Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
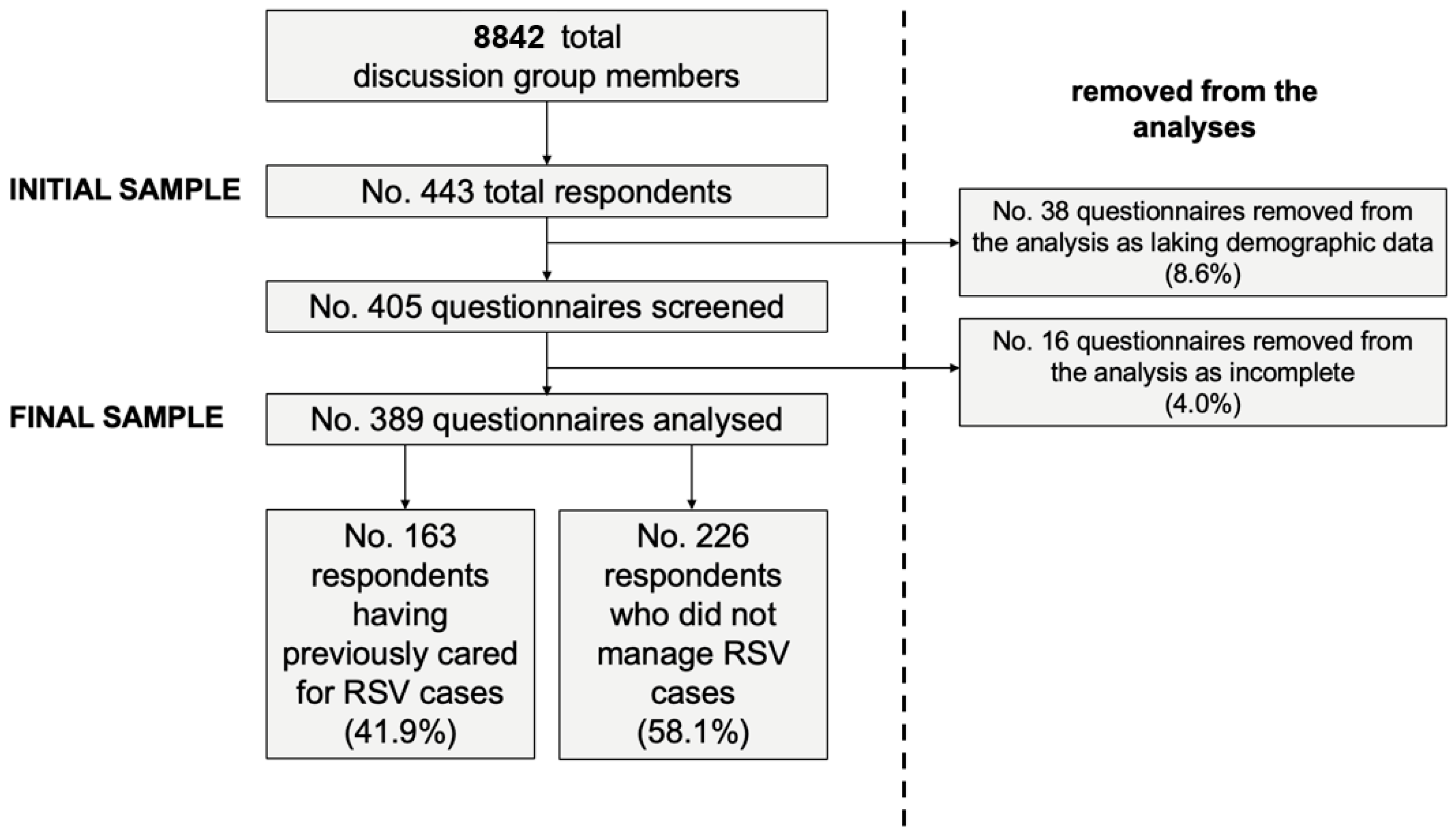
2.3. Sample Size
2.4. Questionnaire
- Characteristics of the participants: age, sex, seniority, Italian region where the professional mainly worked and lived.
- General Knowledge Test. A series of 25 statements were shown to the study participants (i.e., 19 true-false; 6 multiple-choice). A cumulative score (General Knowledge Score; GKS) was calculated by adding +1 for every correct answer, with a potential range 0 to 25. A similarly designed knowledge test was previously applied for KAP studies in healthcare settings and effectively adapted to a broad array of medical settings [54,65,66,67,68].
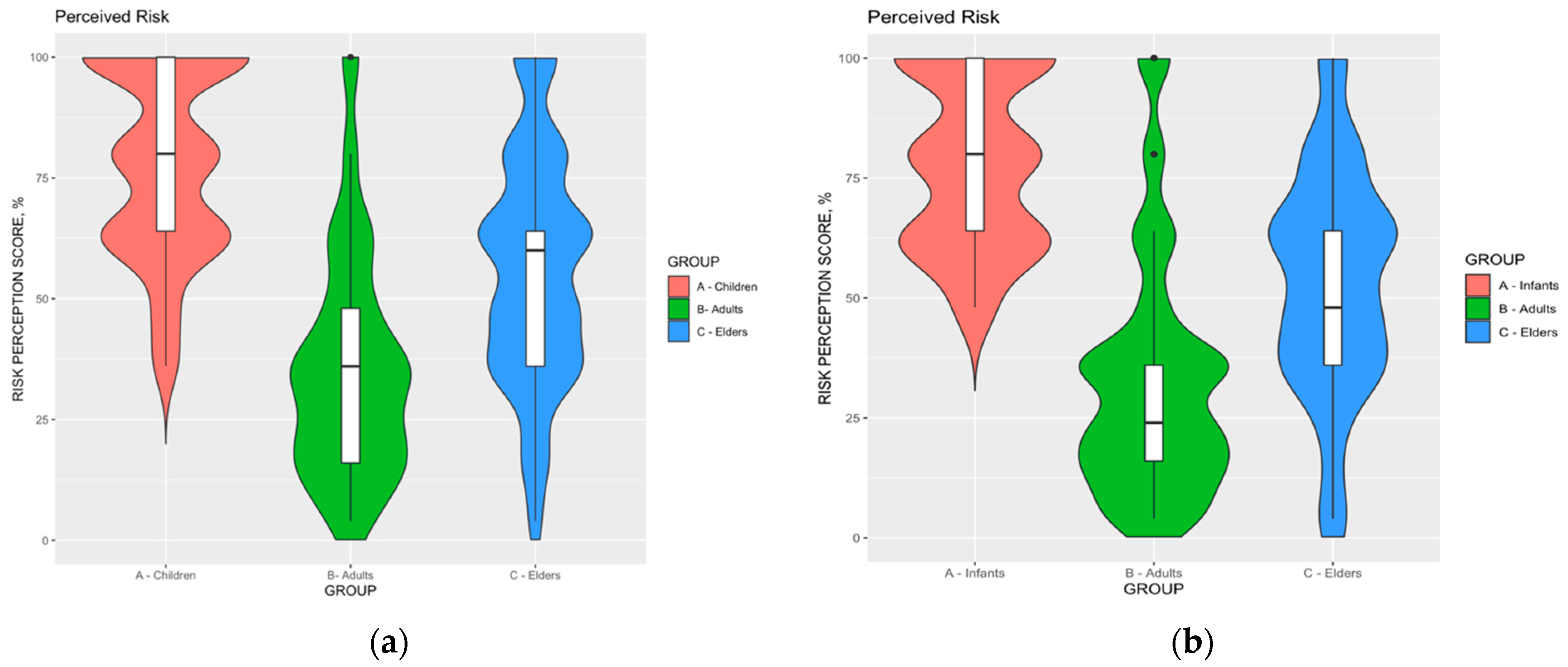
- Risk perception. According to the original definition of Yates, the perceived risk may be acknowledged as the function of the perceived probability of an event (F) and its expected consequences (C) [69]. Participants were therefore asked to rate the perceived severity (C) and the perceived frequency (F) of RSV infections through a fully labeled 5-points Likert scale (range: from “not significant”, 1, to “very significant”, 5). Distinctive estimates were calculated for infants (age 0 to 8 years), adults (age 18 to 64 years), and elderly (age 65 and more). Three Risk Perception Scores (RPS) were therefore calculated as the mathematical product of C and F (i.e., RPS = C x F, potential range 1 to 25).
- Attitudes towards mAb. Participants were initially asked to self-rate their attitude towards RSV mAb as a prophylactic option. Respondents were then asked whether they acknowledged mAb as a valuable option for preventing RSV natural infection, and for avoiding severe infections including LRTI. All of the aforementioned items were rated through a 5-points fully labeled Likert scale that ranged from “totally disagree” (1) to “totally agree” (5). Attitudes were then dichotomized in “somewhat agreeing” (i.e., agree to totally agree) vs. “somewhat disagreeing” (i.e., totally disagree to neutral).
- Practices. Participants were eventually asked about their interactions with RSV in the previous 5 years, and more precisely if they: (a) managed any RSV case in their daily practice; (b) diagnosed at least one case of RSV infection; (c) required any hospitalization for LRTI cases associated with RSV cases infections; or (d) required any shot of mAb for RSV immunoprophylaxis. All the aforementioned iterations were assessed as dichotomous items (i.e., ever vs. never).
2.5. Ethical Considerations
2.6. Data Analysis
3. Results
3.1. Descriptive Analysis: General Characteristics of the Sample
3.2. General Knowledge Test
3.3. Attitudes
3.4. Previous Interactions with RSV
3.5. Univariate Analysis
3.6. Regression Analysis
- (a)
- Model 1 assessed the whole of the sample (i.e., 389 pediatricians) about the outcome variable of having had any previous experience in the managing of RSV cases, and assuming as explanatory variables: seniority ≥ 10 years, working in hospital settings; the region of residence; and reporting higher RPS for adults.
- (b)
- Model 2 assessed all participants having reportedly managed any RSV case in the previous 5 years (i.e., 163 pediatricians). The analyses identified the previous delivery of mAb prophylactic therapy as the outcome variable, while the following explanatory variables were eventually included: belonging to an older age group (≥40 years); greater seniority (≥10 years); working in hospital settings; male gender; region of residence; higher GKS; and higher RPS for children.
4. Discussion
Limits of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Abu Raya, B.; Baraldi, E.; Flanagan, K.; Martinon Torres, F.; Tsolia, M.; Zielen, S. RSV Prevention in All Infants: Which Is the Most Preferable Strategy? Front. Immunol. 2022, 13, 880368. [Google Scholar] [CrossRef]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccines Immunother. 2022, 18, 2079322. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Leader, S.; Kohlhase, K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–632. [Google Scholar] [CrossRef]
- Na’Amnih, W.; Kassem, E.; Tannous, S.; Kagan, V.; Jbali, A.; Hanukayev, E.; Freimann, S.; Obolski, U.; Muhsen, K. Incidence and risk factors of hospitalisations for respiratory syncytial virus among children aged less than 2 years. Epidemiology Infect. 2022, 150, e45. [Google Scholar] [CrossRef]
- Jans, J.; Wicht, O.; Widjaja, I.; Ahout, I.M.L.; de Groot, R.; Guichelaar, T.; Luytjes, W.; de Jonge, M.I.; de Haan, C.A.M.; Ferwerda, G. Characteristics of RSV-Specific Maternal Antibodies in Plasma of Hospitalized, Acute RSV Patients under Three Months of Age. PLoS ONE 2017, 12, e0170877. [Google Scholar] [CrossRef]
- Chida-Nagai, A.; Sato, H.; Sato, I.; Shiraishi, M.; Sasaki, D.; Izumi, G.; Yamazawa, H.; Cho, K.; Manabe, A.; Takeda, A. Risk factors for hospitalisation due to respiratory syncytial virus infection in children receiving prophylactic palivizumab. Eur. J. Pediatr. 2021, 181, 539–547. [Google Scholar] [CrossRef]
- Azzari, C.; Baraldi, E.; Bonanni, P.; Bozzola, E.; Coscia, A.; Lanari, M.; Manzoni, P.; Mazzone, T.; Sandri, F.; Lisi, G.C.; et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital. J. Pediatr. 2021, 47, 198. [Google Scholar] [CrossRef]
- Pellegrinelli, L.; Galli, C.; Bubba, L.; Cereda, D.; Anselmi, G.; Binda, S.; Gramegna, M.; Pariani, E. Respiratory syncytial virus in influenza-like illness cases: Epidemiology and molecular analyses of four consecutive winter seasons (2014-2015/2017-2018) in Lombardy (Northern Italy). J. Med. Virol. 2020, 92, 2999–3006. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef]
- Andeweg, S.P.; Schepp, R.M.; van de Kassteele, J.; Mollema, L.; Berbers, G.A.M.; van Boven, M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci. Rep. 2021, 11, 8953. [Google Scholar] [CrossRef]
- Mazur, N.I.; Martinón-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower respiratory tract infection caused by respiratory syncytial virus: Current management and new therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Giersing, B.K.; Karron, R.A.; Vekemans, J.; Kaslow, D.C.; Moorthy, V.S. Meeting report: WHO consultation on Respiratory Syncytial Virus (RSV) vaccine development, Geneva, 25–26 April 2016. Vaccine 2019, 37, 7355–7362. [Google Scholar] [CrossRef]
- Mosalli, R.; Alqarni, S.A.; Khayyat, W.W.; Alsaidi, S.T.; Almatrafi, A.S.; Bawakid, A.S.; Paes, B. Respiratory syncytial virus nosocomial outbreak in neonatal intensive care: A review of the incidence, management, and outcomes. Am. J. Infect. Control. 2021, 50, 801–808. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef]
- Debes, S.; Haug, J.B.; de Blasio, B.F.; Jonassen, C.M.; Dudman, S.G. Etiology of viral respiratory tract infections in hospitalized adults, and evidence of the high frequency of prehospitalization antibiotic treatment in Norway. Health Sci. Rep. 2021, 4, e403. [Google Scholar] [CrossRef] [PubMed]
- Obolski, U.; Kassem, E.; Na’Amnih, W.; Tannous, S.; Kagan, V.; Muhsen, K. Unnecessary antibiotic treatment of children hospitalised with respiratory syncytial virus (RSV) bronchiolitis: Risk factors and prescription patterns. J. Glob. Antimicrob. Resist. 2021, 27, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Andabaka, T.; Nickerson, J.W.; Rojas-Reyes, M.X.; Rueda, J.D.; Vrca, V.B.; Barsic, B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst. Rev. 2013, 2013, CD006602. [Google Scholar] [CrossRef]
- Frogel, M.P.; Stewart, D.L.; Hoopes, M.; Fernandes, A.W.; Mahadevia, P.J. A Systematic Review of Compliance with Palivizumab Administration for RSV Immunoprophylaxis. J. Manag. Care Pharm. 2010, 16, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Olchanski, N.; Hansen, R.N.; Pope, E.; D’Cruz, B.; Fergie, J.; Goldstein, M.; Krilov, L.R.; McLaurin, K.K.; Nabrit-Stephens, B.; Oster, G.; et al. Palivizumab Prophylaxis for Respiratory Syncytial Virus: Examining the Evidence Around Value. Open Forum Infect. Dis. 2018, 5, ofy031. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S.; Stirling, R.; Tunis, M.; Sander, B. Cost-effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, e20184064. [Google Scholar] [CrossRef]
- Viguria, N.; Navascués, A.; Juanbeltz, R.; Echeverría, A.; Ezpeleta, C.; Castilla, J. Effectiveness of palivizumab in preventing respiratory syncytial virus infection in high-risk children. Hum. Vaccines Immunother. 2021, 17, 1867–1872. [Google Scholar] [CrossRef]
- Luna, M.S.; Manzoni, P.; Paes, B.; Baraldi, E.; Cossey, V.; Kugelman, A.; Chawla, R.; Dotta, A.; Fernández, R.R.; Resch, B.; et al. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr. Respir. Rev. 2018, 33, 35–44. [Google Scholar] [CrossRef]
- Mitchell, I.; Li, A.; Bjornson, C.L.; Lanctot, K.L.; Paes, B.A.; the CARESS investigators. Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada. Am. J. Perinatol. 2021, 39, 1668–1677. [Google Scholar] [CrossRef]
- Zylbersztejn, A.; Almossawi, O.; Gudka, N.; Tompsett, D.; De Stavola, B.; Standing, J.F.; Smyth, R.; Hardelid, P. Access to palivizumab among children at high risk of respiratory syncytial virus complications in English hospitals. Br. J. Clin. Pharmacol. 2021, 88, 1246–1257. [Google Scholar] [CrossRef]
- Batista, J.D.L.; Ferreira, M.A.P.; Xavier, C.D.S.; de Souza, I.T.A.; Cruz, L.N.; Polanczyk, C.A. A post-incorporation study on the use of palivizumab in the Brazilian public health system. Rev. Do Inst. De Med. Trop. De São Paulo 2021, 63, e5. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.C.; Long, S.S. Committee on Infectious Diseases and Committee on Fetus and Newborn Revised Indications for the Use of Palivizumab and Respiratory Syncytial Virus Immune Globulin Intravenous for the Prevention of Respiratory Syncytial Virus Infections. Pediatrics 2003, 112, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Cutrera, R.; Wolfler, A.; Picone, S.; Rossi, G.A.; Gualberti, G.; Merolla, R.; Del Vecchio, A.; Villani, A.; Midulla, F.; Dotta, A. Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016–2017 epidemic season: A systematic review of seven Italian reports. Ital. J. Pediatr. 2019, 45, 139. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee; Brady, M.T.; Byington, C.L.; Dele Davies, H.; Edwards, K.M.; Jackson, M.A.; Maldonado, Y.A.; Murray, D.L.; Orenstein, W.A.; et al. Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 2014, 134, e620–e638. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. New Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef]
- Voirin, N.; Virlogeux, V.; Demont, C.; Kieffer, A. Potential Impact of Nirsevimab on RSV Transmission and Medically Attended Lower Respiratory Tract Illness Caused by RSV: A Disease Transmission Model. Infect. Dis. Ther. 2021, 11, 277–292. [Google Scholar] [CrossRef]
- Domachowske, J.; Madhi, S.A.; Simões, E.A.; Atanasova, V.; Cabañas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. New Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Breakthrough therapy designation of nirsevimab for the prevention of lower respiratory tract illness caused by respiratory syncytial virus infections (RSV). Expert Opin. Investig. Drugs 2021, 31, 23–29. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef]
- Calderaro, A.; De Conto, F.; Buttrini, M.; Piccolo, G.; Montecchini, S.; Maccari, C.; Martinelli, M.; Di Maio, A.; Ferraglia, F.; Pinardi, F.; et al. Human respiratory viruses, including SARS-CoV-2, circulating in the winter season 2019–2020 in Parma, Northern Italy. Int. J. Infect. Dis. 2020, 102, 79–84. [Google Scholar] [CrossRef]
- Sherman, A.C.; Babiker, A.; Sieben, A.J.; Pyden, A.; Steinberg, J.; Kraft, C.S.; Koelle, K.; Kanjilal, S. The Effect of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Mitigation Strategies on Seasonal Respiratory Viruses: A Tale of 2 Large Metropolitan Centers in the United States. Clin. Infect. Dis. 2020, 72, e154–e157. [Google Scholar] [CrossRef]
- Kuitunen, I.M.; Artama, M.M.; Mäkelä, L.; Backman, K.M.; Heiskanen-Kosma, T.M.; Renko, M.M. Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland During Early 2020. Pediatr. Infect. Dis. J. 2020, 39, e423–e427. [Google Scholar] [CrossRef]
- Van Brusselen, D.; de Troeyer, K.; ter Haar, E.; van der Auwera, A.; Poschet, K.; van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; van Herendael, B.; et al. Bronchiolitis in COVID-19 Times: A Nearly Absent Disease? Eur. J. Pediatr. 2021, 180, 1969–1973. [Google Scholar] [CrossRef]
- Britton, P.N.; Hu, N.; Saravanos, G.; Shrapnel, J.; Davis, J.; Snelling, T.; Dalby-Payne, J.; Kesson, A.M.; Wood, N.; Macartney, K.; et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc. Health 2020, 4, e42–e43. [Google Scholar] [CrossRef]
- Ninot, G. Defining Non-Pharmacological Interventions. In Non-Pharmacological Interventions an Essential Answer to Current Demographic, Health, and Environmental Transitions; Ninot, G., Ed.; Springer Nature AG: Cham, Switzerland, 2021; pp. 1–44. ISBN 987-3-030-60971-9. [Google Scholar] [CrossRef]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory syncytial virus: Paying the immunity debt with interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Foley, D.A.; Phuong, L.K.; Peplinski, J.; Lim, S.M.; Lee, W.H.; Farhat, A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch. Dis. Child. 2021, 107, e1–e7. [Google Scholar] [CrossRef]
- Foley, D.A.; Yeoh, D.K.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Moore, H.C.; Blyth, C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019—Related Public Health Measures. Clin. Infect. Dis. 2021, 1, ciaa1906. [Google Scholar] [CrossRef]
- Lumley, S.F.; Richens, N.; Lees, E.; Cregan, J.; Kalimeris, E.; Oakley, S.; Morgan, M.; Segal, S.; Dawson, M.; Walker, A.S.; et al. Changes in pediatric respiratory infections at a UK teaching hospital 2016–2021; impact of the SARS-CoV-2 pandemic. J. Infect. 2021, 84, 40–47. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Zaniboni, A.; Ranzieri, S. Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatr. Rep. 2022, 14, 147–165. [Google Scholar] [CrossRef]
- Giles, M.L.; Buttery, J.; Davey, M.-A.; Wallace, E. Pregnant women’s knowledge and attitude to maternal vaccination including group B streptococcus and respiratory syncytial virus vaccines. Vaccine 2019, 37, 6743–6749. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Calvert, A.; Metz, J.; Kilich, E.; MacLeod, R.; Beadon, K.; Heath, P.T.; Khalil, A.; Finn, A.; Snape, M.D.; et al. Attitudes of Pregnant Women and Healthcare Professionals Toward Clinical Trials and Routine Implementation of Antenatal Vaccination Against Respiratory Syncytial Virus: A Multicenter Questionnaire Study. Pediatr. Infect. Dis. J. 2019, 38, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Wicker, S. Personal attitudes and misconceptions, not official recommendations guide occupational physicians’ vaccination decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (StroBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Cecchini, E.; Schino, S.; Gambadoro, N.; Ricciardi, L.; Trio, O.; Biondi-Zoccai, G.; Sangiorgi, G. Facing the pandemic with a smile: The case of Memedical and its impact on cardiovascular professionals. Minerva Cardioangiol. 2022. Epub Ahead of Print. [Google Scholar] [CrossRef]
- Hurley, L.P.; Allison, M.A.; Kim, L.; O’Leary, S.T.; Crane, L.A.; Brtnikova, M.; Beaty, B.L.; Allen, K.E.; Poser, S.; Lindley, M.C.; et al. Primary care physicians’ perspectives on respiratory syncytial virus (RSV) disease in adults and a potential RSV vaccine for adults. Vaccine 2018, 37, 565–570. [Google Scholar] [CrossRef]
- Reeves, R.; Hardelid, P.; Panagiotopoulos, N.; Minaji, M.; Warburton, F.; Pebody, R. Burden of hospital admissions caused by respiratory syncytial virus (RSV) in infants in England: A data linkage modelling study. J. Infect. 2019, 78, 468–475. [Google Scholar] [CrossRef]
- Barbati, F.; Moriondo, M.; Pisano, L.; Calistri, E.; Lodi, L.; Ricci, S.; Giovannini, M.; Canessa, C.; Indolfi, G.; Azzari, C. Epidemiology of Respiratory Syncytial Virus-Related Hospitalization Over a 5-Year Period in Italy: Evaluation of Seasonality and Age Distribution Before Vaccine Introduction. Vaccines 2020, 8, 15. [Google Scholar] [CrossRef]
- Rainisch, G.; Adhikari, B.; Meltzer, M.I.; Langley, G. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine 2019, 38, 251–257. [Google Scholar] [CrossRef]
- Palmer, L.; Hall, C.B.; Katkin, J.P.; Shi, N.; Masaquel, A.S.; McLaurin, K.K.; Mahadevia, P.J. Healthcare costs within a year of respiratory syncytial virus among medicaid infants. Pediatr. Pulmonol. 2010, 45, 772–781. [Google Scholar] [CrossRef]
- Shi, T.; Arnott, A.; Semogas, I.; Falsey, A.R.; Openshaw, P.; Wedzicha, J.A.; Campbell, H.; Nair, H.; Zhang, S.; Li, Y.; et al. The Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 2019, 222, S563–S569. [Google Scholar] [CrossRef]
- Staadegaard, L.; Caini, S.; Wangchuk, S.; Thapa, B.; de Almeida, W.A.F.; de Carvalho, F.C.; Njouom, R.; A Fasce, R.; Bustos, P.; Kyncl, J.; et al. The Global Epidemiology of RSV in Community and Hospitalized Care: Findings From 15 Countries. Open Forum Infect. Dis. 2021, 8, ofab159. [Google Scholar] [CrossRef]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic review on respiratory syncytial virus epidemiology in adults and the elderly in Latin America. Int. J. Infect. Dis. 2019, 90, 170–180. [Google Scholar] [CrossRef]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef]
- Riccò, M.; Razio, B.; Panato, C.; Poletti, L.; Signorelli, C. Knowledge, Attitudes and Practices of Agricultural Workers towards Tetanus Vaccine: A Field Report. Ann Ig 2017, 29, 239–255. [Google Scholar]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Mandate or Not Mandate: Knowledge, Attitudes, and Practices of Italian Occupational Physicians towards SARS-CoV-2 Immunization at the Beginning of Vaccination Campaign. Vaccines 2021, 9, 889. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. [Google Scholar] [CrossRef]
- Yates, F.J.; Stone, E.R. The Risk Construct. In Risk-Taking Behaviour; Yates, F.J., Ed.; John Wiley & Sons: Chichester, UK, 1992; pp. 1–25. ISBN 0471922501. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 24 September 2022).
- Weiner, J.H. Respiratory Syncytial Virus Infection and Palivizumab: Are Families Receiving Accurate Information? Am. J. Perinatol. 2009, 27, 219–223. [Google Scholar] [CrossRef]
- Rybak, A.; Levy, C.; Jung, C.; Béchet, S.; Batard, C.; Hassid, F.; Zouari, M.; Cahn-Sellem, F.; Bangert, M.; Cohen, R. Delayed Bronchiolitis Epidemic in French Primary Care Setting Driven by Respiratory Syncytial Virus: Preliminary Data from the Oursyn Study, March 2021. Pediatr. Infect. Dis. J. 2021, 40, e511–e514. [Google Scholar] [CrossRef]
- Bozzola, E.; Ciarlitto, C.; Guolo, S.; Brusco, C.; Cerone, G.; Antilici, L.; Schettini, L.; Piscitelli, A.L.; Vittucci, A.C.; Cutrera, R.; et al. Respiratory Syncytial Virus Bronchiolitis in Infancy: The Acute Hospitalization Cost. Front. Pediatr. 2021, 8, 594898. [Google Scholar] [CrossRef]
- Di Mattia, G.; Nenna, R.; Mancino, E.; Rizzo, V.; Pierangeli, A.; Villani, A.; Midulla, F. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr. Pulmonol. 2021, 56, 3106–3109. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Dondi, A.; Scarpini, S.; Rocca, A.; Vandini, S.; Poletti, G.; Lanari, M. Current State and Challenges in Developing Respiratory Syncytial Virus Vaccines. Vaccines 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.; Giles, M.L. Maternal RSV Vaccine Development. Where to from Here? Hum. Vaccines Immunother. 2021, 17, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef]
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Srikantiah, P. Respiratory syncytial virus: Promising progress against a leading cause of pneumonia. Lancet Glob. Health 2021, 9, e1644–e1645. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Trial to Evaluate the Efficacy and Safety of RSVpreF in Infants Born to Women Vaccinated During Pregnancy. [Internet]; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04424316 (accessed on 24 September 2022).
- ClinicalTrials.gov. A phase III Double-Blind Study to Assess Safety and Efficacy of an RSV Maternal Unadjuvanted Vaccine, in Pregnant Women and Infants Born to Vaccinated Mothers (GRACE). [Internet]; 2022. Available online: https://clinicaltrials.gov/ct2/show/results/NCT04605159 (accessed on 24 September 2022).
- Baraldi, E.; Lanari, M.; Manzoni, P.; Rossi, G.A.; Vandini, S.; Rimini, A.; Romagnoli, C.; Colonna, P.; Biondi, A.; Biban, P.; et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital. J. Pediatr. 2014, 40, 65. [Google Scholar] [CrossRef]
- Janz, N.K.; Becker, M.H. The Health Belief Model: A Decade Later. Health Educ. Q. 1984, 11, 1–47. [Google Scholar] [CrossRef]
- Rosenstock, I.M. Historical Origins of the Health Belief Model. Health Educ. Monogr. 1974, 2, 328–335. [Google Scholar] [CrossRef]
- Carpenter, C.J. A Meta-Analysis of the Effectiveness of Health Belief Model Variables in Predicting Behavior. Health Commun. 2010, 25, 661–669. [Google Scholar] [CrossRef]
- Mo, P.K.H.; Lau, J.T.F. Influenza vaccination uptake and associated factors among elderly population in Hong Kong: The application of the Health Belief Model. Health Educ. Res. 2015, 30, 706–718. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, attitudes, beliefs and practices of occupational physicians towards vaccinations of health care workers: A cross sectional pilot study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S. Tetanus Vaccination Status and Vaccine Hesitancy in Amateur Basketball Players (Italy, 2020). Vaccines 2022, 10, 131. [Google Scholar] [CrossRef]
- Begley, K.M.; Leis, A.M.; Petrie, J.G.; Johnson, E.; McSpadden, E.; Lamerato, L.E.; Wei, M.; Martin, E.T. Epidemiology of RSV-A and RSV-B in Adults and Children with Medically-Attended Acute Respiratory Illness over Three Seasons. medRxiv 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.11.04.22281968v1 (accessed on 22 September 2022). [CrossRef]
- Lively, J.Y.; Curns, A.T.; Weinberg, G.A.; Edwards, K.M.; Staat, M.A.; Prill, M.M.; Gerber, S.I.; Langley, G.E. Respiratory syncytial virus–associated outpatient visits among children younger than 24 months. J. Pediatr. Infect. Dis. Soc. 2019, 8, 284–286. [Google Scholar] [CrossRef]
- Arriola, C.S.; Kim, L.; Langley, G.; Anderson, E.J.; Openo, K.; Martin, A.M.; Lynfield, R.; Bye, E.; Como-Sabetti, K.; Reingold, A.; et al. Estimated Burden of Community-Onset Respiratory Syncytial Virus–Associated Hospitalizations Among Children Aged <2 Years in the United States, 2014–2015. J. Pediatr. Infect. Dis. Soc. 2019, 9, 587–595. [Google Scholar] [CrossRef]
- Byington, C.L.; Wilkes, J.; Korgenski, K.; Sheng, X. Respiratory Syncytial Virus–Associated Mortality in Hospitalized Infants and Young Children. Pediatrics 2015, 135, e24–e31. [Google Scholar] [CrossRef]
- Allen, K.E.; Beekmann, S.E.; Polgreen, P.; Poser, S.; Pierre, J.S.; Santibañez, S.; Gerber, S.I.; Kim, L. Survey of diagnostic testing for respiratory syncytial virus (RSV) in adults: Infectious disease physician practices and implications for burden estimates. Diagn. Microbiol. Infect. Dis. 2018, 92, 206–209. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Demont, C.; Petrica, N.; Bardoulat, I.; Duret, S.; Watier, L.; Chosidow, A.; Lorrot, M.; Kieffer, A.; Lemaitre, M. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect. Dis. 2021, 21, 730. [Google Scholar] [CrossRef]
- Auvinen, R.; Syrjänen, R.; Ollgren, J.; Nohynek, H.; Skogberg, K. Clinical characteristics and population-based attack rates of respiratory syncytial virus versus influenza hospitalizations among adults—An observational study. Influ. Other Respir. Viruses 2021, 16, 276–288. [Google Scholar] [CrossRef]
- Yung, C.-F.; Lee, K.-S.; Thein, T.-L.; Tan, L.-K.; Gan, V.; Wong, J.; Lye, D.; Ng, L.-C.; Leo, Y.-S. Dengue Serotype-Specific Differences in Clinical Manifestation, Laboratory Parameters and Risk of Severe Disease in Adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Long, C.E.; Schnabel, K.C. Respiratory Syncytial Virus Infections in Previously Healthy Working Adults. Clin. Infect. Dis. 2001, 33, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Tramuto, F.; Maida, C.M.; Di Naro, D.; Randazzo, G.; Vitale, F.; Restivo, V.; Costantino, C.; Amodio, E.; Casuccio, A.; Graziano, G.; et al. Respiratory Syncytial Virus: New Challenges for Molecular Epidemiology Surveillance and Vaccination Strategy in Patients with ILI/SARI. Vaccines 2021, 9, 1334. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Pariani, E.; Amendola, A.; Piatti, A.; Anselmi, G.; Ranghiero, A.; Bubba, L.; Rosa, A.M.; Pellegrinelli, L.; Binda, S.; Coppola, L.; et al. Ten Years (2004–2014) of Influenza Surveillance in Northern Italy. Hum. Vaccines Immunother. 2015, 11, 198–205. [Google Scholar] [CrossRef]
- Mortensen, G.L.; Harrod-Lui, K. Parental knowledge about respiratory syncytial virus (RSV) and attitudes to infant immunization with monoclonal antibodies. Expert Rev. Vaccines 2022, 21, 1523–1531. [Google Scholar] [CrossRef]
- Heiervang, E.; Goodman, R. Advantages and limitations of web-based surveys: Evidence from a child mental health survey. Soc. Psychiatry Psychiatr. Epidemiol. 2009, 46, 69–76. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, S.; Wang, L.; Zhao, Y.; Liu, H.; Yao, D.; Xu, Y.; Lv, Q.; Hao, G.; Xu, Y.; et al. Knowledge, Attitudes, and Practices Regarding Zika: Paper- and Internet-Based Survey in Zhejiang, China. JMIR Public Health Surveill. 2017, 3, e81. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; Di Palma, P.; Minutolo, G.; Ranzieri, S.; Zaffina, S.; Baldassarre, A.; Restivo, V. Managing of Migraine in the Workplaces: Knowledge, Attitudes and Practices of Italian Occupational Physicians. Medicina 2022, 58, 686. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Balzarini, F. Challenges faced by the Italian medical workforce. Lancet 2020, 395, e55–e56. [Google Scholar] [CrossRef]
- Vicarelli, G.; Pavolini, E. Health workforce governance in Italy. Health Policy 2015, 119, 1606–1612. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Balzarini, F.; A Capozzi, V.; Volpi, L. Knowledge, attitudes, beliefs and practices of obstetrics-gynecologists on seasonal influenza and pertussis immunizations in pregnant women: Preliminary results from North-Western Italy. Minerva Obstet. Gynecol. 2019, 71, 288–297. [Google Scholar] [CrossRef]
- Zingg, A.; Siegrist, M. Measuring people’s knowledge about vaccination: Developing a one-dimensional scale. Vaccine 2012, 30, 3771–3777. [Google Scholar] [CrossRef]
- Krumpal, I. Determinants of social desirability bias in sensitive surveys: A literature review. Qual. Quant. 2011, 47, 2025–2047. [Google Scholar] [CrossRef]
- Sharp, A.; Minaji, M.; Panagiotopoulos, N.; Reeves, R.; Charlett, A.; Pebody, R. Estimating the burden of adult hospital admissions due to RSV and other respiratory pathogens in England. Influ. Other Respir. Viruses 2021, 16, 125–131. [Google Scholar] [CrossRef]
- Cromer, D.; van Hoek, A.J.; Newall, A.; Pollard, A.J.; Jit, M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: A modelling and cost-effectiveness analysis for England. Lancet Public Health 2017, 2, e367–e374. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, attitudes, beliefs and practices of Occupational Physicians towards seasonal influenza vaccination: A cross-sectional study from North-Eastern Italy. J. Prev. Med. Hyg. 2017, 58, E141–E154. [Google Scholar]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Bragazzi, N.L.; Balzarini, F. Pertussis immunization in healthcare workers working in pediatric settings: Knowledge, Attitudes and Practices (KAP) of Occupational Physicians. Preliminary results from a web-based survey (2017). J. Prev. Med. Hyg. 2020, 61, e66–e75. [Google Scholar]
- Bozzola, E. Respiratory Syncytial Virus Resurgence in Italy: The Need to Protect All Neonates and Young Infants. Int. J. Environ. Res. Public Health 2021, 19, 380. [Google Scholar] [CrossRef]
- Rovetta, A. Reliability of Google Trends: Analysis of the Limits and Potential of Web Infoveillance During COVID-19 Pandemic and for Future Research. Front. Res. Metrics Anal. 2021, 6, 670226. [Google Scholar] [CrossRef]
- Riccò, M.; Zaniboni, A.; Satta, E.; Ranzieri, S.; Cerviere, M.P.; Marchesi, F.; Peruzzi, S. West Nile Virus Infection: A Cross-Sectional Study on Italian Medical Professionals during Summer Season 2022. Trop. Med. Infect. Dis. 2022, 7, 404. [Google Scholar] [CrossRef] [PubMed]


| Variable | No./389 | Average ± SD |
|---|---|---|
| Gender | ||
| Male | 141, 36.2% | |
| Female | 238, 61.2% | |
| Not stated | 10, 2.6% | |
| Region | ||
| North-Western Italy | 69, 17.7% | |
| North-Eastern Italy | 127, 32.6% | |
| Central Italy | 122, 31.4% | |
| Southern Italy | 35, 9.0% | |
| Major Islands | 36, 9.3% | |
| Age (years) | 40.1 ± 9.1 | |
| Seniority as PDL (years) | 13.9 ± 9.0 | |
| Previously managed RSV cases | 163, 41.9% | |
| Previously diagnosed RSV cases | 134, 34.4% | |
| Previously required hospitalization for RSV | 127, 32.6% | |
| Previously required mAb immunoprophylaxis for RSV | 56, 14.4% | |
| Acknowledging RSV infection as frequent/very frequent in … | ||
| … infants | 360, 92.5% | |
| … adults | 117, 30.1% | |
| … elderly | 213, 54.8% | |
| Acknowledging RSV infection as severe/very severe in … | ||
| … infants | 330, 84.8% | |
| … adults | 110, 28.3% | |
| … elderly | 256, 65.8% | |
| General Knowledge Score (%) | 54.0 ± 14.2 | |
| General Knowledge Score > median (52.0%) | 179, 46.0% | |
| Risk Perception Score for infants | 78.3 ± 19.5 | |
| Risk Perception Score for infants > median (80.0%) | 142, 36.5% | |
| Risk Perception Score for adults | 35.5 ± 22.9 | |
| Risk Perception Score for adults > median (36.0%) | 119, 30.6% | |
| Risk Perception Score for elderly | 56.1 ± 23.9 | |
| Risk Perception Score for elderly > median (60.0%) | 187, 48.1% | |
| Favorable/Highly favorable to RSV vaccination when made available | 366, 94.1% | |
| Attitude towards mAb (favorable/highly favorable) | 291, 74.8% | |
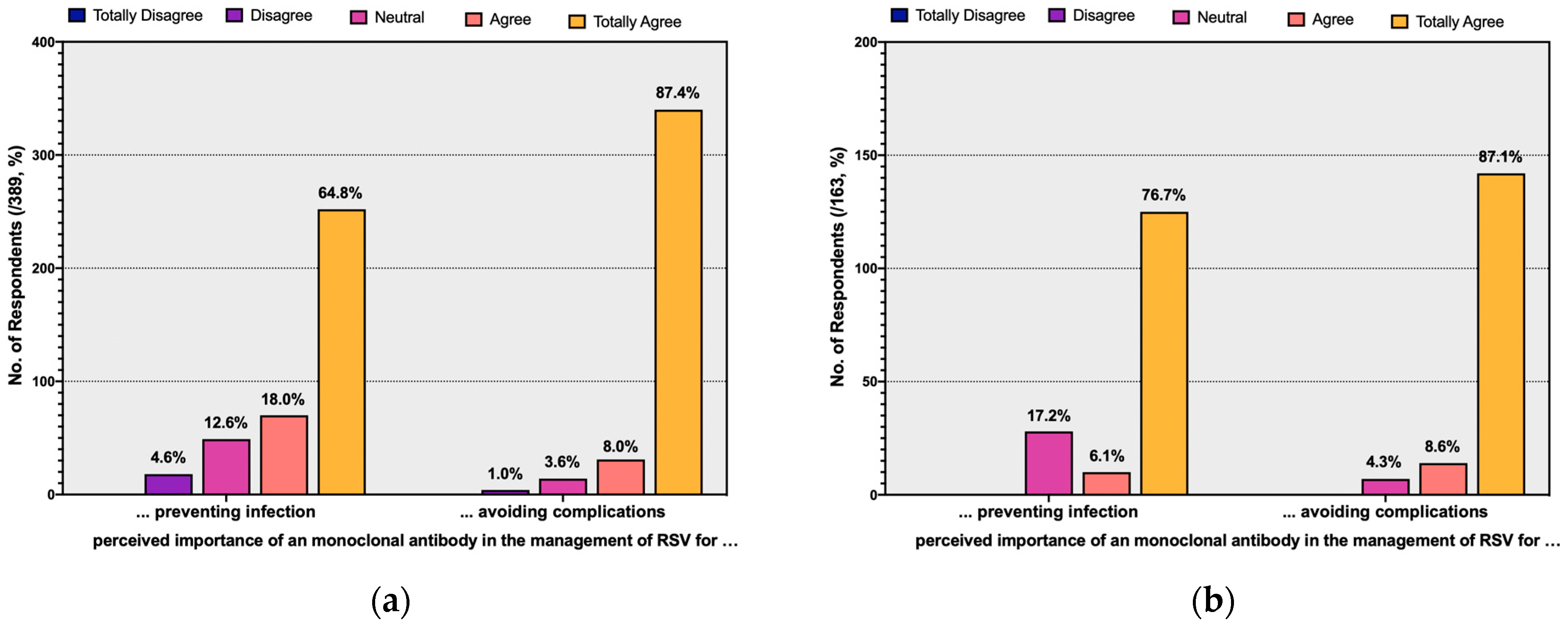
| Acknowledging as significant/very significant aspects for mAb … | ||
| … avoiding natural infection | 322, 82.8% | |
| … avoiding complications (i.e., LRTI) | 371, 95.4% |
| Variable | GKS | RPS Infants | RPS Adults | RPS Elderly |
|---|---|---|---|---|
| GKS | - | 0.021 (p = 0.676) | 0.155 (p = 0.002) | −0.099 (p = 0.052) |
| RPS infants | 0.021 (p = 0.676) | - | 0.050 (p = 0.329) | 0.174 (p = 0.001) |
| RPS adults | 0.155 (p = 0.002) | 0.050 (p = 0.329) | - | 0.446 (p < 0.001) |
| RPS elderly | −0.099 (p = 0.052) | 0.174 (p = 0.001) | 0.446 (p < 0.001) | - |
| Variable | Previously Managed RSV | p Value | |
|---|---|---|---|
| Ever (No./163, %) | Never (No./226, %) | ||
| Age ≥ 40 years | 68, 41.7% | 102, 45.1% | 0.503 |
| Seniority ≥ 10 years | 85, 52.1% | 161, 71.2% | <0.001 |
| Working in Hospital Settings | 79, 48.5% | 27, 11.9% | <0.001 |
| Male Gender | 64, 39.3% | 77, 34.1% | 0.293 |
| Region of residence | <0.001 | ||
| North-Western Italy | 22, 13.5% | 47, 20.8% | |
| North-Eastern Italy | 56, 34.4% | 71, 31.4% | |
| Central Italy | 49, 30.1% | 73, 32.3% | |
| Southern Italy | 7, 4.3% | 28, 12.4% | |
| Major Islands | 29, 17.8% | 7, 3.1% | |
| Higher Knowledge Status | 81, 49.7% | 129, 57.1% | 0.149 |
| Higher Risk Perception | |||
| Infants | 54, 33.1% | 88, 38.9% | 0.240 |
| Adults | 39, 23.9% | 80, 35.4% | 0.015 |
| Elderly | 78, 47.9% | 109, 48.2% | 0.941 |
| Favorable attitude towards mAb | 123, 75.5% | 168, 74.3% | 0.801 |
| Variable | Previous Use of mAb | p Value | |
|---|---|---|---|
| Ever (No./56, %) | Never (No./107, %) | ||
| Age ≥ 40 years | 17, 30.4% | 51, 47.7% | 0.033 |
| Seniority ≥ 10 years | 24, 42.9% | 61, 57.0% | 0.086 |
| Working in Hospital Settings | 36, 64.3% | 43, 40.2% | 0.003 |
| Male Gender | 16, 28.6% | 77, 34.1% | 0.043 |
| Region of residence | 0.001 | ||
| North-Western Italy | 8, 14.3% | 14, 13.1% | |
| North-Eastern Italy | 13, 23.2% | 43, 40.2% | |
| Central Italy | 12, 21.4% | 37, 34.6% | |
| Southern Italy | 4, 7.1% | 3, 2.8% | |
| Major Islands | 19, 33.9% | 10, 9.3% | |
| Higher Knowledge Status | 41, 73.2% | 41, 38.3% | <0.001 |
| Higher Risk Perception | |||
| Infants | 25, 44.6% | 29, 27.1% | 0.024 |
| Adults | 13, 23.2% | 26, 24.3% | 0.877 |
| Elderly | 22, 39.3% | 56, 52.3% | 0.113 |
| Attitude towards use of mAb | 43, 76.8% | 80, 74.8% | 0.776 |
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | |
| Age ≥ 40 years | - | - | 1.138 | 0.209; 6.200 |
| Seniority ≥ 10 years | 1.206 | 0.691; 2.103 | 0.412 | 0.066; 2.554 |
| Working in Hospital Settings | 7.962 | 4.222; 15.012 | 3.917 | 1.233; 12.436 |
| Male Gender | - | - | 0.168 | 0.054; 0.522 |
| Region of residence | ||||
| North-Western Italy | 1.000 | REFERENCE | 1.000 | REFERENCE |
| North-Eastern Italy | 3.314 | 1.583; 6.935 | 0.262 | 0.050; 1.375 |
| Central Italy | 2.644 | 1.258; 5.556 | 0.503 | 0.086; 2.941 |
| Southern Italy | 1.551 | 0.532; 4.526 | 1.099 | 0.111; 10.845 |
| Major Islands | 14.373 | 4.861; 42.498 | 11.283 | 1.732; 73.487 |
| Higher Knowledge Status | - | - | 33.933 | 7.756; 148.457 |
| Higher Risk Perception | ||||
| Children | - | - | 7.295 | 1.977; 26.924 |
| Adults | 0.632 | 0.364; 1.096 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Corrado, S.; Cerviere, M.P.; Ranzieri, S.; Marchesi, F. Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians. Pediatr. Rep. 2023, 15, 154-174. https://doi.org/10.3390/pediatric15010013
Riccò M, Corrado S, Cerviere MP, Ranzieri S, Marchesi F. Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians. Pediatric Reports. 2023; 15(1):154-174. https://doi.org/10.3390/pediatric15010013
Chicago/Turabian StyleRiccò, Matteo, Silvia Corrado, Milena Pia Cerviere, Silvia Ranzieri, and Federico Marchesi. 2023. "Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians" Pediatric Reports 15, no. 1: 154-174. https://doi.org/10.3390/pediatric15010013
APA StyleRiccò, M., Corrado, S., Cerviere, M. P., Ranzieri, S., & Marchesi, F. (2023). Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians. Pediatric Reports, 15(1), 154-174. https://doi.org/10.3390/pediatric15010013








