IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis
Abstract
:1. Background
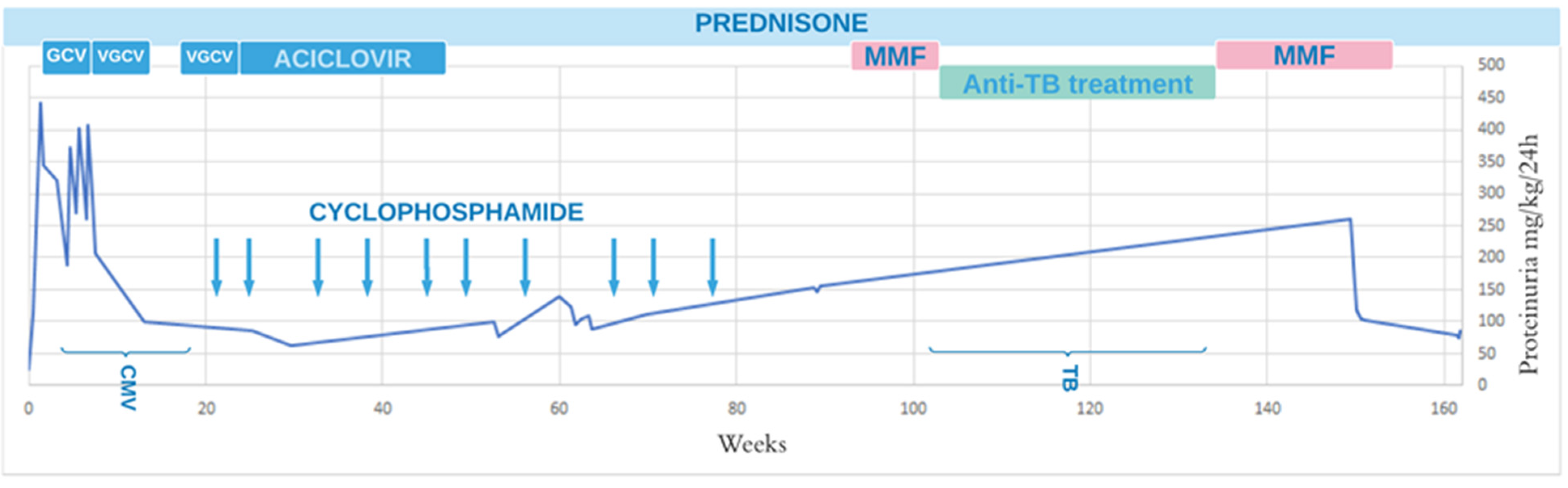
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Das, L.; Hoh, S.F.; Gao, X.; Book, Y.X.; Arkachaisri, T. Urinalysis monitoring in children with Henoch-Schönlein purpura: Is it time to revise? Int. J. Rheum. Dis. 2019, 22, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Huang, W.Y.; Hao, S.; Niu, X.L.; Wang, P.; Wu, Y.; Zhu, G.H. A single-center analysis of Henoch-Schonlein purpura nephritis with nephrotic proteinuria in children. Pediatr. Rheumatol. Online J. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oni, L.; Sampath, S. Childhood IgA Vasculitis (Henoch Schonlein Purpura)-Advances and Knowledge Gaps. Front Pediatr. 2019, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Ono, A.; Ohara, S.; Suzuki, Y.; Suyama, K.; Suzuki, J.; Hosoya, M. Henoch-Schönlein purpura nephritis in childhood: Pathogenesis, prognostic factors and treatment. Fukushima J. Med. Sci. 2013, 59, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, H.; Takahashi, S.; Kawakubo, Y.; Kinukawa, N.; Funaki, S.; Harada, K. Adolescent with Henoch-Schönlein purpura glomerulonephritis and intracranial hemorrhage possibly secondary to the reactivation of latent CMV. Pediatr. Int. 2008, 50, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.-Z.; Wang, R.-C.; Yang, J.; Hao, H.-L.; Xue, L.-Y. Pulmonary tuberculosis presenting as henoch–schönlein purpura. Medicine 2020, 99, e22583. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Komeda, Y.; Watanabe, T.; Kudo, M. Purpura-free small intestinal IgA vasculitis complicated by cytomegalovirus reactivation. BMJ Case Rep. 2020, 13, e235042. [Google Scholar] [CrossRef] [PubMed]
- Heineke, M.H.; Ballering, A.V.; Jamin, A.; Ben Mkaddem, S.; Monteiro, R.C.; Van Egmond, M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun. Rev. 2017, 16, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Narchi, H. Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: A systematic review. Arch. Dis. Child. 2005, 90, 916–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, S.M.; Joosse, P.; Unlü, C.; Steller, E.P. Henoch-Schönlein disease localized in the appendix. Indian J. Pediatr. 2013, 80, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Al Hamdani, S.F.; Salman, A.H. A Rare Complication of Henoch-Schönlein Purpura: Acute Appendicitis Treated Conservatively—A Case Report and Literature Review. Case Rep. Acute Med. 2020, 3, 17–24. [Google Scholar] [CrossRef]
- Migliori, G.B.; Sotgiu, G.; Rosales-Klintz, S.; Centis, R.; D’Ambrosio, L.; Abubakar, I.; Bothamley, G.; Caminero, J.A.; Cirillo, D.M.; Dara, M.; et al. ERS/ECDC Statement: European Union standards for tuberculosis care, 2017 update. Eur. Respir. J. 2018, 51, 1702678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tao, Y. Tuberculosis-associated IgA nephropathy. J. Int. Med. Res. 2018, 46, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Asberg, A.; Rollag, H.; Hartmann, A. Valganciclovir for the prevention and treatment of CMV in solid organ transplant recipients. Expert Opin. Pharmacother. 2010, 11, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Patient’s Results | Normal Values |
|---|---|---|
| WBC (thou/µL) | 10.3 | 4.0–10.0 |
| Hb (g/dL) | 10.4 | 14.0–18.0 |
| PLT (thou/µL) | 325 | 140–400 |
| CRP (mg/dL) | 1.3 | 0.0–1.0 |
| albumin (g/dL) | 2.4 | 3.7–5.6 |
| total protein (g/dL) | 4.5 | 6.0–8.0 |
| creatinine (mg/dL) | 0.8 | 0.2–0.7 |
| urea (mg/dL) | 31 | 17.1–45.0 |
| Na (mmol/L) | 137 | 132–145 |
| K (mmol/L) | 4.3 | 3.5–5.1 |
| Ca (mEq/L) | 4 | 4.58–5.32 |
| AspAT (U/L) | 24 | 15–40 |
| ALT (U/L) | 23 | 10–45 |
| cholesterol (mg/dL) | 118 | 106–224 |
| triglycerides (mg/dL) | 128 | 34–165 |
| APTT (s) | 38.42 | 28–40 |
| INR | 1.64 | 0.9–1.25 |
| fibrinogen (g/L) | 2.12 | 2.0–4.0 |
| D-dimers | 12,428.77 | 170–550 |
| Urinalysis: | - | |
| protein (mg/dL) | 120 | - |
| RBC (HPF) | 15–18 | - |
| leukocytes (HPF) | 0 | - |
| 24-h urine collection/protein (mg/kg/day) | 24 (1.2 g/day) | - |
| IgA (mg/dL) | ||
| IgG (mg/dL) | 268.7 | 85–194 |
| IgM (mg/dL) | 869 | 706–1440 |
| C3 (mg/dL) | 60 | 44–113.1 |
| C4 (mg/dL) | 95 | 88–165 |
| ASO | 20.6 | 14–44 |
| ANA | 291 | <200 |
| ANCA | absent | - |
| absent | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizerska-Wasiak, M.; Winiarska, M.; Nogal, K.; Cichoń-Kawa, K.; Pańczyk-Tomaszewska, M.; Małdyk, J. IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis. Pediatr. Rep. 2021, 13, 416-420. https://doi.org/10.3390/pediatric13030048
Mizerska-Wasiak M, Winiarska M, Nogal K, Cichoń-Kawa K, Pańczyk-Tomaszewska M, Małdyk J. IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis. Pediatric Reports. 2021; 13(3):416-420. https://doi.org/10.3390/pediatric13030048
Chicago/Turabian StyleMizerska-Wasiak, Małgorzata, Maria Winiarska, Karolina Nogal, Karolina Cichoń-Kawa, Małgorzata Pańczyk-Tomaszewska, and Jadwiga Małdyk. 2021. "IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis" Pediatric Reports 13, no. 3: 416-420. https://doi.org/10.3390/pediatric13030048
APA StyleMizerska-Wasiak, M., Winiarska, M., Nogal, K., Cichoń-Kawa, K., Pańczyk-Tomaszewska, M., & Małdyk, J. (2021). IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis. Pediatric Reports, 13(3), 416-420. https://doi.org/10.3390/pediatric13030048





