Cardiovascular Risk in Pediatrics: A Dynamic Process during the First 1000 Days of Life
Abstract
1. Introduction
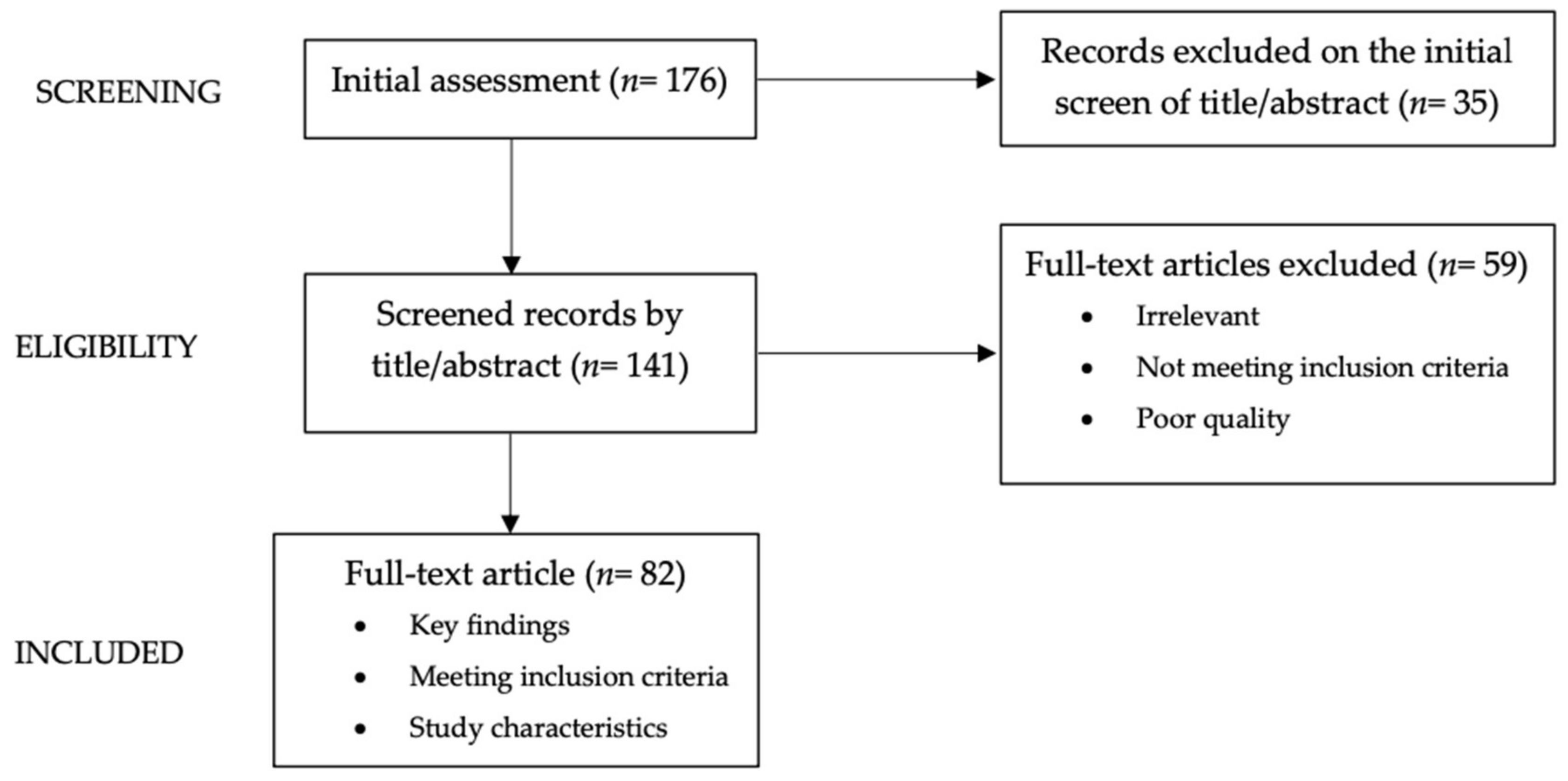
2. Methods
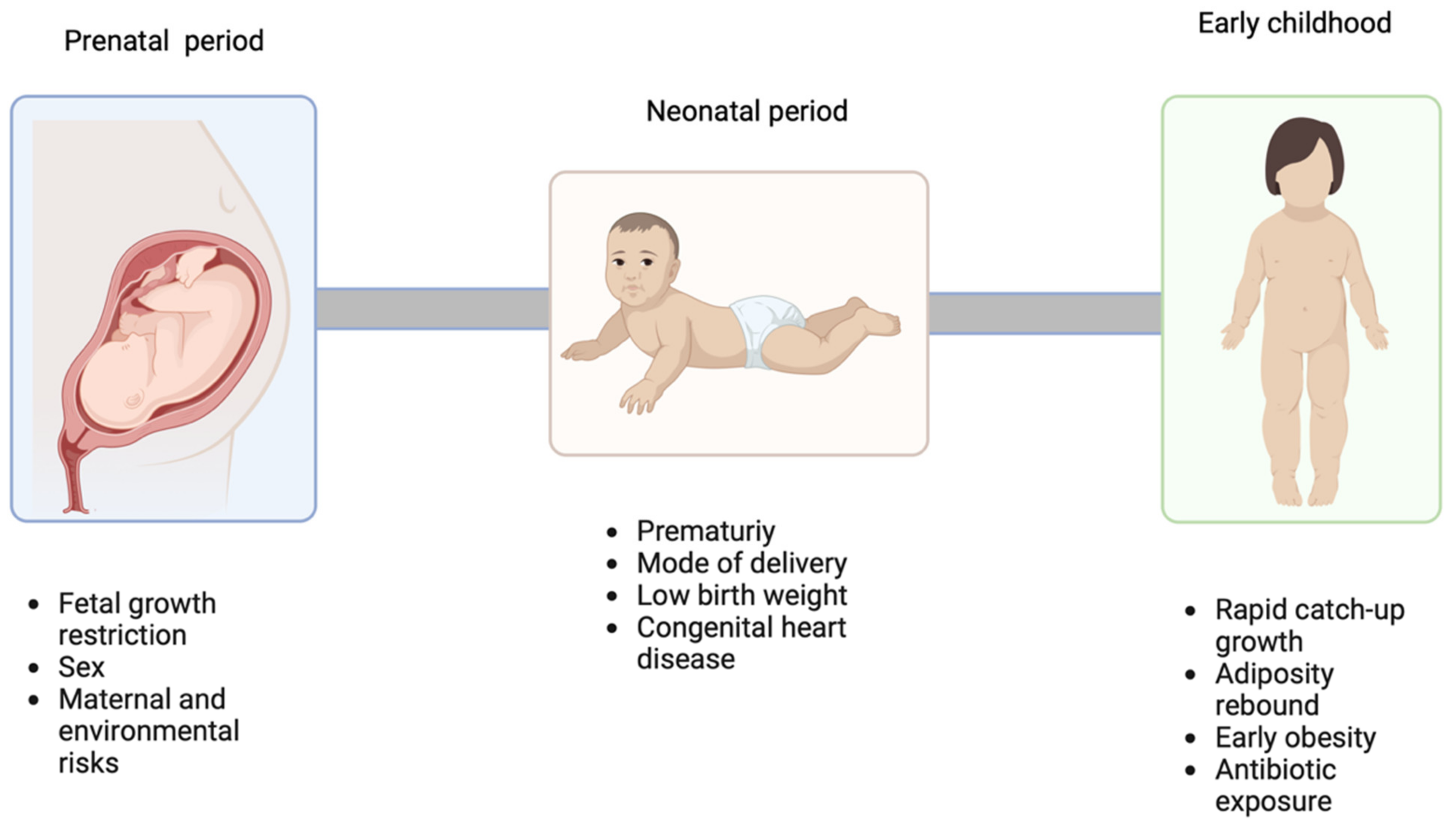
3. Prenatal and Neonatal Cv Risk Factors
3.1. Fetal Growth Restriction
3.2. High Birth Weight
3.3. Prematurity
3.4. Maternal Cardiovascular Disease Risk Factors
3.5. Mode of Delivery
3.6. Sex Differences
3.7. Congenital Heart Disease
4. Early Childhood Cardiovascular Risk Factors
4.1. Rapid Catch-Up Growth
4.2. Adiposity Rebound
4.3. Early Obesity
4.4. Infants Antibiotics Exposure
5. Preventative Measures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wacker-Gussmann, A.; Oberhoffer-Fritz, R. Cardiovascular Risk Factors in Childhood and Adolescence. J. Clin. Med. 2022, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 September 2023).
- Faienza, M.F.; Urbano, F.; Lassandro, G.; Valente, F.; D’Amato, G.; Portincasa, P.; Giordano, P. The Cardiovascular Disease (CVD) Risk Continuum from Prenatal Life to Adulthood: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8282. [Google Scholar] [CrossRef] [PubMed]
- Candelino, M.; Tagi, V.M.; Chiarelli, F. Cardiovascular Risk in Children: A Burden for Future Generations. Ital. J. Pediatr. 2022, 48, 57. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global Burden of Cardiovascular Diseases: Part I: General Considerations, the Epidemiologic Transition, Risk Factors, and Impact of Urbanization. Circulation 2001, 104, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Fetal Origins of Cardiovascular Disease. Ann. Med. 1999, 31 (Suppl. S1), 3–6. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef]
- Palinski, W. Effect of Maternal Cardiovascular Conditions and Risk Factors on Offspring Cardiovascular Disease. Circulation 2014, 129, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and Regional Estimates of Term and Preterm Babies Born Small for Gestational Age in 138 Low-Income and Middle-Income Countries in 2010. Lancet Glob. Health 2013, 1, e26–e36. [Google Scholar] [CrossRef]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low Birth Weight: Case Definition & Guidelines for Data Collection, Analysis, and Presentation of Maternal Immunization Safety Data. Vaccine 2017, 35, 6492–6500. [Google Scholar] [CrossRef]
- Pfab, T.; Slowinski, T.; Godes, M.; Halle, H.; Priem, F.; Hocher, B. Low Birth Weight, a Risk Factor for Cardiovascular Diseases in Later Life, Is Already Associated with Elevated Fetal Glycosylated Hemoglobin at Birth. Circulation 2006, 114, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Haghdoost, A.A.; Jamshidi, F.; Aliramezany, M.; Moosazadeh, M. Low Birthweight or Rapid Catch-up Growth: Which Is More Associated with Cardiovascular Disease and Its Risk Factors in Later Life? A Systematic Review and Cryptanalysis. Paediatr. Int. Child Health 2015, 35, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Syddall, H.E.; Sayer, A.A.; Simmonds, S.J.; Osmond, C.; Cox, V.; Dennison, E.M.; Barker, D.J.P.; Cooper, C. Birth Weight, Infant Weight Gain, and Cause-Specific Mortality: The Hertfordshire Cohort Study. Am. J. Epidemiol. 2005, 161, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Ronalds, G.; Clark, H.; Smith, G.D.; Leon, D.A. Birth Weight Is Inversely Associated with Incident Coronary Heart Disease and Stroke among Individuals Born in the 1950s: Findings from the Aberdeen Children of the 1950s Prospective Cohort Study. Circulation 2005, 112, 1414–1418. [Google Scholar] [CrossRef]
- Wollmann, H.A. Intrauterine Growth Restriction: Definition and Etiology. Horm. Res. 1998, 49 (Suppl. S2), 1–6. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Munoz, F.M.; Bardají, A.; Boghossian, N.S.; Khalil, A.; Mousa, H.; Nesin, M.; Nisar, M.I.; Pool, V.; Spiegel, H.M.L.; et al. Small for Gestational Age: Case Definition & Guidelines for Data Collection, Analysis, and Presentation of Maternal Immunisation Safety Data. Vaccine 2017, 35, 6518–6528. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Delvecchio, M.; Zito, A.; De Palma, F.; Cortese, F.; Nitti, A.; Massari, E.; Gesualdo, M.; Ricci, G.; et al. Vascular Function and Myocardial Performance Indices in Children Born Small for Gestational Age. Circ. J. 2016, 80, 958–963. [Google Scholar] [CrossRef]
- Vickers, M.H. Early Life Nutrition, Epigenetics and Programming of Later Life Disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef]
- Crispi, F.; Figueras, F.; Cruz-Lemini, M.; Bartrons, J.; Bijnens, B.; Gratacos, E. Cardiovascular Programming in Children Born Small for Gestational Age and Relationship with Prenatal Signs of Severity. Am. J. Obstet. Gynecol. 2012, 207, 121.e1–121.e9. [Google Scholar] [CrossRef]
- Hocher, B. Fetal Programming of Cardiovascular Diseases in Later Life—Mechanisms beyond Maternal Undernutrition: Perspectives. J. Physiol. 2007, 579, 287–288. [Google Scholar] [CrossRef]
- Landsberg, L. Insulin Resistance and Hypertension. Clin. Exp. Hypertens. 1999, 21, 885–894. [Google Scholar] [CrossRef]
- Ramalho, S.H.R.; Shah, A.M. Lung Function and Cardiovascular Disease: A Link. Trends Cardiovasc. Med. 2021, 31, 93–98. [Google Scholar] [CrossRef]
- Abman, S.H. Bronchopulmonary Dysplasia: “A Vascular Hypothesis”. Am. J. Respir. Crit. Care Med. 2001, 164, 1755–1756. [Google Scholar] [CrossRef] [PubMed]
- Revanna, G.K.; Kunjunju, A.; Sehgal, A. Bronchopulmonary Dysplasia Associated Pulmonary Hypertension: Making the Best Use of Bedside Echocardiography. Prog. Pediatr. Cardiol. 2017, 46, 39–43. [Google Scholar] [CrossRef]
- Durward, A.; Macrae, D. Long Term Outcome of Babies with Pulmonary Hypertension. Semin. Fetal. Neonatal Med. 2022, 27, 101384. [Google Scholar] [CrossRef]
- Willemsen, R.H.; de Kort, S.W.K.; van der Kaay, D.C.M.; Hokken-Koelega, A.C.S. Independent Effects of Prematurity on Metabolic and Cardiovascular Risk Factors in Short Small-for-Gestational-Age Children. J. Clin. Endocrinol. Metab. 2008, 93, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of Fetal and Child Health on Kidney Development and Long-Term Risk of Hypertension and Kidney Disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Perkovic, V.; Cass, A.; Chang, C.L.; Poulter, N.R.; Spector, T.; Haysom, L.; Craig, J.C.; Salmi, I.A.; Chadban, S.J.; et al. Is Low Birth Weight an Antecedent of CKD in Later Life? A Systematic Review of Observational Studies. Am. J. Kidney Dis. 2009, 54, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Patel, S.R.; Mattoo, T.K.; Valentini, R.P.; Aggarwal, S. The Impact of Change in Volume and Left-Ventricular Hypertrophy on Left-Ventricular Mechanical Dyssynchrony in Children with End-Stage Renal Disease. Pediatr. Cardiol. 2012, 33, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.C.; McNair, R.; Skepper, J.N.; Figg, N.; Schurgers, L.J.; Deanfield, J.; Rees, L.; Shanahan, C.M. Chronic Mineral Dysregulation Promotes Vascular Smooth Muscle Cell Adaptation and Extracellular Matrix Calcification. J. Am. Soc. Nephrol. JASN 2010, 21, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Brodszki, J.; Länne, T.; Marsál, K.; Ley, D. Impaired Vascular Growth in Late Adolescence after Intrauterine Growth Restriction. Circulation 2005, 111, 2623–2628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dodson, R.B.; Rozance, P.J.; Petrash, C.C.; Hunter, K.S.; Ferguson, V.L. Thoracic and Abdominal Aortas Stiffen through Unique Extracellular Matrix Changes in Intrauterine Growth Restricted Fetal Sheep. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H429–H437. [Google Scholar] [CrossRef]
- Longo, S.; Bollani, L.; Decembrino, L.; Di Comite, A.; Angelini, M.; Stronati, M. Short-Term and Long-Term Sequelae in Intrauterine Growth Retardation (IUGR). J. Matern.-Fetal Neonatal Med. 2013, 26, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Verburg, B.O.; Jaddoe, V.W.V.; Wladimiroff, J.W.; Hofman, A.; Witteman, J.C.M.; Steegers, E.A.P. Fetal Hemodynamic Adaptive Changes Related to Intrauterine Growth: The Generation R Study. Circulation 2008, 117, 649–659. [Google Scholar] [CrossRef]
- Leipälä, J.A.; Boldt, T.; Turpeinen, U.; Vuolteenaho, O.; Fellman, V. Cardiac Hypertrophy and Altered Hemodynamic Adaptation in Growth-Restricted Preterm Infants. Pediatr. Res. 2003, 53, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Tintu, A.; Rouwet, E.; Verlohren, S.; Brinkmann, J.; Ahmad, S.; Crispi, F.; van Bilsen, M.; Carmeliet, P.; Staff, A.C.; Tjwa, M.; et al. Hypoxia Induces Dilated Cardiomyopathy in the Chick Embryo: Mechanism, Intervention, and Long-Term Consequences. PLoS ONE 2009, 4, e5155. [Google Scholar] [CrossRef]
- Ong, K.K. Catch-up Growth in Small for Gestational Age Babies: Good or Bad? Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 30–34. [Google Scholar] [CrossRef]
- Woo, J.G. Infant Growth and Long-Term Cardiometabolic Health: A Review of Recent Findings. Curr. Nutr. Rep. 2019, 8, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Baetens, D.; Orci, L.; Unger, R.H. Lipotoxic Heart Disease in Obese Rats: Implications for Human Obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar] [CrossRef]
- Zhou, M.-S.; Schulman, I.H.; Raij, L. Nitric Oxide, Angiotensin II, and Hypertension. Semin. Nephrol. 2004, 24, 366–378. [Google Scholar] [CrossRef]
- Walsh, J.M.; McAuliffe, F.M. Prediction and Prevention of the Macrosomic Fetus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 162, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, S.; Koivunen, R.; Gissler, M.; Nuojua-Huttunen, S.; Suikkari, A.-M.; Hydén-Granskog, C.; Martikainen, H.; Tiitinen, A.; Hartikainen, A.-L. Perinatal Outcome of Children Born after Frozen and Fresh Embryo Transfer: The Finnish Cohort Study 1995–2006. Hum. Reprod. Oxf. Engl. 2010, 25, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Araki, R.; Tani, H.; Ishihara, O.; Kuwahara, A.; Irahara, M.; Yoshimura, Y.; Kuramoto, T.; Saito, H.; Nakaza, A.; et al. Implications of Assisted Reproductive Technologies on Term Singleton Birth Weight: An Analysis of 25,777 Children in the National Assisted Reproduction Registry of Japan. Fertil. Steril. 2013, 99, 450–455. [Google Scholar] [CrossRef]
- Pinborg, A.; Henningsen, A.A.; Loft, A.; Malchau, S.S.; Forman, J.; Andersen, A.N. Large Baby Syndrome in Singletons Born after Frozen Embryo Transfer (FET): Is It Due to Maternal Factors or the Cryotechnique? Hum. Reprod. Oxf. Engl. 2014, 29, 618–627. [Google Scholar] [CrossRef]
- Mäkinen, S.; Söderström-Anttila, V.; Vainio, J.; Suikkari, A.-M.; Tuuri, T. Does Long in Vitro Culture Promote Large for Gestational Age Babies? Hum. Reprod. Oxf. Engl. 2013, 28, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth Weight and Subsequent Risk of Type 2 Diabetes: A Meta-Analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef]
- Cnattingius, S.; Villamor, E.; Lagerros, Y.T.; Wikström, A.-K.; Granath, F. High Birth Weight and Obesity--a Vicious Circle across Generations. Int. J. Obes. 2012, 36, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Agarwala, A.; Novak, E.; Brown, D.L. Association of High Birth Weight with Incident Heart Failure in the ARIC Study. J. Am. Heart Assoc. 2019, 8, e011524. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Smith, G.N. Pregnancy Complications, Cardiovascular Risk Factors, and Future Heart Disease. Obstet. Gynecol. Clin. N. Am. 2020, 47, 487–495. [Google Scholar] [CrossRef]
- Kumar, V.H.S. Cardiovascular Morbidities in Adults Born Preterm: Getting to the Heart of the Matter! Children 2022, 9, 1843. [Google Scholar] [CrossRef] [PubMed]
- Bensley, J.G.; Stacy, V.K.; De Matteo, R.; Harding, R.; Black, M.J. Cardiac Remodelling as a Result of Pre-Term Birth: Implications for Future Cardiovascular Disease. Eur. Heart J. 2010, 31, 2058–2066. [Google Scholar] [CrossRef]
- Chehade, H.; Simeoni, U.; Guignard, J.-P.; Boubred, F. Preterm Birth: Long Term Cardiovascular and Renal Consequences. Curr. Pediatr. Rev. 2018, 14, 219–226. [Google Scholar] [CrossRef]
- Lazdam, M.; de la Horra, A.; Diesch, J.; Kenworthy, Y.; Davis, E.; Lewandowski, A.J.; Szmigielski, C.; Shore, A.; Mackillop, L.; Kharbanda, R.; et al. Unique Blood Pressure Characteristics in Mother and Offspring after Early Onset Preeclampsia. Hypertension 2012, 60, 1338–1345. [Google Scholar] [CrossRef]
- Geelhoed, J.J.M.; Fraser, A.; Tilling, K.; Benfield, L.; Davey Smith, G.; Sattar, N.; Nelson, S.M.; Lawlor, D.A. Preeclampsia and Gestational Hypertension Are Associated With Childhood Blood Pressure Independently of Family Adiposity Measures: The Avon Longitudinal Study of Parents and Children. Circulation 2010, 122, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Macdonald-Wallis, C.; Fraser, A.; Nelson, S.M.; Hingorani, A.; Davey Smith, G.; Sattar, N.; Deanfield, J. Cardiovascular Biomarkers and Vascular Function during Childhood in the Offspring of Mothers with Hypertensive Disorders of Pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. Eur. Heart J. 2012, 33, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.; Miranda, J.; Paules, C.; Garcia-Otero, L.; Vellvé, K.; Kalapotharakos, G.; Sepulveda-Martinez, A.; Crovetto, F.; Gomez, O.; Gratacós, E.; et al. Fetal Cardiac Remodeling and Dysfunction Is Associated with Both Preeclampsia and Fetal Growth Restriction. Am. J. Obstet. Gynecol. 2020, 222, 79.e1–79.e9. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Glass, C.K.; Witztum, J.L.; Deutsch, R.; D’Armiento, F.P.; Palinski, W. Influence of Maternal Hypercholesterolaemia during Pregnancy on Progression of Early Atherosclerotic Lesions in Childhood: Fate of Early Lesions in Children (FELIC) Study. Lancet 1999, 354, 1234–1241. [Google Scholar] [CrossRef]
- Geelhoed, J.J.; el Marroun, H.; Verburg, B.O.; van Osch-Gevers, L.; Hofman, A.; Huizink, A.C.; Moll, H.A.; Verhulst, F.C.; Helbing, W.A.; Steegers, E.A.; et al. Maternal Smoking during Pregnancy, Fetal Arterial Resistance Adaptations and Cardiovascular Function in Childhood. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Huh, S.Y.; Taveras, E.M.; Rich-Edwards, J.W.; Gillman, M.W. Associations of Maternal Prenatal Smoking with Child Adiposity and Blood Pressure. Obes. Res. 2005, 13, 2021–2028. [Google Scholar] [CrossRef]
- Whincup, P.H.; Kaye, S.J.; Owen, C.G.; Huxley, R.; Cook, D.G.; Anazawa, S.; Barrett-Connor, E.; Bhargava, S.K.; Birgisdottir, B.E.; Carlsson, S.; et al. Birth Weight and Risk of Type 2 Diabetes: A Systematic Review. JAMA 2008, 300, 2886–2897. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Relton, C.; Sattar, N.; Nelson, S.M. Maternal Adiposity—A Determinant of Perinatal and Offspring Outcomes? Nat. Rev. Endocrinol. 2012, 8, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-Castillejos, A.; Gomez-Salgado, J.; Rodriguez-Almagro, J.; Ortiz-Esquinas, I.; Hernandez-Martinez, A. Relationship between Maternal Body Mass Index with the Onset of Breastfeeding and Its Associated Problems: An Online Survey. Int. Breastfeed. J. 2020, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Broe, A.; Pottegård, A.; Lamont, R.F.; Jørgensen, J.S.; Damkier, P. Increasing Use of Antibiotics in Pregnancy during the Period 2000–2010: Prevalence, Timing, Category, and Demographics. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial Impact on Cholesterol and Bile Acid Metabolism: Current Status and Future Prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef]
- Qi, X.; Wu, S.; Zhang, H.; Yue, H.; Xu, S.; Ji, F.; Qi, G. Effects of Dietary Conjugated Linoleic Acids on Lipid Metabolism and Antioxidant Capacity in Laying Hens. Arch. Anim. Nutr. 2011, 65, 354–365. [Google Scholar] [CrossRef]
- Vik, K.L.; Romundstad, P.; Carslake, D.; Smith, G.D.; Nilsen, T.I.L. Comparison of Father-Offspring and Mother-Offspring Associations of Cardiovascular Risk Factors: Family Linkage within the Population-Based HUNT Study, Norway. Int. J. Epidemiol. 2014, 43, 760–771. [Google Scholar] [CrossRef]
- Begum, T.; Fatima, Y.; Anuradha, S.; Hasan, M.; Mamun, A.A. Longitudinal Association between Caesarean Section Birth and Cardio-Vascular Risk Profiles among Adolescents in Australia. Aust. N.Z. J. Public Health 2022, 46, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.Y.; Wild, A.E.; Roberts, P.; Willis, A.C.; Fleming, T.P. Maternal Undernutrition during the Preimplantation Period of Rat Development Causes Blastocyst Abnormalities and Programming of Postnatal Hypertension. Dev. Camb. Engl. 2000, 127, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.L.; Ingelfinger, J.R.; Nyengaard, J.R.; Rasch, R. Maternal Protein Restriction Suppresses the Newborn Renin-Angiotensin System and Programs Adult Hypertension in Rats. Pediatr. Res. 2001, 49, 460–467. [Google Scholar] [CrossRef]
- Grigore, D.; Ojeda, N.B.; Robertson, E.B.; Dawson, A.S.; Huffman, C.A.; Bourassa, E.A.; Speth, R.C.; Brosnihan, K.B.; Alexander, B.T. Placental Insufficiency Results in Temporal Alterations in the Renin Angiotensin System in Male Hypertensive Growth Restricted Offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R804–R811. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.L.; Ingelfinger, J.R.; Rasch, R. Modest Maternal Protein Restriction Fails to Program Adult Hypertension in Female Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1131–R1136. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Dubay, C.; Lomniczi, A.; Kaidar, G.; Matagne, V.; Sandau, U.S.; Dissen, G.A. Gene Networks and the Neuroendocrine Regulation of Puberty. Mol. Cell. Endocrinol. 2010, 324, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Forsén, T.; Eriksson, J.G.; Tuomilehto, J.; Osmond, C.; Barker, D.J. Growth in Utero and during Childhood among Women Who Develop Coronary Heart Disease: Longitudinal Study. BMJ 1999, 319, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Tutarel, O. Acquired Heart Conditions in Adults with Congenital Heart Disease: A Growing Problem. Heart 2014, 100, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; D’Ottavio, A.; Spears, T.; Chiswell, K.; Hartman, R.J.; Krasuski, R.A.; Kemper, A.R.; Meyer, R.E.; Hoffman, T.M.; Walsh, M.J.; et al. Causes of Death and Cardiovascular Comorbidities in Adults With Congenital Heart Disease. J. Am. Heart Assoc. 2020, 9, e016400. [Google Scholar] [CrossRef]
- Giannakoulas, G.; Dimopoulos, K.; Engel, R.; Goktekin, O.; Kucukdurmaz, Z.; Vatankulu, M.A.; Bedard, E.; Diller, G.P.; Papaphylactou, M.; Francis, D.P.; et al. Burden of Coronary Artery Disease in Adults With Congenital Heart Disease and Its Relation to Congenital and Traditional Heart Risk Factors. Am. J. Cardiol. 2009, 103, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Larson-Nath, C.; Goday, P. Malnutrition in Children With Chronic Disease. Nutr. Clin. Pract. 2019, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, B.; Radhakrishnan, R.; Sarala, D.A.; Sundaram, K.R.; Kumar, R.K. What Determines Nutritional Recovery in Malnourished Children after Correction of Congenital Heart Defects? Pediatrics 2009, 124, e294–e299. [Google Scholar] [CrossRef]
- Bigras, J.-L. Cardiovascular Risk Factors in Patients With Congenital Heart Disease. Can. J. Cardiol. 2020, 36, 1458–1466. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Panera, N.; Agostoni, C. Low Birth Weight and Catch-up-Growth Associated with Metabolic Syndrome: A Ten Year Systematic Review. Pediatr. Endocrinol. Rev. 2008, 6, 241–247. [Google Scholar] [PubMed]
- Gluckman, P.D.; Hanson, M.A. The Developmental Origins of the Metabolic Syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef]
- Lurbe, E.; Aguilar, F.; Álvarez, J.; Redon, P.; Torró, M.I.; Redon, J. Determinants of Cardiometabolic Risk Factors in the First Decade of Life: A Longitudinal Study Starting at Birth. Hypertension 2018, 71, 437–443. [Google Scholar] [CrossRef]
- Ong, K.K.; Loos, R.J.F. Rapid Infancy Weight Gain and Subsequent Obesity: Systematic Reviews and Hopeful Suggestions. Acta Paediatrica 2006, 95, 904–908. [Google Scholar] [CrossRef]
- Ong, K.K.; Petry, C.J.; Emmett, P.M.; Sandhu, M.S.; Kiess, W.; Hales, C.N.; Ness, A.R.; Dunger, D.B.; ALSPAC Study Team. Insulin Sensitivity and Secretion in Normal Children Related to Size at Birth, Postnatal Growth, and Plasma Insulin-like Growth Factor-I Levels. Diabetologia 2004, 47, 1064–1070. [Google Scholar] [CrossRef]
- Li, X.; Keown-Stoneman, C.D.G.; Lebovic, G.; Maguire, J.L.; Omand, J.A.; Sievenpiper, J.L.; Birken, C.S. TARGet Kids! Collaboration Body Mass Index Mediates the Association between Growth Trajectories and Cardiometabolic Risk in Children. Child. Obes. Print 2021, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Christian Flemming, G.M.; Bussler, S.; Körner, A.; Kiess, W. Definition and Early Diagnosis of Metabolic Syndrome in Children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Roufarshbaf, M.; Soheili, S.; Payghambarzadeh, F.; Masjedi, M. Association of Childhood Obesity and the Immune System: A Systematic Review of Reviews. Child. Obes. Print 2017, 13, 332–346. [Google Scholar] [CrossRef]
- Taylor, R.W.; Grant, A.M.; Goulding, A.; Williams, S.M. Early Adiposity Rebound: Review of Papers Linking This to Subsequent Obesity in Children and Adults. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 607–612. [Google Scholar] [CrossRef]
- Hughes, A.R.; Sherriff, A.; Ness, A.R.; Reilly, J.J. Timing of Adiposity Rebound and Adiposity in Adolescence. Pediatrics 2014, 134, e1354–e1361. [Google Scholar] [CrossRef] [PubMed]
- Totzauer, M.; Escribano, J.; Closa-Monasterolo, R.; Luque, V.; Verduci, E.; ReDionigi, A.; Langhendries, J.-P.; Martin, F.; Xhonneux, A.; Gruszfeld, D.; et al. Different Protein Intake in the First Year and Its Effects on Adiposity Rebound and Obesity throughout Childhood: 11 Years Follow-up of a Randomized Controlled Trial. Pediatr. Obes. 2022, 17, e12961. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, O.; Ichikawa, G.; Koyama, S.; Sairenchi, T. Childhood Obesity: Rapid Weight Gain in Early Childhood and Subsequent Cardiometabolic Risk. Clin. Pediatr. Endocrinol. Case Rep. Clin. Investig. 2020, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wibaek, R.; Vistisen, D.; Girma, T.; Admassu, B.; Abera, M.; Abdissa, A.; Mudie, K.; Kæstel, P.; Jørgensen, M.E.; Wells, J.C.K.; et al. Body Mass Index Trajectories in Early Childhood in Relation to Cardiometabolic Risk Profile and Body Composition at 5 Years of Age. Am. J. Clin. Nutr. 2019, 110, 1175–1185. [Google Scholar] [CrossRef]
- Arisaka, O.; Ichikawa, G.; Koyama, S.; Sairenchi, T. Is Childhood Cardiometabolic Status a Risk Factor from Early Infancy or Toddler Age? J. Pediatr. 2017, 188, 314–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guzzetti, C.; Ibba, A.; Casula, L.; Pilia, S.; Casano, S.; Loche, S. Cardiovascular Risk Factors in Children and Adolescents With Obesity: Sex-Related Differences and Effect of Puberty. Front. Endocrinol. 2019, 10, 591. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Garrison, R.J.; Kannel, W.B.; Stokes, J.; Castelli, W.P. Incidence and Precursors of Hypertension in Young Adults: The Framingham Offspring Study. Prev. Med. 1987, 16, 235–251. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking Adipogenesis during White Adipose Tissue Development, Expansion and Regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Haynes, W.G. Interaction between Leptin and Sympathetic Nervous System in Hypertension. Curr. Hypertens. Rep. 2000, 2, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.L.; Correia, M.; Morgan, D.A.; Shaffer, R.A.; Haynes, W.G. State-of-the-Art-Lecture: Obesity-Induced Hypertension: New Concepts from the Emerging Biology of Obesity. Hypertension 1999, 33, 537–541. [Google Scholar] [CrossRef]
- Reneau, J.; Goldblatt, M.; Gould, J.; Kindel, T.; Kastenmeier, A.; Higgins, R.; Rengel, L.R.; Schoyer, K.; James, R.; Obi, B.; et al. Effect of Adiposity on Tissue-Specific Adiponectin Secretion. PLoS ONE 2018, 13, e0198889. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Sonmez, A.; Caglar, K.; Celik, T.; Yenicesu, M.; Eyileten, T.; Acikel, C.; Oguz, Y.; Yavuz, I.; Vural, A. Effect of Antihypertensive Agents on Plasma Adiponectin Levels in Hypertensive Patients with Metabolic Syndrome. Nephrology 2007, 12, 147–153. [Google Scholar] [CrossRef]
- Wardle, J.; Carnell, S.; Haworth, C.M.; Plomin, R. Evidence for a Strong Genetic Influence on Childhood Adiposity despite the Force of the Obesogenic Environment. Am. J. Clin. Nutr. 2008, 87, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; O’Rahilly, S. Mutations in Ligands and Receptors of the Leptin-Melanocortin Pathway That Lead to Obesity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 569–577. [Google Scholar] [CrossRef]
- Choquet, H.; Meyre, D. Genomic Insights into Early-Onset Obesity. Genome Med. 2010, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, M.; Papandreou, D.; Vasios, G.K.; Pavlidou, E.; Antasouras, G.; Psara, E.; Taha, Z.; Poulios, E.; Giaginis, C. Exclusive Breastfeeding for at Least Four Months Is Associated with a Lower Prevalence of Overweight and Obesity in Mothers and Their Children after 2–5 Years from Delivery. Nutrients 2022, 14, 3599. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The Association between Breastfeeding and Childhood Obesity: A Meta-Analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef]
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and Protein Intakes of Breast-Fed and Formula-Fed Infants during the First Year of Life and Their Association with Growth Velocity: The DARLING Study. Am. J. Clin. Nutr. 1993, 58, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.-P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower Protein Content in Infant Formula Reduces BMI and Obesity Risk at School Age: Follow-up of a Randomized Trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Gingras, V.; Aris, I.M.; Rifas-Shiman, S.L.; Switkowski, K.M.; Oken, E.; Hivert, M.-F. Timing of Complementary Feeding Introduction and Adiposity Throughout Childhood. Pediatrics 2019, 144, e20191320. [Google Scholar] [CrossRef]
- WHO; UNICEF (Eds.) Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003; ISBN 978-92-4-156221-8. [Google Scholar]
- Baron, R.; Taye, M.; Besseling-van der Vaart, I.; Ujčič-Voortman, J.; Szajewska, H.; Seidell, J.C.; Verhoeff, A. SAWANTI working group The Relationship of Prenatal and Infant Antibiotic Exposure with Childhood Overweight and Obesity: A Systematic Review. J. Dev. Orig. Health Dis. 2020, 11, 335–349. [Google Scholar] [CrossRef]
- McCaig, L.F.; Besser, R.E.; Hughes, J.M. Trends in Antimicrobial Prescribing Rates for Children and Adolescents. JAMA 2002, 287, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, D.J. Antibiotic Residues in Poultry Tissues and Eggs: Human Health Concerns? Poult. Sci. 2003, 82, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Ternak, G. Antibiotics May Act as Growth/Obesity Promoters in Humans as an Inadvertent Result of Antibiotic Pollution? Med. Hypotheses 2005, 64, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Ajslev, T.A.; Andersen, C.S.; Gamborg, M.; Sørensen, T.I.A.; Jess, T. Childhood Overweight after Establishment of the Gut Microbiota: The Role of Delivery Mode, Pre-Pregnancy Weight and Early Administration of Antibiotics. Int. J. Obes. 2011, 35, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, M.J. Infant Antibiotic Exposures and Early-Life Body Mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Bridgman, S.L.; Becker, A.B.; Kozyrskyj, A.L. Infant Antibiotic Exposure and the Development of Childhood Overweight and Central Adiposity. Int. J. Obes. 2014, 38, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Sciaccaluga, C.; Cameli, M.; Cecere, A.; Ciccone, M.M.; Di Francesco, S.; Ganau, A.; Imbalzano, E.; Liga, R.; Palermo, P.; et al. When Should Cardiovascular Prevention Begin? The Importance of Antenatal, Perinatal and Primordial Prevention. Eur. J. Prev. Cardiol. 2021, 28, 361–369. [Google Scholar] [CrossRef]
- Koletzko, B.; Cremer, M.; Flothkötter, M.; Graf, C.; Hauner, H.; Hellmers, C.; Kersting, M.; Krawinkel, M.; Przyrembel, H.; Röbl-Mathieu, M.; et al. Diet and Lifestyle Before and During Pregnancy—Practical Recommendations of the Germany-Wide Healthy Start—Young Family Network. Geburtshilfe Frauenheilkd. 2018, 78, 1262–1282. [Google Scholar] [CrossRef]
- Simon, A.; Pratt, M.; Hutton, B.; Skidmore, B.; Fakhraei, R.; Rybak, N.; Corsi, D.J.; Walker, M.; Velez, M.P.; Smith, G.N.; et al. Guidelines for the Management of Pregnant Women with Obesity: A Systematic Review. Obes. Rev. 2020, 21, e12972. [Google Scholar] [CrossRef]
- RANZCOG Excellence in Women’s Health Management of Obesity in Pregnancy 2022. Available online: https://ranzcog.edu.au/wp-content/uploads/2022/05/Guidance-for-Healthy-Weight-Gain-in-Pregnancy.pdf (accessed on 10 September 2023).
- Lee, Y.S.; Biddle, S.; Chan, M.F.; Cheng, A.; Cheong, M.; Chong, Y.S.; Foo, L.L.; Lee, C.H.; Lim, S.C.; Ong, W.S.; et al. Health Promotion Board-Ministry of Health Clinical Practice Guidelines: Obesity. Singap. Med. J. 2016, 57, 472. [Google Scholar] [CrossRef] [PubMed]
- ACOG Practice Bulletin No 156: Obesity in Pregnancy. Obstet. Gynecol. 2015, 126, e112–e126. [CrossRef] [PubMed]
- Hokken-Koelega, A.C.S.; van der Steen, M.; Boguszewski, M.C.S.; Cianfarani, S.; Dahlgren, J.; Horikawa, R.; Mericq, V.; Rapaport, R.; Alherbish, A.; Braslavsky, D.; et al. International Consensus Guideline on Small for Gestational Age: Etiology and Management From Infancy to Early Adulthood. Endocr. Rev. 2023, 44, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.T. Long-Term Care, from Neonatal Period to Adulthood, of Children Born Small for Gestational Age. Clin. Pediatr. Endocrinol. Case Rep. Clin. Investig. 2019, 28, 97–103. [Google Scholar] [CrossRef]
- Santiago, A.C.; da Cunha, L.P.; Vieira, N.S.; Moreira, L.M.; de Oliveira, P.R.; Lyra, P.P.; Alves, C.D. Breastfeeding in Children Born Small for Gestational Age and Future Nutritional and Metabolic Outcomes: A Systematic Review. J. Pediatr. 2019, 95, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Forsen, T.J.; Kajantie, E.; Eriksson, J.G. Maternal and Social Origins of Hypertension. Hypertension 2007, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J.L. Epigenetic Modification of the Renin-Angiotensin System in the Fetal Programming of Hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef]
- Khan, I.; Dekou, V.; Hanson, M.; Poston, L.; Taylor, P. Predictive Adaptive Responses to Maternal High-Fat Diet Prevent Endothelial Dysfunction but Not Hypertension in Adult Rat Offspring. Circulation 2004, 110, 1097–1102. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Grigore, D.; Yanes, L.L.; Iliescu, R.; Robertson, E.B.; Zhang, H.; Alexander, B.T. Testosterone Contributes to Marked Elevations in Mean Arterial Pressure in Adult Male Intrauterine Growth Restricted Offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R758–R763. [Google Scholar] [CrossRef] [PubMed]
| Risk Factor | Reference | Results |
|---|---|---|
| Prenatal and neonatal CV risk factors | ||
| Fetal growth restriction | Syddall et al., 2005 [14] | Syddall et al. underscored, in their cohort study, a significant association between lower birthweight and an elevated risk of circulatory disease-related mortality in both men and women. |
| Lawlor et al., 2005 [15] | There is a reverse relationship between birth weight and the risk of coronary heart disease and stroke in a population born during a period when environmental conditions, as reflected by low infant mortality rates, were comparatively favorable for infants. | |
| Faienza et al., 2016 [18] | SGA individuals showed vascular abnormalities and subtle cardiac changes compared with AGA individuals, increasing their cardiovascular risk. | |
| Brodszki et al., 2005 [32] | Inadequate intrauterine growth due to placental insufficiency appears to result in impaired vascular development that persists into early adulthood, affecting both males and females. The smaller dimensions of the aorta and the elevated resting heart rate observed in adolescents who experienced IUGR could have implications for their future cardiovascular well-being. | |
| Dodson et al., 2014 [33] | Intrauterine growth restriction resulting from placental insufficiency leads to heightened vascular stiffness through remodeling at the end of gestation, potentially laying the groundwork for changes in vascular growth and development. | |
| Verburg et al., 2008 [35] | Reduced fetal growth is linked to adaptive adjustments in fetal cardiovascular function. The alterations in cardiac structure and cardiac output align with a progressive rise in afterload and diminished arterial flexibility, even before clinical signs of fetal growth restriction become evident. These early changes may play a role in the heightened risk of cardiovascular disease in adulthood. | |
| Leipälä et al., 2003 [36] | IUGR is linked to changes in cardiovascular adaptation and the development of septal and left ventricular hypertrophy in low-birth-weight newborns. While the findings suggest that SGA fetuses can undergo significant cardiovascular adaptation, there may still be an elevated risk of circulatory issues in the future. | |
| Tintu et al., 2009 [37] | This study explored the impact of prenatal hypoxia on chick embryos, revealing that it leads to cardiomyopathy characterized by enlarged heart chambers, reduced heart muscle mass, and increased cell death. These cardiac abnormalities persist into adulthood and are associated with elevated VEGF levels. The findings underscore the significant role of VEGF in hypoxia-induced cardiomyopathy, which poses a lasting risk for cardiovascular diseases in affected individuals. | |
| High birth weight | Cnattingius et al., 2012 [48] | Prenatal factors play a significant role in the obesity epidemic, and preventing LGA births could help break the cycle of intergenerational obesity. |
| Rashid et al., 2019 [49] | High birth weight is associated with an increased risk of heart failure and potential mortality, regardless of traditional risk factors. Therefore, it is important to inquire about a history of high birth weight in both young and older adults as a preventive measure for heart failure. | |
| Prematurity | Willemsen et al., 2008 [27] | In a group of short children born SGA, preterm birth has varying impacts on multiple cardiovascular risk factors. Specifically, preterm SGA children exhibited elevated systolic and diastolic blood pressure but lower levels of body fat. They also displayed increased insulin secretion and a higher disposition index compared with their term-born SGA counterparts. |
| Bensley et al., 2010 [52] | Pre-term birth initiates changes in myocardial structure, ultimately leading to long-term cardiac vulnerability. | |
| Maternal cardiovascular disease risk factors | Lazdam et al., 2012 [54] | Early-onset preeclampsia is associated with elevated postnatal diastolic blood pressure, a greater increase in blood pressure over the years, and higher nocturnal blood pressure in later life. Offspring born to mothers with early-onset preeclampsia also have higher systolic blood pressure compared with those born to mothers with late-onset preeclampsia. |
| Geelhoed et al., 2010 [55] | Gestational blood pressure disorders are linked to higher blood pressure in offspring. The mechanisms connecting preeclampsia and gestational hypertension to offspring blood pressure may differ, with preeclampsia possibly affecting intrauterine growth restriction. | |
| Lawlor et al., 2012 [56] | Preeclampsia and gestational hypertension elevate offspring blood pressure in infancy, indicating shared risk factors between mothers and infants, unrelated to cardiometabolic abnormalities. These effects are not solely due to long-term consequences of pregnancy hypertensive disorders. | |
| Youssef et al., 2020 [57] | Fetuses of preeclamptic mothers, regardless of their growth patterns, displayed cardiovascular issues similar to fetal growth restriction. More research is required to understand the mechanisms behind fetal cardiac adaptation in these cases. | |
| Barker et al., 2007 [131] | Hypertension can develop through two distinct pathways: fetal malnutrition (making the child susceptible to postnatal stress) and maternal metabolic dysfunction, particularly in protein metabolism. | |
| Bogdarina et al., 2007 [132] | Offspring from mothers fed a low protein diet exhibited increased expression of AT1b receptor mRNA and protein in the adrenal gland. The increased AT1b receptor expression is believed to play a role in hypertension development and may result from fetal programming. | |
| Khan et al., 2004 [133] | In this study, researchers examined ‘predictive adaptive’ responses in rodents with adult offspring from fat-fed mothers displaying metabolic syndrome traits. When these offspring were raised on a high-fat diet, their vascular function and heart rates improved, but elevated blood pressure persisted in female offspring. Therefore, predictive adaptive responses may not completely prevent high blood pressure. | |
| Geelhoed et al., 2011 [59] | Adaptive alterations in fetal arterial resistance could be part of the mechanisms connecting maternal smoking during pregnancy to both low birth weight and cardiovascular developmental changes in their children. | |
| Oken et al., 2005 [60] | Mothers who smoked before or during early pregnancy had children with slightly higher systolic blood pressure, but only those who smoked during early pregnancy had more overweight children. The mechanisms linking smoking to child weight gain and blood pressure may differ. | |
| Vik et al., 2014 [69] | Researchers examined the influence of parental factors on cardiovascular risk factors in their offspring. They compared the associations between fathers and offspring and mothers and offspring. The results showed that these associations were largely similar, suggesting that there are no strong maternal effects transmitted through intrauterine mechanisms. | |
| Mode of delivery | Begum et al., 2022 [70] | C-section-born children had higher scores (waist circumference, systolic blood pressure, HDL cholesterol levels, fat mass index, and a composite metabolic syndrome score) for several CVD risk indicators compared with those born vaginally. Additionally, children with a high BMI trajectory had increased CVD risk, particularly in the C-section group. This suggests that C-sections were independently associated with elevated CVD risk profiles in children, which were further exacerbated by a high BMI trajectory. |
| Sex differences | Grigore et al., 2007 [73] | In a rat model of late gestational reduced uterine perfusion, male offspring with IUGR developed high blood pressure. The study investigates the role of the RAAS in this process. Researchers found that early RAAS blockade using an ACE inhibitor prevents hypertension in adult IUGR male offspring, highlighting the RAAS’s involvement in established hypertension. They also observed temporal changes in the RAAS in IUGR offspring, particularly in the intra-renal RAAS, which may be influenced by factors like sex hormones and which contribute to the development and persistence of hypertension in this model. |
| Ojeda et al., 2006 [134] | In a rat model of IUGR induced by placental insufficiency, only male IUGR offspring develop hypertension in adulthood. This study investigates the role of testosterone and the RAAS in this hypertension. At 16 weeks of age, male IUGR offspring have higher testosterone levels and elevated BP. Gonadectomy reduces BP in IUGR males but not in controls. Treatment with an ACE inhibitor, enalapril, lowers BP in both intact and castrated IUGR males, but the response is more significant in intact males, suggesting that testosterone, in conjunction with the RAAS, contributes to hypertension in adult male IUGR offspring. | |
| Congenital heart disease | Goldstein et al., 2020 [78] | In adults with CHD, mortality risks vary depending on the severity of their condition. Severe CHD is associated with a higher likelihood of early mortality. Individuals with nonsevere CHD tend to have longer life expectancy but still face risks of mortality from both cardiovascular and non-cardiovascular causes. It is crucial to undergo long-term follow-up, including personalized screening and risk management strategies. |
| Giannakoulas et al., 2009 [79] | In adults with CHD, the risk of developing CAD increases as they age. CAD prevalence in adult CHD patients is similar to the general population. Traditional CVD risk factors applied to this population emphasize the importance of CAD prevention | |
| Early childhood CV risk factors | ||
| Rapid catch-up growth | Lurbe et al., 2018 [85] | In this prospective study, researchers explored how BW, growth patterns, and cardiometabolic risk factors were interconnected within a cohort monitored from birth to age 10. While BW served as a reflection of early fetal experiences and exhibited enduring effects, the pace of weight gain emerged as a pivotal factor in the development of obesity, metabolic disorders, and cardiovascular issues. |
| Ong et al., 2004 [87] | Lower BW may contribute to IR, particularly when coupled with rapid early weight gain. Additionally, smaller birth size, lower IGF-I levels, and shorter childhood stature were associated with reduced compensatory insulin secretion. | |
| Li et al., 2021 [88] | The study provided strong evidence that the influence of BMI trajectories on CMR operated indirectly through concurrent BMI. Researchers should select the appropriate analytical method based on their study hypothesis to accurately assess the overall or direct impact of growth patterns on cardiometabolic disease risk in children. | |
| Adiposity rebound | Hughes et al., 2014 [92] | The study aimed to explore the relationship between the timing of AR in childhood and adiposity indicators (BMI and fat mass) at age 15. The findings revealed that early AR, occurring between 3.5 and 5 years, was strongly associated with higher BMI and fat mass during adolescence. Interventions to prevent excessive adiposity should focus on addressing modifiable factors in early childhood to delay the timing of AR. |
| Totzauer et al., 2022 [93] | Infants fed with lower protein formula had lower BMI trajectories compared with those fed with conventional higher protein formula. Therefore, feeding infants with lower protein formula can lead to healthier BMI outcomes and similar values at adiposity rebound as observed in breastfed infants. | |
| Wibaek et al., 2019 [95] | Early childhood growth patterns are linked to the development of obesity and CMR, emphasizing the importance of interventions targeting young children with unfavorable growth patterns in low-income countries. | |
| Early obesity | Guzzetti et al., 2019 [97] | Gender and puberty affect the frequency of CVRF abnormalities, even during prepubertal stages. Identifying individuals with a higher risk of metabolic complications is crucial for the development of tailored prevention strategies. |
| Wardle et al., 2008 [106] | Genetic factors play a substantial role in BMI and abdominal adiposity in children born during the pediatric obesity epidemic. To address obesity effectively, early prevention may target family dynamics, while long-term weight management will require individual commitment and broader societal efforts to modify environments, especially for genetically predisposed children. | |
| Mantzorou et al., 2022 [109] | Breastfeeding exclusively for at least 4 months has favorable outcomes, including a reduced risk of childhood overweight and obesity, along with benefits for postnatal maternal weight control. It is important to convey these advantages to expectant and new mothers and implement supportive measures to promote breastfeeding initiation and continuity for all mothers and their babies. | |
| Yan et al., 2014 [110] | Breastfeeding is a significant protective factor against childhood obesity. | |
| Weber et al., 2014 [112] | Choosing a low-protein infant formula has demonstrated a correlation with reduced BMI and a lowered risk of childhood obesity among school-aged children. Therefore, it is crucial to avoid infant foods that offer excessive protein intake as a potential approach to address childhood obesity. | |
| Gingras et al., 2019 [113] | Introducing CF early is related to elevated adiposity measurements in both breastfed and formula-fed children, while introducing CF later was associated with increased adiposity in formula-fed children. | |
| Infants antibiotics exposure | Ternak et al., 2005 [118] | The usage of antibiotics, both in humans and animals, has significantly increased over the years. Animal studies have demonstrated that antibiotics can promote growth by affecting gut flora, and there are indications that similar effects might occur in humans. This hypothesis warrants further research. |
| Trasande et al., 2013 [120] | Early antibiotic exposure during the first 6 months of life is associated with increased body mass from 10 to 38 months, but later exposures in infancy show no consistent link to body mass changes. Given the prevalence of antibiotic use in infants and rising concerns about childhood obesity, further research is needed to explore the long-term effects on body mass and cardiovascular health. | |
| Azad et al., 2014 [121] | In boys, early-life antibiotic use was linked to a higher likelihood of being overweight and having excess central body fat during preadolescence. This suggests the importance of prudent antibiotic usage, especially in infancy. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Mannarino, S.; Garella, V.; Rossi, V.; Biganzoli, E.M.; Zuccotti, G. Cardiovascular Risk in Pediatrics: A Dynamic Process during the First 1000 Days of Life. Pediatr. Rep. 2023, 15, 636-659. https://doi.org/10.3390/pediatric15040058
Calcaterra V, Mannarino S, Garella V, Rossi V, Biganzoli EM, Zuccotti G. Cardiovascular Risk in Pediatrics: A Dynamic Process during the First 1000 Days of Life. Pediatric Reports. 2023; 15(4):636-659. https://doi.org/10.3390/pediatric15040058
Chicago/Turabian StyleCalcaterra, Valeria, Savina Mannarino, Vittoria Garella, Virginia Rossi, Elia Mario Biganzoli, and Gianvincenzo Zuccotti. 2023. "Cardiovascular Risk in Pediatrics: A Dynamic Process during the First 1000 Days of Life" Pediatric Reports 15, no. 4: 636-659. https://doi.org/10.3390/pediatric15040058
APA StyleCalcaterra, V., Mannarino, S., Garella, V., Rossi, V., Biganzoli, E. M., & Zuccotti, G. (2023). Cardiovascular Risk in Pediatrics: A Dynamic Process during the First 1000 Days of Life. Pediatric Reports, 15(4), 636-659. https://doi.org/10.3390/pediatric15040058








