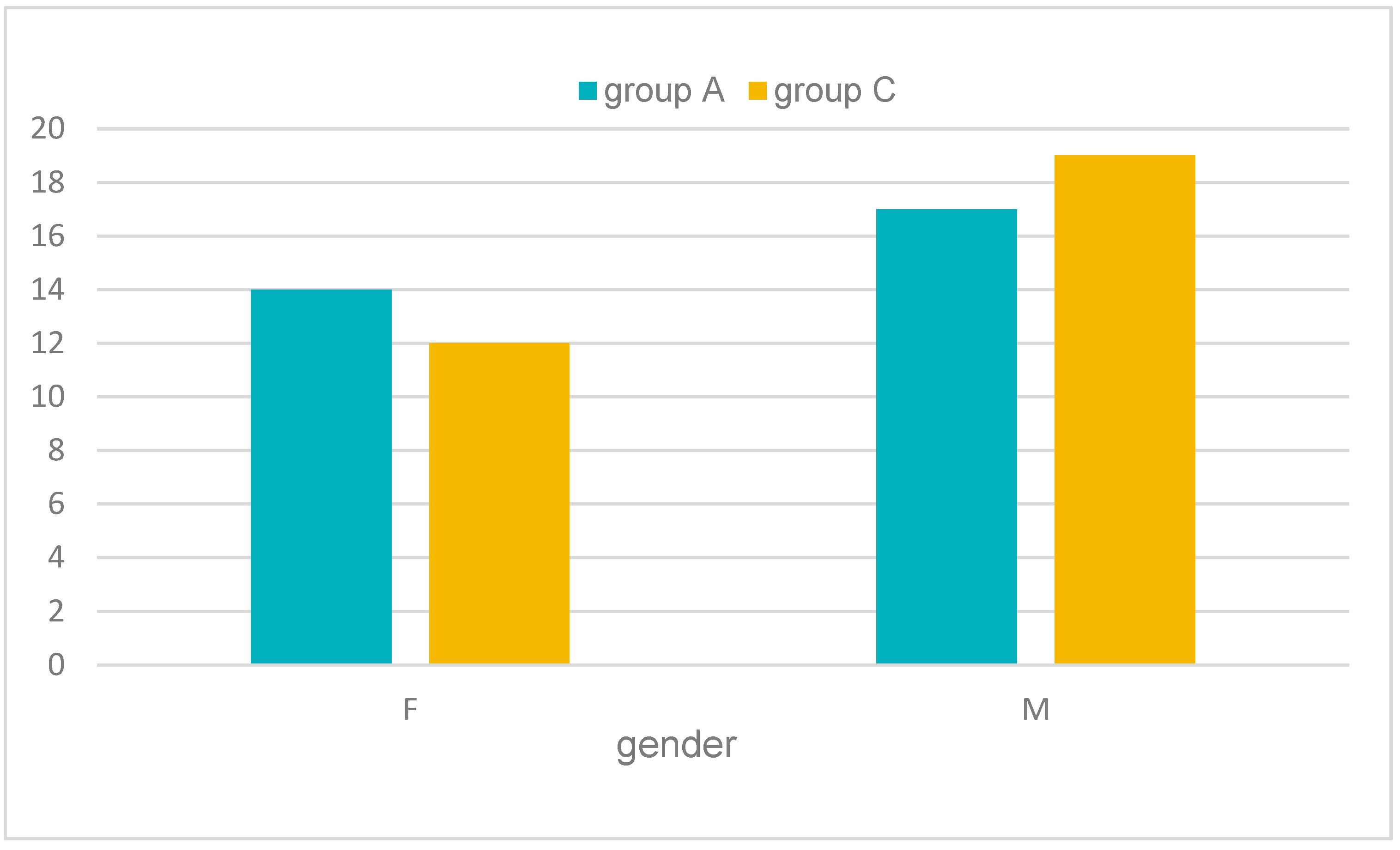



| Age | |||||||
|---|---|---|---|---|---|---|---|
| Groups | N | Min | 1st Qu. | Median | Mean | 3rd Qu. | Max |
| all | 62 | 7 | 10 | 13.5 | 13.13 | 16 | 17 |
| group A | 31 | 7 | 10.5 | 13 | 13.06 | 16 | 17 |
| group C | 31 | 7 | 10 | 14 | 13.19 | 16 | 17 |
| Gender | |||||
|---|---|---|---|---|---|
| Groups | N | F | M | ||
| all | 62 | 26 | 41.94% | 36 | 58.06% |
| group A | 31 | 14 | 45.16% | 17 | 54.84% |
| group C | 31 | 12 | 38.71% | 19 | 61.29% |
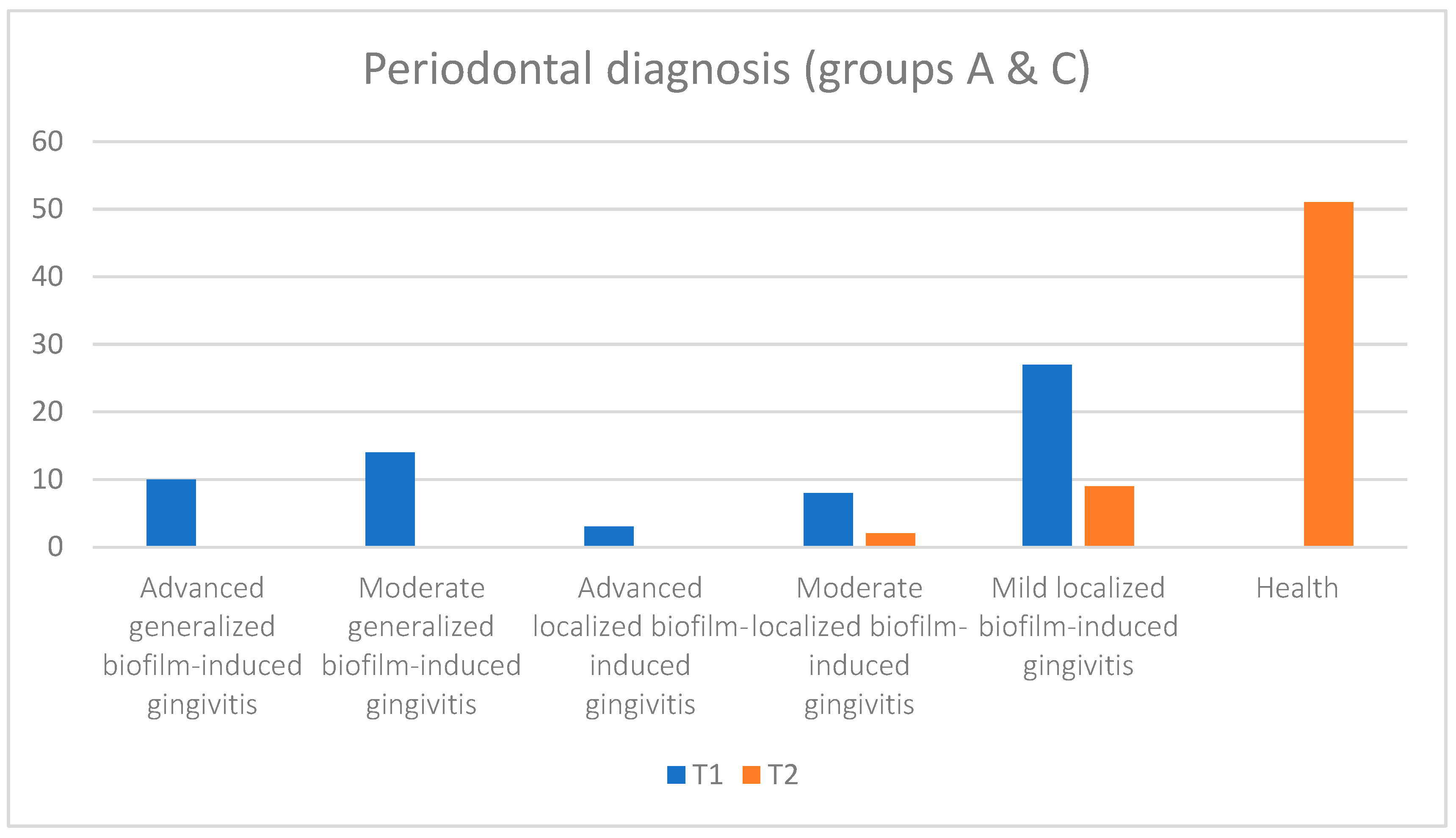
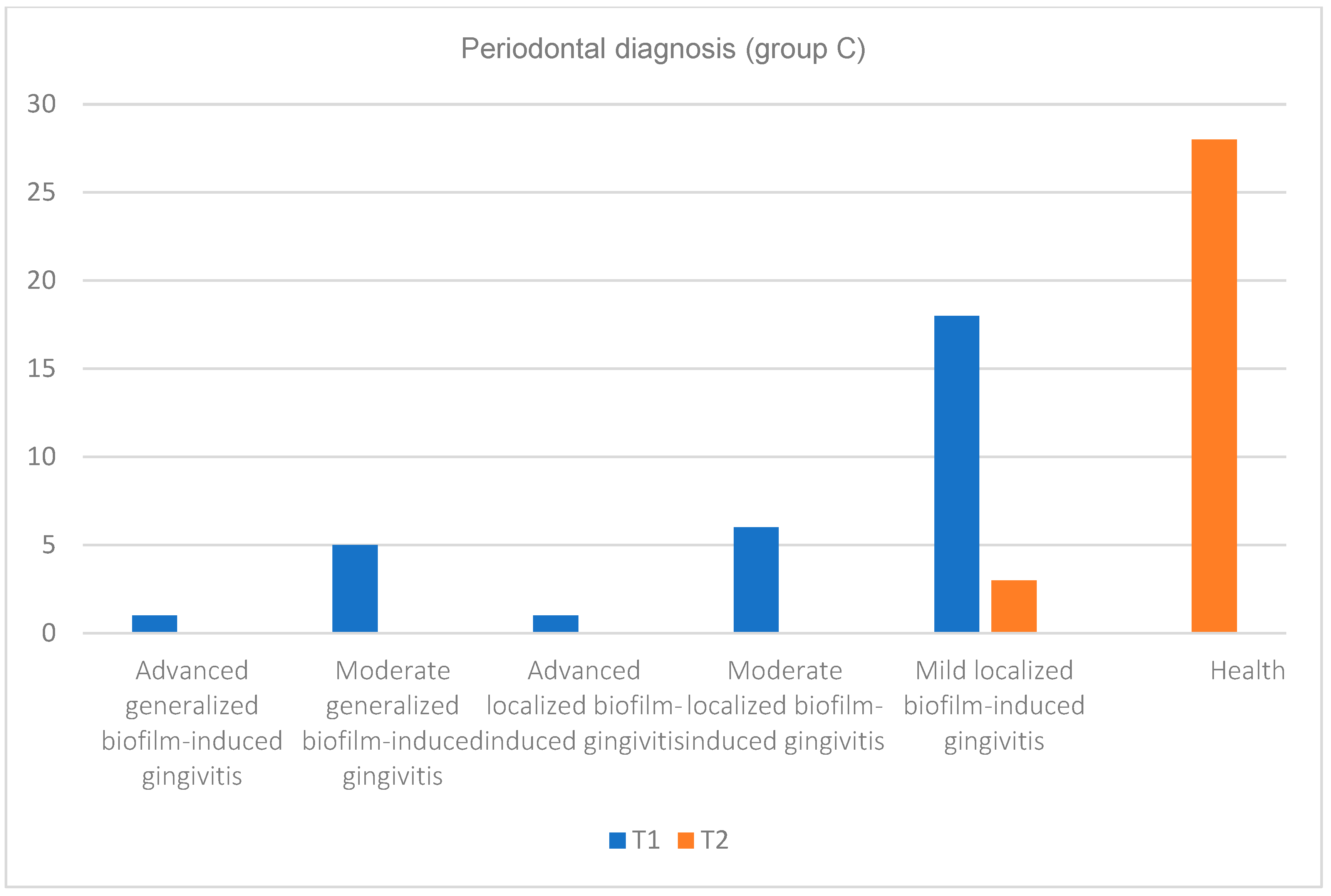
| Periodontal Diagnosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced Generalized Biofilm-Induced Gingivitis | Moderate Generalized Biofilm-Induced Gingivitis | Advanced Localized Biofilm-Induced Gingivitis | Moderate Localized Biofilm-Induced Gingivitis | Mild Localized Biofilm-Induced Gingivitis | Health | ||||||||
| Groups | N | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 |
| all | 62 | 10 | 0 | 14 | 0 | 3 | 0 | 8 | 2 | 27 | 9 | 0 | 51 |
| group A | 31 | 9 | 0 | 9 | 0 | 2 | 0 | 2 | 2 | 9 | 6 | 0 | 23 |
| group C | 31 | 1 | 0 | 5 | 0 | 1 | 0 | 6 | 0 | 18 | 3 | 0 | 28 |
| Gingival Index | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gums with Normal Appearance (Cod 0) | Gums with Mild Inflammation (Cod 1) | Gums with Moderate Inflammation (Cod 2) | Gums with Advanced Inflammation (Cod 3) | ||||||
| Groups | N | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 |
| all | 62 | 0 | 51 | 26 | 9 | 23 | 2 | 13 | 0 |
| group A | 31 | 0 | 23 | 9 | 6 | 11 | 2 | 11 | 0 |
| group C | 31 | 0 | 28 | 17 | 3 | 12 | 0 | 2 | 0 |
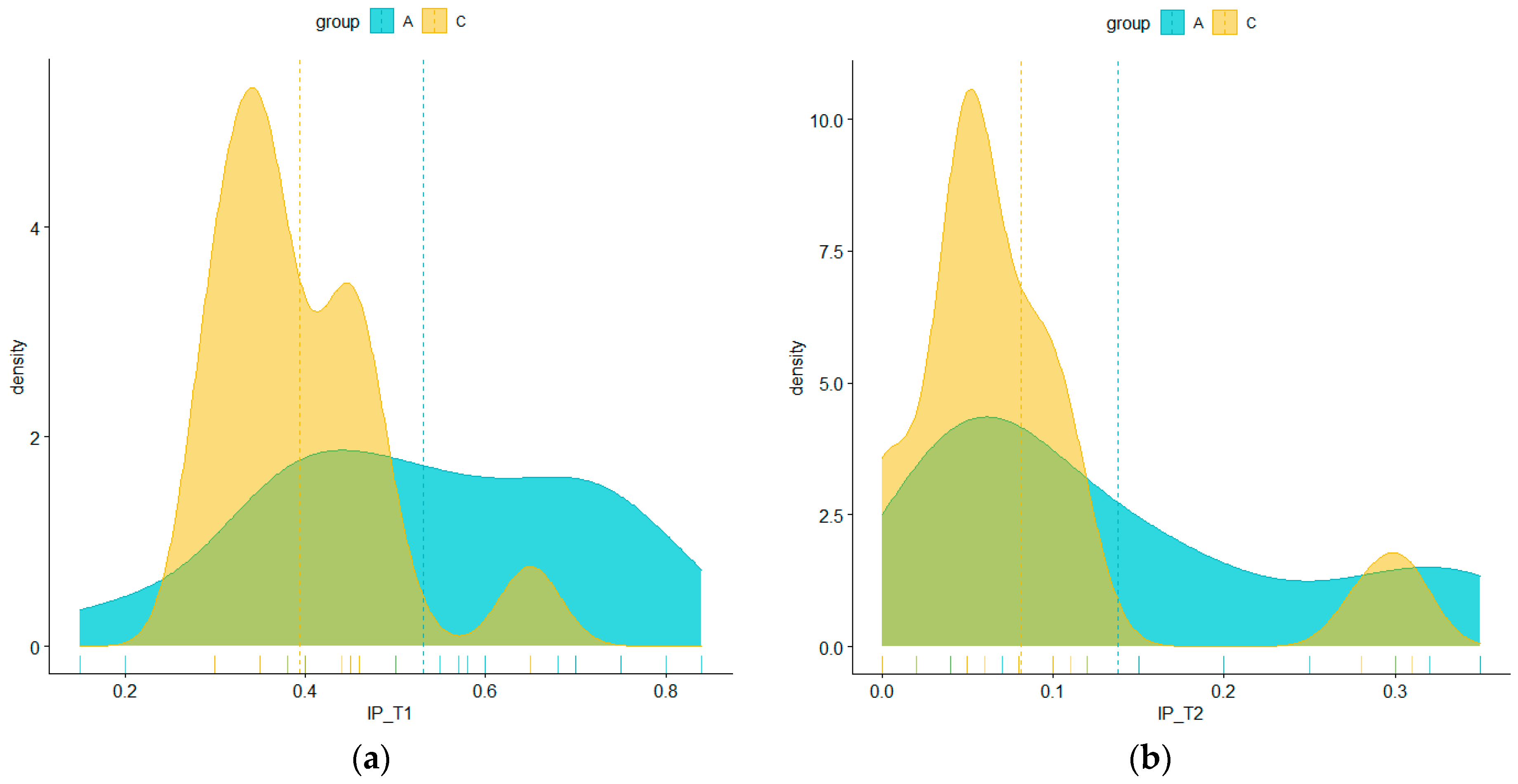
| IP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 1st Qu. | Median | Mean | 3rd Qu. | Max | ||||||||
| Groups | N | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 |
| all | 62 | 15.00% | 0.00% | 35.00% | 5.00% | 42.00% | 6.50% | 46.21% | 10.94% | 56.50% | 14.25% | 84.00% | 35.00% |
| group A | 31 | 15.00% | 2.00% | 40.00% | 5.00% | 50.00% | 10.00% | 53.06% | 13.77% | 70.00% | 20.00% | 84.00% | 35.00% |
| group C | 31 | 30.00% | 0.00% | 35.00% | 5.00% | 35.00% | 5.00% | 39.35% | 8.10% | 45.00% | 10.00% | 65.00% | 31.00% |
| IP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | t | df | p Value | Signif | ||||
| Groups | N | Mean | ±SD | Mean | ±SD | ||||
| all | 62 | 46.21% | 15.67% | 10.94% | 9.94% | 14.97 | 102.28 | 1.28 × 10-27 | **** |
| group A | 31 | 53.06% | 17.84% | 13.77% | 11.06% | 10.42 | 50.09 | 3.83 × 10-14 | **** |
| group C | 31 | 39.35% | 9.16% | 8.10% | 7.88% | 14.40 | 58.71 | 6.28 × 10-21 | **** |
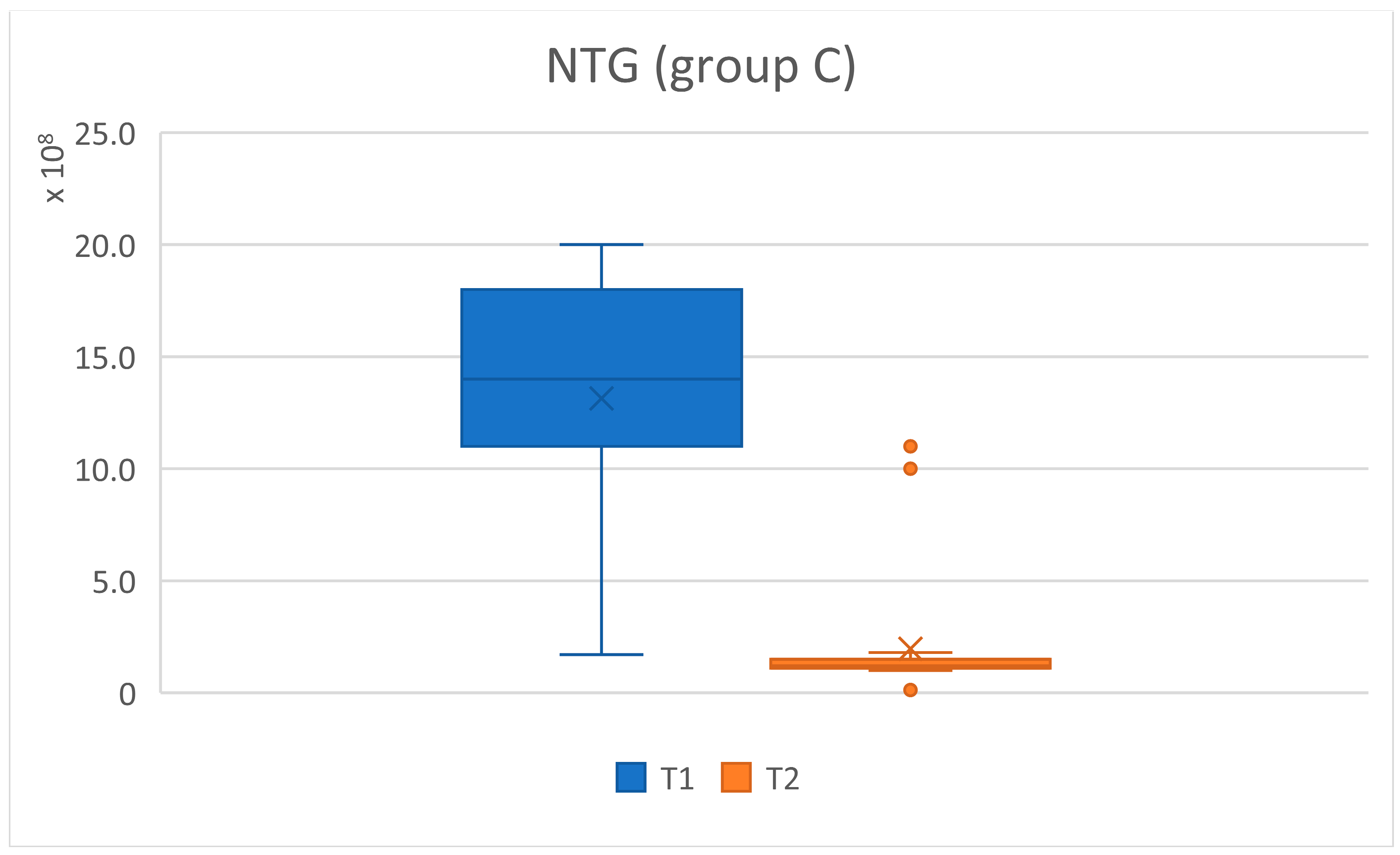
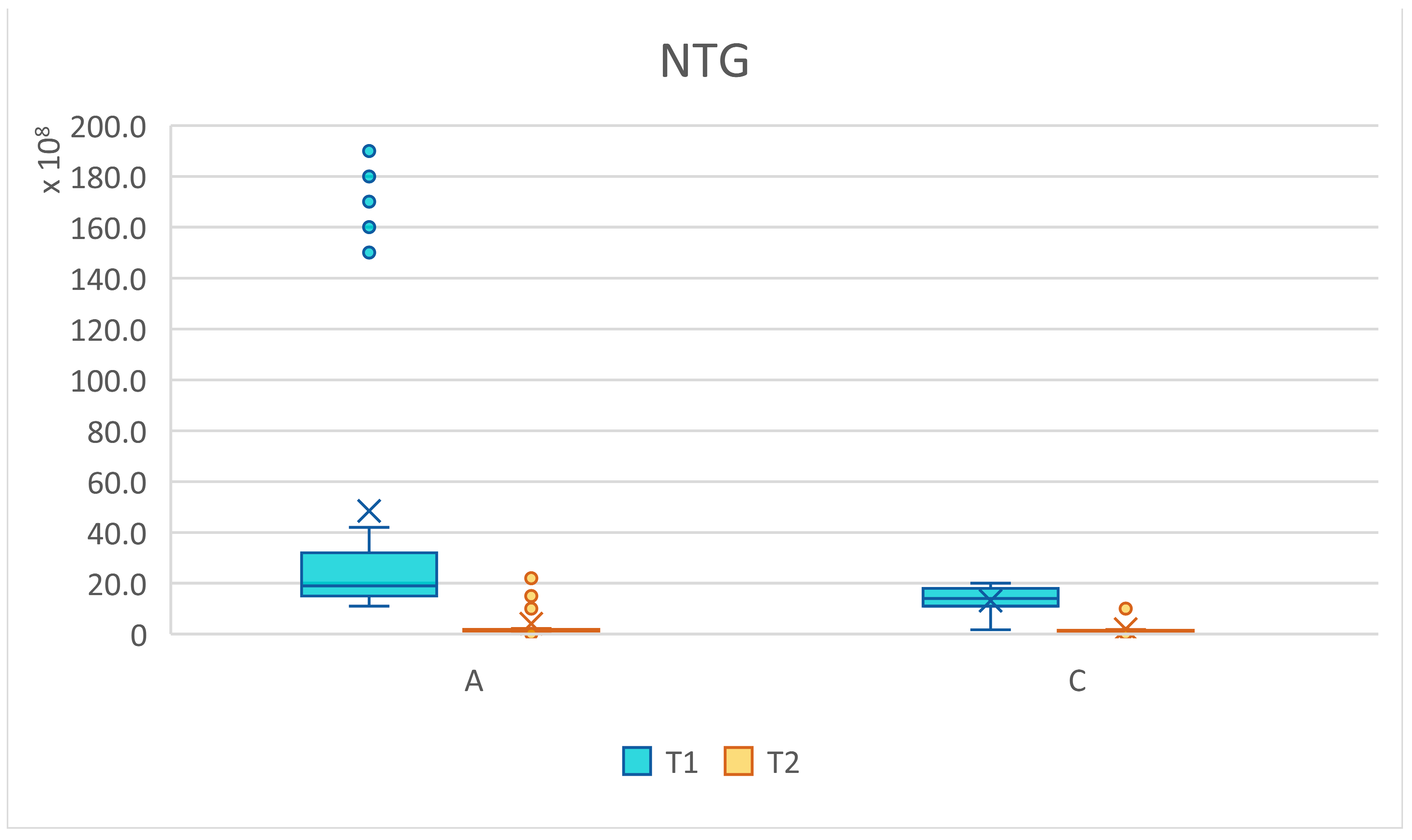
| NTG | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | t | df | p Value | Signif | ||||
| Groups | N | Mean | ±SD | Mean | ±SD | ||||
| all | 62 | 3.08 × 109 | 4.66 × 109 | 3.05 × 108 | 4.90 × 108 | 4.66 | 62.35 | 0.0000169 | **** |
| group A | 31 | 4.85 × 109 | 6.12 × 109 | 4.13 × 108 | 6.20 × 108 | 4.02 | 30.62 | 0.000354 | *** |
| group C | 31 | 1.31 × 109 | 5.86 × 108 | 1.96 × 108 | 2.83 × 108 | 9.56 | 43.24 | 3.16 × 10-12 | **** |
| T1 | T2 | t | df | p Value | Signif | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator | N | #n.n. | Mean/Val | ±SD | #n.n. | Mean | ±SD | ||||
| Aa | 62 | 51/11Aa | 13,181.82 | 6896.64 | 57/5Aa | 1320 | 438.18 | 5.68 | 10.62 | 0.000191 | *** |
| Pg | 62 | 17/45 Pg | 38,984.44 | 34,805.29 | 44/18Pg | 7722.22 | 7493.81 | 5.70 | 52.95 | 5.35 × 10-7 | **** |
| Td | 62 | 16/46 Td | 35,613.04 | 50,352.79 | 26/36Td | 4206.94 | 3819.53 | 4.21 | 45.66 | 0.000117 | *** |
| Tf | 62 | 10/52 Tf | 13,707.69 | 7989.23 | 32/32Tf | 1529.33 | 2093.62 | 10.39 | 62.31 | 3.13 × 10-15 | **** |
| Pi | 62 | 25/37 Pi | 29,364.86 | 27,264.38 | 37/25Pi | 1032.40 | 724.93 | 6.32 | 36.08 | 2.60 × 10-7 | **** |
| Pm | 62 | 41/21 Pm | 22,266.67 | 17,722.06 | 46/16 | 1711.25 | 2588.38 | 5.24 | 21.11 | 3.33 × 10-5 | **** |
| Fn | 62 | 13/49 Fn | 7757.14 | 7825.23 | 45/17 | 719.41 | 586.28 | 6.25 | 49.53 | 9.41 × 10-8 | **** |
| En | 62 | 54/8 En | 6212.50 | 1389.18 | 57/5En | 410 | 610.04 | 10.33 | 10.27 | 9.47 × 10-7 | **** |
| Cg | 62 | 0/62 Cg | 19,258.06 | 6316.61 | 0/62Cg | 5285.32 | 5227.73 | 13.42 | 117.88 | 7.30 × 10-24 | **** |
| Parameter | N (%) Patients Total Lot T1 | N (%) Patients Total Lot T2 | N (%) Patients Lot A T1 | N (%) Patients Lot A T2 | N (%) Patients Lot C T1 | N (%) Patients Lot C T2 |
|---|---|---|---|---|---|---|
| 11 (17.7%) | 5 (8.1%) | 7 (11.3%) | 4 (6.5%) | 4 (6.5%) | 1 (1.6) |
| 45 (72.6%) | 18 (29.0) | 25 (40.3%) | 10 (16.1) | 20 (32.3%) | 8 (12.9%) |
| 46 (74.2%) | 36 (58.1%) | 26 (41.9) | 19 (30.6) | 20 (32.3%) | 19 (30.6) |
| 52 (83.9%) | 32 (51.6%) | 26 (41.9) | 16 (25.8%) | 26 (41.9%) | 16 (25.8%) |
| 37 (59.7%) | 25 (40.3%) | 20 (32.3%) | 13 (21.0%) | 17 (27.4%) | 12 (19.4%) |
| 21 (33.9) | 16 (25.8%) | 12 (19.4%) | 9 (14.5%) | 9 (14.5%) | 7 (11.3%) |
| 49 (79%) | 17 (27.4%) | 25 (40.3%) | 9 (14.5%) | 24 (38.7%) | 8 (12.9%) |
| 8 (12.9%) | 5 (8.1%) | 5 (8.1%) | 3 (4.8%) | 3 (4.8%) | 2 (3.2%) |
| 62 (100%) | 62 (100%) | 31 (50%) | 31 (50%) | 31 (50%) | 31 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chipirliu, O.; Crăciun, M.V.; Matei, M.N. Clinical Study and Microbiological Analysis of Periodontopathogenic Microflora Analyzed among Children and Adolescents with Cardiovascular Diseases Compared to Group with Good General Status. Pediatr. Rep. 2024, 16, 482-503. https://doi.org/10.3390/pediatric16020041
Chipirliu O, Crăciun MV, Matei MN. Clinical Study and Microbiological Analysis of Periodontopathogenic Microflora Analyzed among Children and Adolescents with Cardiovascular Diseases Compared to Group with Good General Status. Pediatric Reports. 2024; 16(2):482-503. https://doi.org/10.3390/pediatric16020041
Chicago/Turabian StyleChipirliu, Oana, Marian Viorel Crăciun, and Madalina Nicoleta Matei. 2024. "Clinical Study and Microbiological Analysis of Periodontopathogenic Microflora Analyzed among Children and Adolescents with Cardiovascular Diseases Compared to Group with Good General Status" Pediatric Reports 16, no. 2: 482-503. https://doi.org/10.3390/pediatric16020041
APA StyleChipirliu, O., Crăciun, M. V., & Matei, M. N. (2024). Clinical Study and Microbiological Analysis of Periodontopathogenic Microflora Analyzed among Children and Adolescents with Cardiovascular Diseases Compared to Group with Good General Status. Pediatric Reports, 16(2), 482-503. https://doi.org/10.3390/pediatric16020041






