Administration of Clinical COVID-19 Mouthwashing Protocol and Potential Modulation of Pediatric Oral Bacterial Prevalence of Selenomonas noxia: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Human Subjects and Informed Consent
2.3. DNA Isolation
2.4. qPCR Screening
2.5. Statistical Analysis
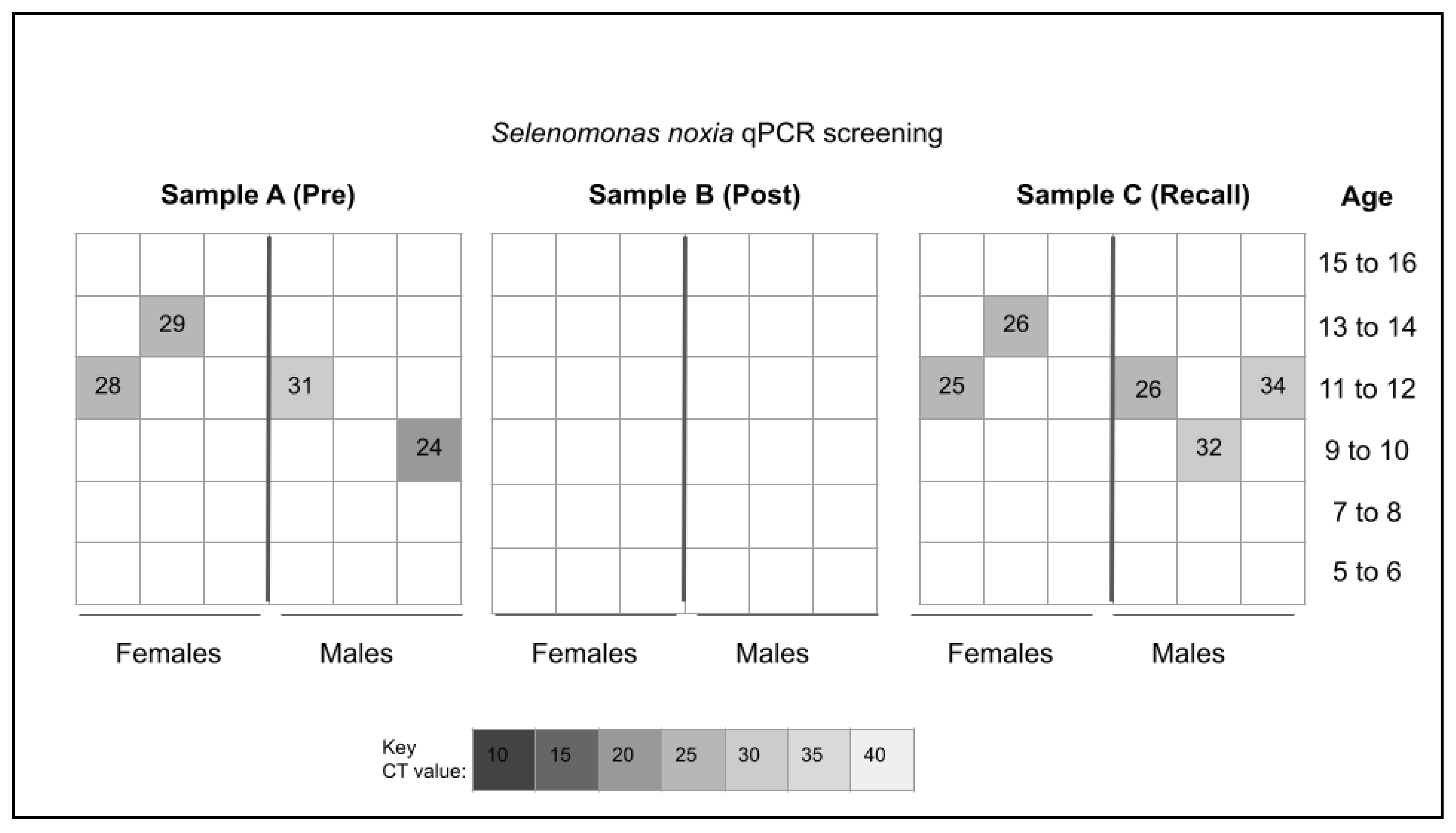
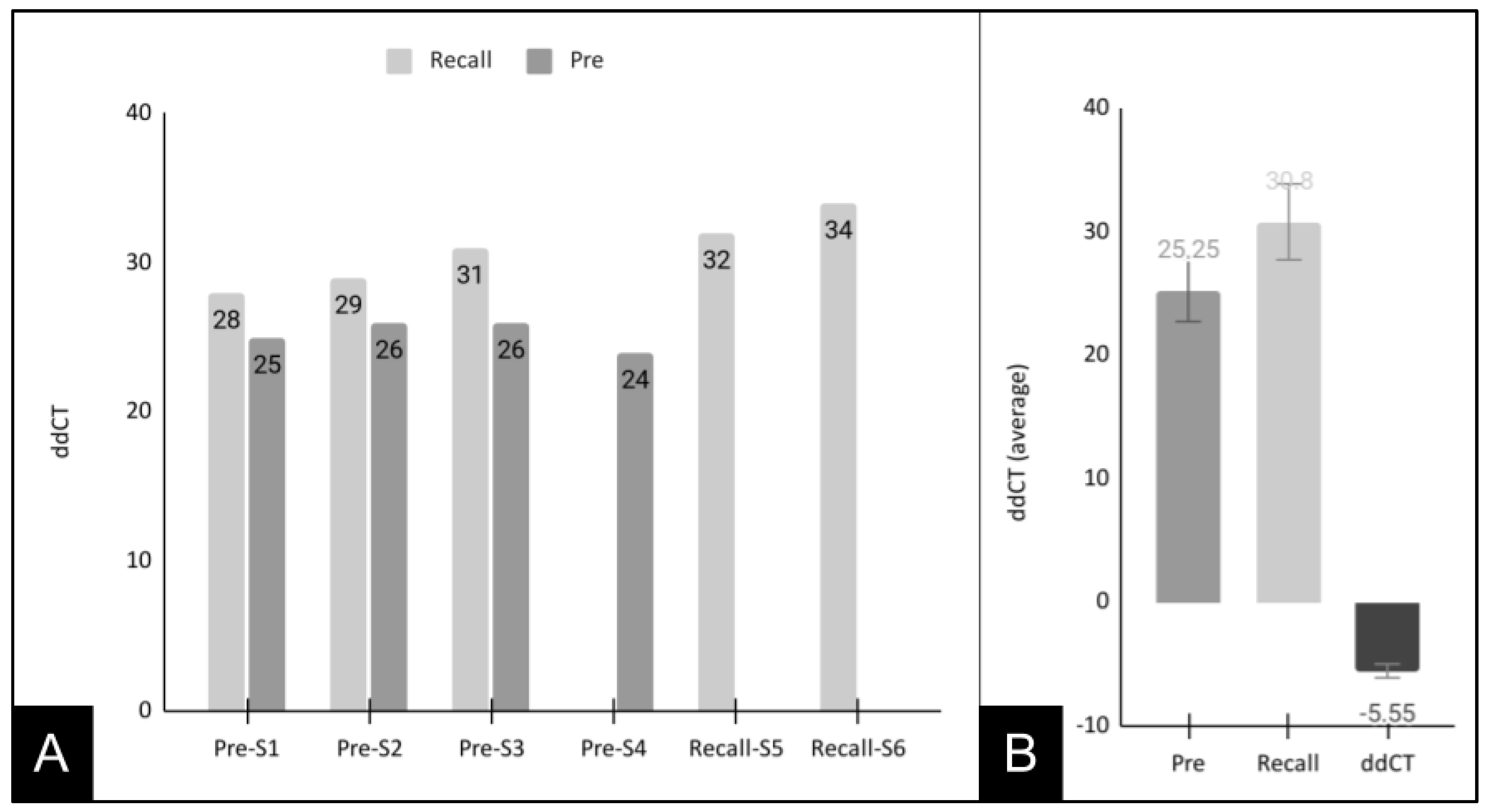
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siles-Garcia, A.A.; Alzamora-Cepeda, A.G.; Atoche-Socola, K.J.; Peña-Soto, C.; Arriola-Guillén, L.E. Biosafety for Dental Patients during Dentistry Care After COVID-19: A Review of the Literature. Disaster Med. Public Health Prep. 2021, 15, e43–e48. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, A.S.; Bhasin, R.; Kochhar, G.K.; Dadlani, H. COVID-19 Pandemic and Dental Practice. Int. J. Dent. 2020, 2020, 8894794. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, R.E.; Tehrani, S.; Revilla-León, M.; Zandinejad, A. Reducing the Risk of COVID-19 Transmission in Dental Offices: A Review. J Prosthodont. 2020, 29, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Bennardo, F.; Antonelli, A.; Barone, S.; Fortunato, L. COVID-19 is a New Challenge for Dental Practitioners: Advice on Patients’ Management from Prevention of Cross Infections to Telemedicine. Open Dent. J. 2020, 14, 298. [Google Scholar] [CrossRef]
- Sette-de-Souza, P.H.; Soares Martins, J.C.; Martins-de-Barros, A.V.; Rodrigues Vieira, B.; Fernandes Costa, M.J.; da Costa Araújo, F.A. A critical appraisal of evidence in the use of preprocedural mouthwash to avoid SARS-CoV-2 transmission during oral interventions. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10222–10224. [Google Scholar]
- Turkistani, K.A.; Turkistani, K.A. Dental Risks and Precautions during COVID-19 Pandemic: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 540–548. [Google Scholar] [CrossRef]
- Alzahrani, M.M.; Bamashmous, S.; Alkharobi, H.; Alghamdi, A.; Alharbi, R.H.; Hassan, A.M.; Darwish, M.; Bukhari, A.; Mahmoud, A.B.; Alfaleh, M.A.; et al. Mouth rinses efficacy on salivary SARS-CoV-2 viral load: A randomized clinical trial. J. Med. Virol. 2023, 95, e28412. [Google Scholar] [CrossRef]
- Farmaha, J.K.; James, J.N.; Frazier, K.; Sahajpal, N.S.; Mondal, A.K.; Bloomquist, D.T.; Kolhe, R.; Looney, S.W.; Bloomquist, R. Reduction of SARS-CoV-2 salivary viral load with pre-procedural mouth rinses: A randomised, controlled, clinical trial. Br. Dent. J. 2023, 234, 593–600. [Google Scholar] [CrossRef]
- Weber, J.; Bonn, E.L.; Auer, D.L.; Kirschneck, C.; Buchalla, W.; Scholz, K.J.; Cieplik, F. Preprocedural mouthwashes for infection control in dentistry-an update. Clin. Oral. Investig. 2023, 27, 33–44. [Google Scholar] [CrossRef]
- Ziaeefar, P.; Bostanghadiri, N.; Yousefzadeh, P.; Gabbay, J.; Shahidi Bonjar, A.H.; Ghazizadeh Ahsaie, M.; Centis, R.; Sabeti, M.; Sotgiu, G.; Migliori, G.B.; et al. The efficacy of mouthwashes in reducing SARS-CoV-2 viral loads in human saliva: A systematic review. New Microbes New Infect. 2022, 49, 101064. [Google Scholar] [CrossRef]
- Idrees, M.; McGowan, B.; Fawzy, A.; Abuderman, A.A.; Balasubramaniam, R.; Kujan, O. Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies. Int. J. Environ. Res. Public Health 2022, 19, 12148. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, A.; Lanave, G.; Marchi, S.; Lorusso, P.; Montomoli, E.; Martella, V.; Camero, M.; Prati, C.; Trombetta, C.M. In vitro virucidal activity of mouthwashes on SARS-CoV-2. Oral Dis. 2022, 28 (Suppl. 2), 2509–2515. [Google Scholar] [CrossRef] [PubMed]
- Meiller, T.F.; Silva, A.; Ferreira, S.M.; Jabra-Rizk, M.A.; Kelley, J.I.; DePaola, L.G. Efficacy of Listerine Antiseptic in reducing viral contamination of saliva. J. Clin. Periodontol. 2005, 32, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Dovrat, S.; Kashi-Zagdoun, E.; Soufiev, Z.; Mendelson, E.; Schlossberg, T. The Solution Is a Solution. Isr. Med. Assoc. J. 2022, 24, 80–84. [Google Scholar]
- Vlachojannis, C.; Chrubasik-Hausmann, S.; Hellwig, E.; Al-Ahmad, A. A Preliminary Investigation on the Antimicrobial Activity of Listerine®, Its Components, and of Mixtures Thereof. Phytother. Res. 2015, 29, 1590–1594. [Google Scholar] [CrossRef]
- Kshitish, D.; Laxman, V.K. The use of ozonated water and 0.2% chlorhexidine in the treatment of periodontitis patients: A clinical and microbiologic study. Indian J. Dent. Res. 2010, 21, 341–348. [Google Scholar]
- Shamsoddin, E. Preprocedural mouth rinses can reduce bacterial contamination in aerosols during periodontal prophylaxis. Evid. Based Dent. 2021, 22, 138–139, Erratum in Evid. Based Dent. 2022, 23, 5. [Google Scholar] [CrossRef]
- Martins, M.L.; Monteiro, A.S.N.; Guimarães, J.E.C.; Guimarães, M.B.C.T.; da Silva, R.F.; Cabral, L.M.; Farah, A.; dePaula, J.; Romanos, M.T.V.; Maia, L.C.; et al. Cytotoxic and antibacterial effect of a red propolis mouthwash, with or without fluoride, on the growth of a cariogenic biofilm. Arch. Oral Biol. 2019, 107, 104512. [Google Scholar] [CrossRef]
- Tanner, A.C. Anaerobic culture to detect periodontal and caries pathogens. J. Oral Biosci. 2015, 57, 18–26. [Google Scholar] [CrossRef]
- López, R.; Dahlén, G.; Retamales, C.; Baelum, V. Clustering of subgingival microbial species in adolescents with periodontitis. Eur. J. Oral Sci. 2011, 119, 141–150. [Google Scholar] [CrossRef]
- Colombo, A.P.; Boches, S.K.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.; et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J. Periodontol. 2009, 80, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Torresyap, G.; Haffajee, A.D.; Uzel, N.G.; Socransky, S.S. Relationship between periodontal pocket sulfide levels and subgingival species. J. Clin. Periodontol. 2003, 30, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Preza, D.; Olsen, I.; Aas, J.A.; Willumsen, T.; Grinde, B.; Paster, B.J. Bacterial profiles of root caries in elderly patients. J. Clin. Microbiol. 2008, 46, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.; Mehretu, A.M.; Buttner, M.P.; Trice, T.; Howard, K.M. Development of a polymerase chain reaction assay for the rapid detection of the oral pathogenic bacterium, Selenomonas noxia. BMC Oral Health 2015, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.; McDaniel, S.; Samiano, B.J.; Marrujo, M.; Kingsley, K.; Howard, K.M. Microbial Screening Reveals Oral Site-Specific Locations of the Periodontal Pathogen Selenomonas noxia. Curr. Issues Mol. Biol. 2021, 43, 29. [Google Scholar] [CrossRef]
- Shen, C.; Simpson, J.; Clawson, J.B.; Lam, S.; Kingsley, K. Prevalence of Oral Pathogen Slackia exigua among Clinical Orthodontic and Non-Orthodontic Saliva Samples. Microorganisms 2023, 11, 867. [Google Scholar] [CrossRef]
- Shen, C.; Clawson, J.B.; Simpson, J.; Kingsley, K. Oral Prevalence of Akkermansia muciniphila Differs among Pediatric and Adult Orthodontic and Non-Orthodontic Patients. Microorganisms 2023, 11, 112. [Google Scholar] [CrossRef]
- Buxser, S. Has resistance to chlorhexidine increased among clinically-relevant bacteria? A systematic review of time course and subpopulation data. PLoS ONE 2021, 16, e0256336. [Google Scholar] [CrossRef]
- De Geest, S.; Laleman, I.; Teughels, W.; Dekeyser, C.; Quirynen, M. Periodontal diseases as a source of halitosis: A review of the evidence and treatment approaches for dentists and dental hygienists. Periodontol 2000 2016, 71, 213–227. [Google Scholar] [CrossRef]
- Lam, O.L.; McGrath, C.; Li, L.S.; Samaranayake, L.P. Effectiveness of oral hygiene interventions against oral and oropharyngeal reservoirs of aerobic and facultatively anaerobic gram-negative bacilli. Am. J. Infect. Control 2012, 40, 175–182. [Google Scholar] [CrossRef]
- Kusahara, D.M.; Friedlander, L.T.; Peterlini, M.A.; Pedreira, M.L. Oral care and oropharyngeal and tracheal colonization by Gram-negative pathogens in children. Nurs. Crit. Care 2012, 17, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Henrique Soares, K.; Firoozi, P.; Maria de Souza, G.; Beatriz Lopes Martins, O.; Gabriel Moreira Falci, S.; Rocha Dos Santos, C.R. Efficacy of Probiotics Compared to Chlorhexidine Mouthwash in Improving Periodontal Status: A Systematic Review and Meta-Analysis. Int. J. Dent. 2023, 2023, 4013004. [Google Scholar] [CrossRef] [PubMed]
- Popolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, M.S.; Aminishakib, P.; Ansari, M. Antiviral mouthwashes: Possible benefit for COVID-19 with evidence-based approach. J. Oral Microbiol. 2020, 12, 1794363. [Google Scholar] [CrossRef] [PubMed]
- Kopycka-Kędzierawski, D.T.; Billings, R.J.; Feng, C.; Ragusa, P.G.; Flint, K.; Watson, G.E.; Wong, C.L.; Manning, S.; Gill, S.R.; O’Connor, T.G. A Prospective Longitudinal Study of Early Childhood Caries Onset in Initially Caries-Free Children. JDR Clin. Trans. Res. 2022, accepted. [Google Scholar] [CrossRef]
- Jensen, E.T.; Bertoni, A.G.; Crago, O.L.; Hoffman, K.L.; Wood, A.C.; Arzumanyan, Z.; Lam, L.K.; Roll, K.; Sandow, K.; Wu, M.; et al. Rationale, design and baseline characteristics of the Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes Obes. Metab. 2020, 22, 1976–1984. [Google Scholar] [CrossRef]
- Xu, H.; Tian, J.; Hao, W.; Zhang, Q.; Zhou, Q.; Shi, W.; Qin, M.; He, X.; Chen, F. Oral Microbiome Shifts From Caries-Free to Caries-Affected Status in 3-Year-Old Chinese Children: A Longitudinal Study. Front Microbiol. 2018, 9, 2009. [Google Scholar] [CrossRef]
- Rogers, M.B.; Firek, B.; Shi, M.; Yeh, A.; Brower-Sinning, R.; Aveson, V.; Kohl, B.L.; Fabio, A.; Carcillo, J.A.; Morowitz, M.J. Disruption of the microbiota across multiple body sites in critically ill children. Microbiome 2016, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Hagenfeld, D.; Kleine Bardenhorst, S.; Matern, J.; Prior, K.; Harks, I.; Eickholz, P.; Lorenz, K.; Kim, T.S.; Kocher, T.; Meyle, J.; et al. Long-term changes in the subgingival microbiota in patients with stage III–IV periodontitis treated by mechanical therapy and adjunctive systemic antibiotics: A secondary analysis of a randomized controlled trial. J. Clin. Periodontol. 2023, accepted. [Google Scholar] [CrossRef]
- Teles, F.R.; Teles, R.P.; Sachdeo, A.; Uzel, N.G.; Song, X.Q.; Torresyap, G.; Singh, M.; Papas, A.; Haffajee, A.D.; Socransky, S.S. Comparison of microbial changes in early redeveloping biofilms on natural teeth and dentures. J. Periodontol. 2012, 83, 1139–1148. [Google Scholar] [CrossRef]
- Teles, F.R.; Teles, R.P.; Uzel, N.G.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Early microbial succession in redeveloping dental biofilms in periodontal health and disease. J. Periodontal. Res. 2012, 47, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Uzel, N.G.; Teles, F.R.; Teles, R.P.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J. Clin. Periodontol. 2011, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Aftab, R.; Dodhia, V.H.; Jeanes, C.; Wade, R.G. Bacterial sensitivity to chlorhexidine and povidone-iodine antiseptics over time: A systematic review and meta-analysis of human-derived data. Sci. Rep. 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, Y.; Xiang, Y.; Hu, T.; Cheng, R.; Cai, H. The efficacy of mouthwashes on oral microorganisms and gingivitis in patients undergoing orthodontic treatment: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral Biol. 2021, 128, 105174. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Agarwal, P.; Nagesh, L. Comparative evaluation of efficacy of 0.2% Chlorhexidine, Listerine and Tulsi extract mouth rinses on salivary Streptococcus mutans count of high school children--RCT. Contemp. Clin. Trials 2011, 32, 802–808. [Google Scholar] [CrossRef]
- Neeraja, R.; Anantharaj, A.; Praveen, P.; Karthik, V.; Vinitha, M. The effect of povidone-iodine and chlorhexidine mouth rinses on plaque Streptococcus mutans count in 6- to 12-year-old school children: An in vivo study. J. Indian Soc. Pedod. Prev. Dent. 2008, 26 (Suppl. 1), S14–S18. [Google Scholar]
- Graves, A.; Grahl, T.; Keiserman, M.; Kingsley, K. Systematic Review and Meta Analysis of the Relative Effect on Plaque Index among Pediatric Patients Using Powered (Electric) versus Manual Toothbrushes. Dent. J. 2023, 11, 46. [Google Scholar] [CrossRef]
- Mavi, J.; Kingsley, K. Analysis of a Pediatric Dental School Patient Population Revealed Increasing Trends of Limited English Proficiency (LEP) Patients: Implications for Pediatric Dental Public Health and Access to Care. Pediatr. Rep. 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Emmett, J.; David, R.; McDaniel, J.; McDaniel, S.; Kingsley, K. Comparison of DNA Extracted from Pediatric Saliva, Gingival Crevicular Fluid and Site-Specific Biofilm Samples. Methods Protoc. 2020, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.M.; Groppo, D.; Halem, S.; Carpino, E. Is obesity an oral bacterial disease? J. Dent. Res. 2009, 88, 519–523. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, D.R.; Silva, P.A.; Colombo, A.P.V.; Silva-Boghossian, C.M. Subgingival microbiota in overweight and obese young adults with no destructive periodontal disease. J. Periodontol. 2021, 92, 1410–1419. [Google Scholar] [CrossRef]
- Rafeeq, R.A.; Saleem, A.E.; Nahidh, M.; Kadhum, A.S.; Al-Huwaizi, A.F.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Clinical management and infection control protocols during the COVID-19 pandemic: An online survey. Technol. Health Care, 2023; online ahead of print. [Google Scholar]
- Mezarina Mendoza, J.P.I.; Trelles Ubillús, B.P.; Salcedo Bolívar, G.T.; Castañeda Palacios, R.D.P.; Herrera Lopez, P.S.G.; Padilla Rodríguez, D.A.; Uchima Koechlin, K.H. Antiviral effect of mouthwashes against SARS-COV-2: A systematic review. Saudi Dent. J. 2022, 34, 167–193. [Google Scholar] [CrossRef]
| Positive Control, Bacterial 16S rRNA | |
| Forward 16S rRNA primer | 5′-ACG CGT CGA CAG AGT TTG ATC CTG GCT-3′ |
| Reverse 16S rRNA primer | 5′-GGG ACT ACC AGG GTA TCT AAT-3′ |
| Selenomonas noxia (SN) primer | |
| Forward primer SN-F1 | 5′-TCT GGG CTA CAC ACGT ACT ACA ATG-3′ |
| Reverse primer SN-R1 | 5′-GCC TGC AAT CCG AAC TGA GA-3′ |
| Demographic Characteristic | Study Sample (n = 97) | UNLV—SDM Clinic Summary | Statistical Analysis |
|---|---|---|---|
| Sex | |||
| Female | 49.5% (n = 47/97) | 52.8% | X2 = 0.371, d.f. = 1 |
| Male | 50.5% (n = 50/97) | 47.2% | p = 0.5422 |
| Race/Ethnicity | |||
| non-Minority (White) | 22.7% (n = 22/97) | 24.7% | X2 = 0.221, d.f. = 1 |
| Minority (non-White) | 77.3% (n = 75/97) | 75.3% | p = 0.6379 |
| Hispanic/Latino | 58.8% (n = 57/97) | 52.4% | |
| Black/African American | 11.3% (n = 11/97) | 12.2% | |
| Asian/Pacific Islander | 3.1% (n = 3/97) | 3.8% | |
| Native American/American Indian | 4.1% (n = 4/97) | 0.1% | |
| Age | |||
| Average Age Range of Age | 9.17 years (5 to 16 years) | 9.04 years (1 to 18 years) |
| Study Sample | DNA Concentration (Average and Standard Deviation) | DNA Purity A260:A280 Ratio |
|---|---|---|
| Sample A (Pre-mouthwash) T1, n = 36 Initial appointment | Average: 1141.7 ng/µL STD +/− 38.5 ng/µL | Average: 1.72 Range: 1.65–1.85 |
| Sample B (Post-mouthwash) T2, n = 36 Initial appointment | Average: 883.9 ng/µL STD +/− 41.7 ng/µL Two-tailed t-test T1:T2, p = 0.0039 | Average: 1.74 Range: 1.61–1.87 |
| Sample C (Recall) T3, n = 36 Recall appointment | Average: 1350.9 ng/µL STD +/− 41.7 ng/µL Two-tailed t-test T1:T3, p = 0.0359 | Average: 1.75 Range: 1.60–1.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodhi, P.; Jiang, Y.; Lin, S.; Downey, J.; Sorenson, C.; Shayegh, M.; Sullivan, V.; Kingsley, K.; Howard, K.M. Administration of Clinical COVID-19 Mouthwashing Protocol and Potential Modulation of Pediatric Oral Bacterial Prevalence of Selenomonas noxia: A Pilot Study. Pediatr. Rep. 2023, 15, 414-425. https://doi.org/10.3390/pediatric15030038
Sodhi P, Jiang Y, Lin S, Downey J, Sorenson C, Shayegh M, Sullivan V, Kingsley K, Howard KM. Administration of Clinical COVID-19 Mouthwashing Protocol and Potential Modulation of Pediatric Oral Bacterial Prevalence of Selenomonas noxia: A Pilot Study. Pediatric Reports. 2023; 15(3):414-425. https://doi.org/10.3390/pediatric15030038
Chicago/Turabian StyleSodhi, Praneeti, Yuxin Jiang, Summer Lin, Jackson Downey, Chase Sorenson, Melika Shayegh, Victoria Sullivan, Karl Kingsley, and Katherine M. Howard. 2023. "Administration of Clinical COVID-19 Mouthwashing Protocol and Potential Modulation of Pediatric Oral Bacterial Prevalence of Selenomonas noxia: A Pilot Study" Pediatric Reports 15, no. 3: 414-425. https://doi.org/10.3390/pediatric15030038
APA StyleSodhi, P., Jiang, Y., Lin, S., Downey, J., Sorenson, C., Shayegh, M., Sullivan, V., Kingsley, K., & Howard, K. M. (2023). Administration of Clinical COVID-19 Mouthwashing Protocol and Potential Modulation of Pediatric Oral Bacterial Prevalence of Selenomonas noxia: A Pilot Study. Pediatric Reports, 15(3), 414-425. https://doi.org/10.3390/pediatric15030038






