Innate Immunity in Children and the Role of ACE2 Expression in SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Innate Immunity to SARS-CoV-2 in Children
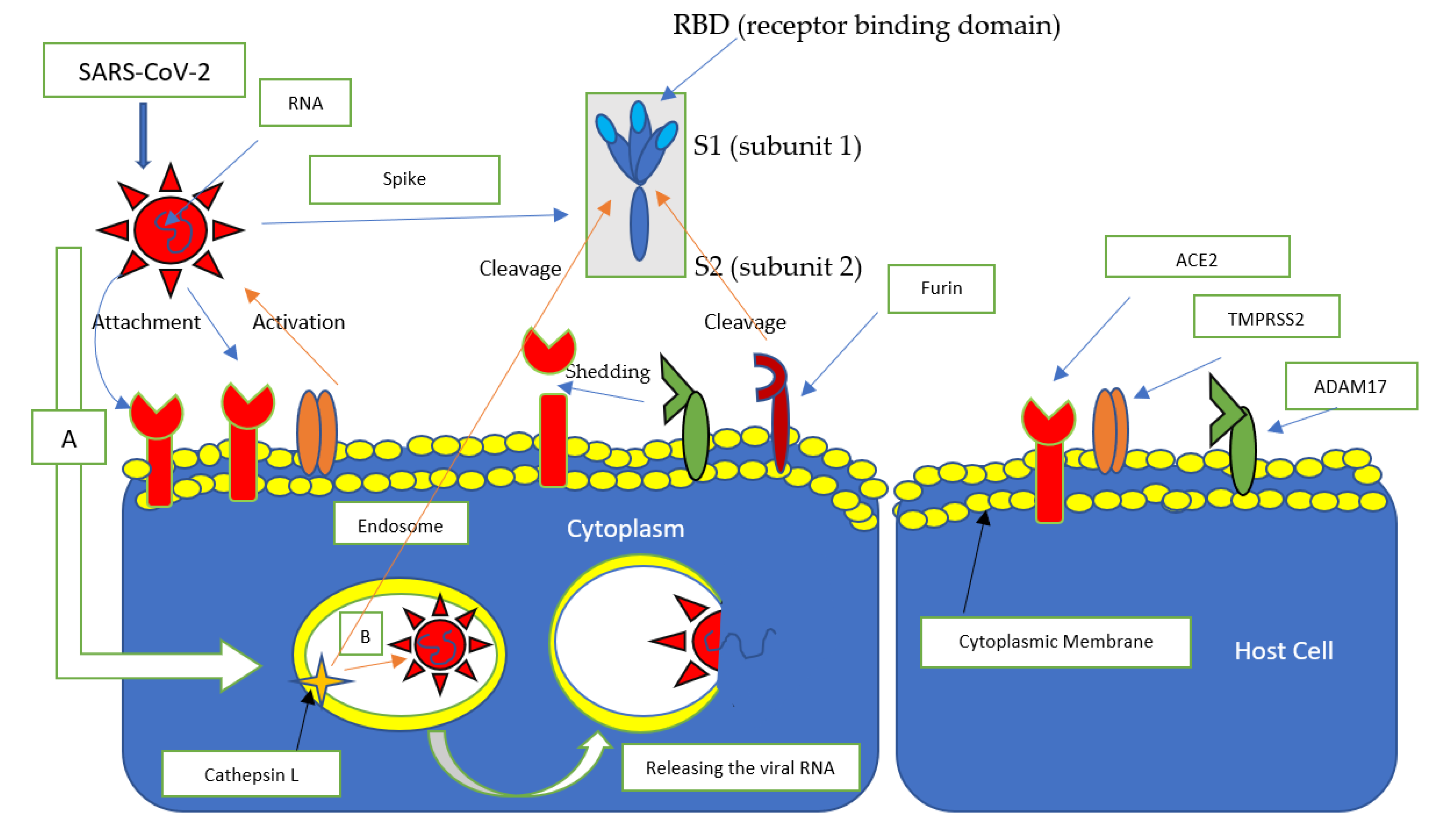
4.2. Role of the Differential Expression of ACE2 in Children in the Etiopathogenesis of COVID-19
4.3. The Role of Infections and Past Infections in the Etiopathogenesis of COVID-19 in Children
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmes, K.V. SARS coronavirus: A new challenge for prevention and therapy. J. Clin. Investig. 2003, 111, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Noor, R. A comparative review of pathogenesis and host innate immunity evasion strategies among the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). Arch. Microbiol. 2021. [Google Scholar] [CrossRef]
- Nassar, M.S.; Bakhrebah, M.A.; Meo, S.A.; Alsuabeyl, M.S.; Zaher, W.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: Epidemiology, pathogenesis and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4956–4961. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef] [Green Version]
- Printz, C. Poor COVID-19 outcomes and deaths linked to advanced age and pre-existing conditions. Cancer 2021, 127, 497. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, Z.; Yang, H.; Zhang, Y.; Xu, F.; Wang, Y. Metabolic Healthy Obesity, Vitamin D Status, and Risk of COVID-19. Aging Dis. 2021, 12, 61–71. [Google Scholar] [CrossRef]
- Bellini, B.; Cresci, B.; Cosentino, C.; Profili, F.; Bartolacci, S.; Scoccimarro, D.; Voller, F.; Balzi, D.; Francesconi, P.; Mannucci, E. Obesity as a risk factor for hospitalization in COronaVirus Disease-19 (COVID-19) patients: Analysis of the Tuscany regional database. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 769–773. [Google Scholar] [CrossRef]
- Tadic, M.; Saeed, S.; Grassi, G.; Taddei, S.; Mancia, G.; Cuspidi, C. Hypertension and COVID-19: Ongoing Controversies. Front. Cardiovasc. Med. 2021, 8, 639222. [Google Scholar] [CrossRef]
- Home, P. Letter to the Editor in Response to article: Clinical considerations for patients with diabetes in times of COVID-19 epidemic (Gupta et al.). Diabetes Metab. Syndr. 2020, 14, 417. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Letter to the editor in response to article: Clinical considerations for patients with diabetes in times of COVID-19 epidemic (Gupta et al.). Diabetes Metab. Syndr. 2020, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; do Vale Moreira, N.C. Letter to the editor in response to article: Clinical considerations for patients with diabetes in times of COVID-19 epidemic (Gupta et al.). Diabetes Metab. Syndr. 2020, 14, 363. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, A.; Singh, A.K.; Misra, A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab. Syndr. 2020, 14, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Udwadia, Z.F.; Koul, P.A.; Dhooria, S. The impact of COVID-19 on patients with preexisting interstitial lung disease: High mortality in these high-risk patients. Lung India 2021, 38, S1–S3. [Google Scholar] [CrossRef]
- Peng, L.; Liang, F.; Xia, Y. Risk of COVID-19 in Patients With Cancer. JAMA Oncol. 2020, 6, 1469–1470. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.L.K.; Yu, J.; Xie, C. Risk of COVID-19 in Patients With Cancer-Reply. JAMA Oncol. 2020, 6, 1472–1473. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef]
- Deng, S.Q.; Peng, H.J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Smits, S.L.; de Lang, A.; van den Brand, J.M.; Leijten, L.M.; van Ijcken, W.F.; Eijkemans, M.J.; van Amerongen, G.; Kuiken, T.; Andeweg, A.C.; Osterhaus, A.D.; et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010, 6, e1000756. [Google Scholar] [CrossRef] [Green Version]
- Taffarel, P.; Jorro Baron, F.; Rodriguez, A.P.; Widmer, J.; Meregallia, C. Multisystem inflammatory syndrome in children related to COVID-19: An update regarding the presentation of two critically ill patients. Arch. Argent. Pediatr. 2021, 119, e26–e35. [Google Scholar] [CrossRef]
- Mehra, B.; Aggarwal, V.; Kumar, P.; Kundal, M.; Gupta, D.; Kumar, A.; Dugaya, S.K. COVID-19-associated Severe Multisystem Inflammatory Syndrome in Children with Encephalopathy and Neuropathy in an Adolescent Girl with the Successful Outcome: An Unusual Presentation. Indian J. Crit. Care Med. 2020, 24, 1276–1278. [Google Scholar] [CrossRef]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Liang, L.; Chen, C.; Nie, S. A child confirmed COVID-19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1565–1566. [Google Scholar] [CrossRef]
- Felsenstein, S.; Hedrich, C.M. SARS-CoV-2 infections in children and young people. Clin. Immunol. 2020, 220, 108588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Huang, L.; Zhang, C.; Luo, R.; Zeng, L.; Liang, H.; Li, Q.; Lu, X.; Wang, X.; et al. Distinct disease severity between children and older adults with COVID-19: Impacts of ACE2 expression, distribution, and lung progenitor cells. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Yonker, L.M.; Neilan, A.M.; Bartsch, Y.; Patel, A.B.; Regan, J.; Arya, P.; Gootkind, E.; Park, G.; Hardcastle, M.; St John, A.; et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J. Pediatr. 2020, 227, 45–52.e45. [Google Scholar] [CrossRef]
- Koch, C.M.; Prigge, A.D.; Anekalla, K.R.; Shukla, A.; Do-Umehara, H.C.; Setar, L.; Chavez, J.; Abdala-Valencia, H.; Politanska, Y.; Markov, N.S.; et al. Immune response to SARS-CoV-2 in the nasal mucosa in children and adults. medRxiv 2021. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Scagnolari, C.; Bitossi, C.; Viscido, A.; Frasca, F.; Oliveto, G.; Scordio, M.; Petrarca, L.; Mancino, E.; Nenna, R.; Riva, E.; et al. ACE2 expression is related to the interferon response in airway epithelial cells but is that functional for SARS-CoV-2 entry? Cytokine 2021, 140, 155430. [Google Scholar] [CrossRef]
- Sajuthi, S.P.; DeFord, P.; Li, Y.; Jackson, N.D.; Montgomery, M.T.; Everman, J.L.; Rios, C.L.; Pruesse, E.; Nolin, J.D.; Plender, E.G.; et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020, 11, 5139. [Google Scholar] [CrossRef]
- Somekh, I.; Yakub Hanna, H.; Heller, E.; Bibi, H.; Somekh, E. Age-Dependent Sensory Impairment in COVID-19 Infection and its Correlation with ACE2 Expression. Pediatr. Infect. Dis. J. 2020, 39, e270–e272. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Swärd, P.; Edsfeldt, A.; Reepalu, A.; Jehpsson, L.; Rosengren, B.E.; Karlsson, M.K. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit. Care 2020, 24, 221. [Google Scholar] [CrossRef]
- Taglauer, E.; Benarroch, Y.; Rop, K.; Barnett, E.; Sabharwal, V.; Yarrington, C.; Wachman, E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta 2020, 100, 69–74. [Google Scholar] [CrossRef]
- Vuille-Dit-Bille, R.N.; Liechty, K.W.; Verrey, F.; Guglielmetti, L.C. SARS-CoV-2 receptor ACE2 gene expression in small intestine correlates with age. Amino Acids 2020, 52, 1063–1065. [Google Scholar] [CrossRef]
- Galván-Peña, S.; Leon, J.; Chowdhary, K.; Michelson, D.A.; Vijaykumar, B.; Yang, L.; Magnuson, A.; Manickas-Hill, Z.; Piechocka-Trocha, A.; Worrall, D.P.; et al. Profound Treg perturbations correlate with COVID-19 severity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ortiz Bezara, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B., Jr.; Meyerholz, D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. bioRxiv 2020, 60, 102976. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Alabed, M.; Temsah, M.H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Schweitzer, K.S.; Crue, T.; Nall, J.M.; Foster, D.; Sajuthi, S.; Correll, K.A.; Nakamura, M.; Everman, J.; Downey, G.P.; Seibold, M.A.; et al. Influenza virus infection increases ACE2 expression and shedding in human small airway epithelial cells. Eur. Respir. J. 2021, 2003988. [Google Scholar] [CrossRef]
- Pavel, A.B.; Wu, J.; Renert-Yuval, Y.; Del Duca, E.; Glickman, J.W.; Miller, R.L.; Paller, A.S.; Krueger, J.G.; Guttman-Yassky, E. SARS-CoV-2 receptor ACE2 protein expression in serum is significantly associated with age. Allergy 2021, 76, 875–878. [Google Scholar] [CrossRef]
- Inde, Z.; Yapp, C.; Joshi, G.N.; Spetz, J.; Fraser, C.; Deskin, B.; Ghelfi, E.; Sodhi, C.; Hackam, D.; Kobzik, L.; et al. Age-dependent regulation of SARS-CoV-2 cell entry genes and cell death programs correlates with COVID-19 disease severity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Lu, X.; Zhang, C.; Huang, L.; Wang, X.; Duan, F.; Liang, H.; Chen, P.; Zeng, L.; et al. Clinical analysis and pluripotent stem cells-based model reveal possible impacts of ACE2 and lung progenitor cells on infants vulnerable to COVID-19. Theranostics 2021, 11, 2170–2181. [Google Scholar] [CrossRef]
- Heinonen, S.; Helve, O.; Andersson, S.; Janér, C.; Süvari, L.; Kaskinen, A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch. Dis. Child. Fetal Neonatal Ed. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Alvez, F. SARS-CoV2 coronavirus: So far polite with children. Debatable immunological and non-immunological evidence. Allergol. Immunopathol. 2020, 48, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Ovali, F. Coronavirus-2019 Disease (COVID-19) in Children. Medeni. Med. J. 2020, 35, 242–252. [Google Scholar] [CrossRef]
- Kikkert, M. Innate Immune Evasion by Human Respiratory RNA Viruses. J. Innate Immun. 2020, 12, 4–20. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Alexandris, N.; Konstantinou, E.; Mesiakaris, K.; Zanidis, C.; Farsalinos, K.; Poulas, K. Imiquimod—A toll like receptor 7 agonist—Is an ideal option for management of COVID 19. Environ. Res. 2020, 188, 109858. [Google Scholar] [CrossRef]
- Yin, X.; Riva, L.; Pu, Y.; Martin-Sancho, L.; Kanamune, J.; Yamamoto, Y.; Sakai, K.; Gotoh, S.; Miorin, L.; De Jesus, P.D.; et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021, 34, 108628. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Costagliola, G.; Spada, E.; Consolini, R. Age-related differences in the immune response could contribute to determine the spectrum of severity of COVID-19. Immun. Inflamm. Dis. 2021. [Google Scholar] [CrossRef]
- Girija, A.S.S. Fox3(+) CD25(+) CD4(+) T regulatory cells (Tregs) may transform the n-CoV’s final destiny to CNS! J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- South, A.M.; Brady, T.M.; Flynn, J.T. ACE2 (Angiotensin-Converting Enzyme 2), COVID-19, and ACE Inhibitor and Ang II (Angiotensin II) Receptor Blocker Use During the Pandemic: The Pediatric Perspective. Hypertension 2020, 76, 16–22. [Google Scholar] [CrossRef]
- Deng, Q.; Rasool, R.U.; Russell, R.M.; Natesan, R.; Asangani, I.A. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. iScience 2021, 24, 102254. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Yeh, I.J.; Phan, N.N.; Yen, M.C.; Liu, H.L.; Wang, C.Y.; Hsu, H.P. Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 infection induces dysregulation of immunity: In silico gene expression analysis. Int. J. Med. Sci. 2021, 18, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Parra-Ortega, I.; Rodriguez-Ortega, D. SARS-CoV-2 impact on oral health: A general view. Bol. Med. Hosp. Infant. Mex. 2021, 78. [Google Scholar] [CrossRef]
- Guo, S.; Yang, J.; Lei, Y.; Liu, B.; Zhang, W.; Zhang, L.; Zuo, Z. Which species does the virus like most: Binding modes study between SARS-CoV-2 S protein and ACE2 receptor. J. Mol. Graph. Model. 2021, 105, 107893. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Z.; Rahman, P. Genetic variability of human angiotensin-converting enzyme 2 (hACE2) among various ethnic populations. Mol. Genet. Genom. Med. 2020, 8, e1344. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Bungau, S.; Kumar, A.; Uddin, M.S.; Kumar, C.; Pal, G.; Sahil; Shrivastava, K.; Zengin, G.; et al. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020, 257, 118075. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Ciaglia, E.; Vecchione, C.; Puca, A.A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020, 8, 206. [Google Scholar] [CrossRef] [Green Version]
- Bénéteau-Burnat, B.; Baudin, B.; Morgant, G.; Baumann, F.C.; Giboudeau, J. Serum angiotensin-converting enzyme in healthy and sarcoidotic children: Comparison with the reference interval for adults. Clin. Chem 1990, 36, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Midulla, F.; Cristiani, L.; Mancino, E. Will children reveal their secret? The coronavirus dilemma. Eur. Respir. J. 2020, 55, 2001617. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.J. Will children reveal their secret? The coronavirus dilemma. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Lee, C.H.; Pinho, M.P.; Buckley, P.R.; Woodhouse, I.B.; Ogg, G.; Simmons, A.; Napolitani, G.; Koohy, H. Potential CD8+ T Cell Cross-Reactivity Against SARS-CoV-2 Conferred by Other Coronavirus Strains. Front. Immunol. 2020, 11, 579480. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, K.; Seto, J.; Matoba, Y.; Aoki, Y.; Tanaka, S.; Ikeda, T.; Matsuzaki, Y.; Itagaki, T.; Mizuta, K. Seasonality of Human Coronavirus OC43, NL63, HKU1, and 229E Infection in Yamagata, Japan, 2010–2019. Jpn. J. Infect. Dis. 2020, 73, 394–397. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Deijs, M.; Milewska, A.; Pyrc, K.; Buelow, E.; van der Bijl, A.; van der Hoek, L. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J. Gen. Virol. 2012, 93, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Steinman, J.B.; Lum, F.M.; Ho, P.P.; Kaminski, N.; Steinman, L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc. Natl. Acad. Sci. USA 2020, 117, 24620–24626. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; Relich, R.F.; Datta, D.; Bond, C.; Goings, M.; Hall, D.; Lei, G.S.; Kedra, J.; John, C.C. Identifying Risk Factors That Distinguish Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection From Common Upper Respiratory Infections in Children. Cureus 2021, 13, e13266. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xing, Y.; Shi, L.; Li, W.; Gao, Y.; Pan, S.; Wang, Y.; Wang, W.; Xing, Q. Coinfection and Other Clinical Characteristics of COVID-19 in Children. Pediatrics 2020, 146. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, F.; Lu, X.; Du, H.; Xu, J.; Han, F.; Zhang, L.; Zhang, M. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected children: A retrospective study. Medicine 2021, 100, e24315. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Deveson, I.W.; Pang, C.N.I.; Yeang, M.; Naing, Z.; Adikari, T.; Hammond, J.M.; Stevanovski, I.; Beukers, A.G.; Verich, A.; et al. Respiratory viral co-infections among SARS-CoV-2 cases confirmed by virome capture sequencing. Sci. Rep. 2021, 11, 3934. [Google Scholar] [CrossRef]
- Peci, A.; Tran, V.; Guthrie, J.L.; Li, Y.; Nelson, P.; Schwartz, K.L.; Eshaghi, A.; Buchan, S.A.; Gubbay, J.B. Prevalence of Co-Infections with Respiratory Viruses in Individuals Investigated for SARS-CoV-2 in Ontario, Canada. Viruses 2021, 13, 130. [Google Scholar] [CrossRef]
- Tang, M.L.; Li, Y.Q.; Chen, X.; Lin, H.; Jiang, Z.C.; Gu, D.L.; Chen, X.; Tang, C.X.; Xie, Z.Q. Co-Infection with Common Respiratory Pathogens and SARS-CoV-2 in Patients with COVID-19 Pneumonia and Laboratory Biochemistry Findings: A Retrospective Cross-Sectional Study of 78 Patients from a Single Center in China. Med. Sci. Monit. 2021, 27, e929783. [Google Scholar] [CrossRef]
- Stowe, J.; Tessier, E.; Zhao, H.; Guy, R.; Muller-Pebody, B.; Zambon, M.; Andrews, N.; Ramsay, M.; Bernal, J.L. Interactions between SARS-CoV-2 and Influenza and the impact of coinfection on disease severity: A test negative design. medRxiv 2020. [Google Scholar] [CrossRef]
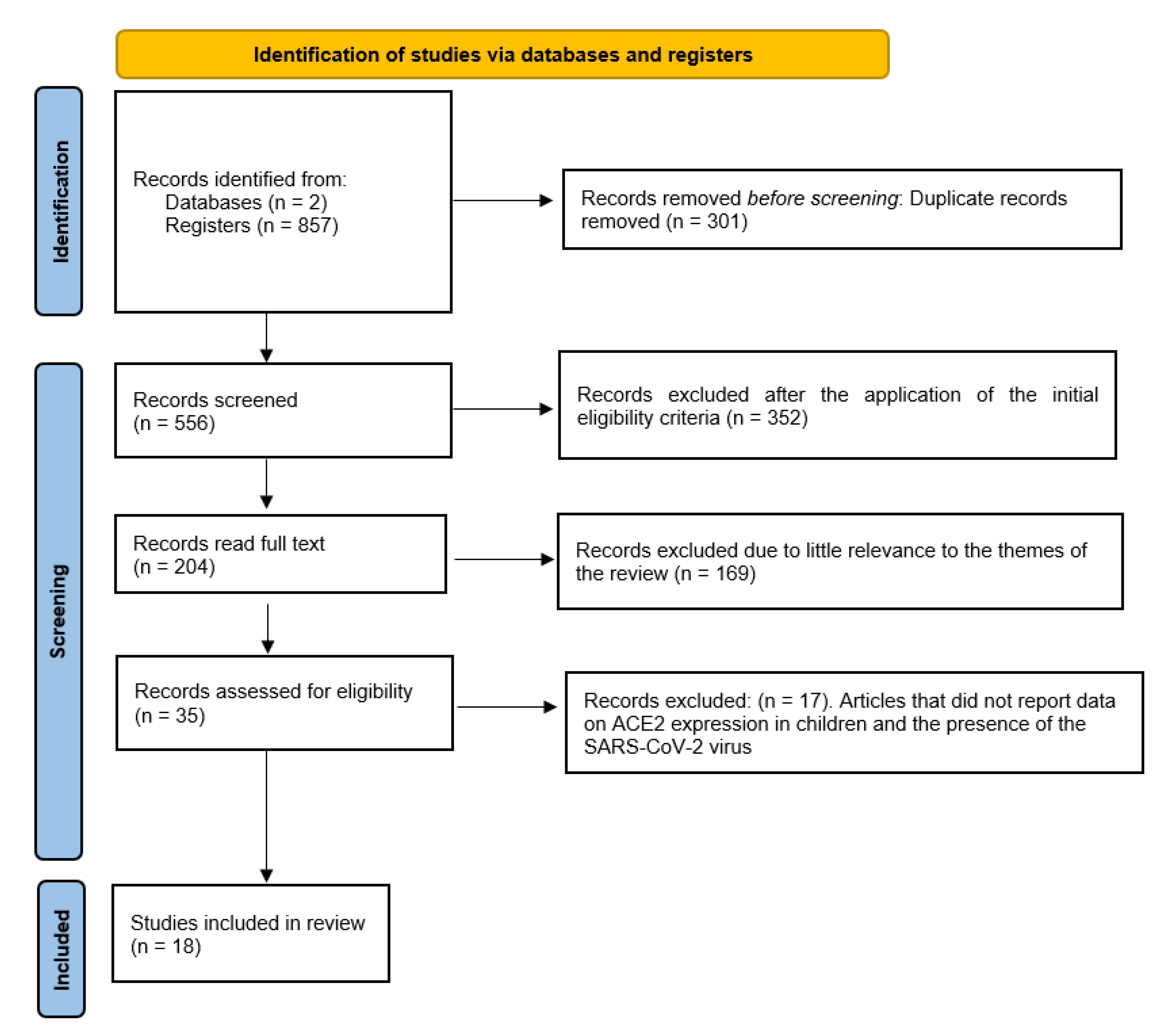
| Category | Exclusion Criteria | Inclusion Criteria |
|---|---|---|
| Publication Language | Not English | English |
| Study type | Reviews, systematic reviews 1, case reports, or case series | Clinical studies, in vitro studies, retrospective studies, prospective studies, cohort studies, clinical trials, and epidemiological studies |
| Data characteristics | Articles that did not report the number of patients/children, did not evaluate ACE2 expression or did not identify SARS-CoV-2 infections | Articles that reported data on the expression of ACE2 in children and the presence of the SARS-CoV-2 virus |
| Year of publication | Published before 2020 | Published in 2020–2021 |
| Database/Provider | Keywords, Search Details | Number of Records | Records after Removal of Overlapping Articles | Records after the Application of the Initial Eligibility Criteria | Articles Deemed Potentially Eligible | Articles Included in the Review that Discussed COVID-19 Issues in Children Regarding ACE2 Receptor Expression |
|---|---|---|---|---|---|---|
| Pubmed | Search: covid 19 AND Immunity innate AND children Sort by: Most Recent (“covid 19”[All Fields] OR “covid 19”[MeSH Terms] OR “covid 19 vaccines”[All Fields] OR “covid 19 vaccines”[MeSH Terms] OR “covid 19 serotherapy”[All Fields] OR “covid 19 serotherapy”[Supplementary Concept] OR “covid 19 nucleic acid testing”[All Fields] OR “covid 19 nucleic acid testing”[MeSH Terms] OR “covid 19 serological testing”[All Fields] OR “covid 19 serological testing”[MeSH Terms] OR “covid 19 testing”[All Fields] OR “covid 19 testing”[MeSH Terms] OR “sars cov 2”[All Fields] OR “sars cov 2”[MeSH Terms] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “ncov”[All Fields] OR “2019 ncov”[All Fields] OR ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields] OR “cov”[All Fields]) AND 2019/11/01:3000/12/31[Date—Publication])) AND (“immunity, innate”[MeSH Terms] OR (“immunity”[All Fields] AND “innate”[All Fields]) OR “innate immunity”[All Fields] OR (“immunity”[All Fields] AND “innate”[All Fields]) OR “immunity innate”[All Fields]) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields] OR “child s”[All Fields] OR “children s”[All Fields] OR “childrens”[All Fields] OR “childs”[All Fields]) Translations covid 19: (“COVID-19” OR “COVID-19”[MeSH Terms] OR “COVID-19 Vaccines” OR “COVID-19 Vaccines”[MeSH Terms] OR “COVID-19 serotherapy” OR “COVID-19 serotherapy”[Supplementary Concept] OR “COVID-19 Nucleic Acid Testing” OR “covid-19 nucleic acid testing”[MeSH Terms] OR “COVID-19 Serological Testing” OR “covid-19 serological testing”[MeSH Terms] OR “COVID-19 Testing” OR “covid-19 testing”[MeSH Terms] OR “SARS-CoV-2” OR “sars-cov-2”[MeSH Terms] OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “NCOV” OR “2019 NCOV” OR ((“coronavirus”[MeSH Terms] OR “coronavirus” OR “COV”) AND 2019/11/01[PDAT]: 3000/12/31[PDAT])) Immunity innate: “immunity, innate”[MeSH Terms] OR (“immunity”[All Fields] AND “innate”[All Fields]) OR “innate immunity”[All Fields] OR (“immunity”[All Fields] AND “innate”[All Fields]) OR “immunity, innate”[All Fields] children: “child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields] OR “child’s”[All Fields] OR “children’s”[All Fields] OR “childrens”[All Fields] OR “childs”[All Fields] | 117 | ||||
| Pubmed | Search: covid 19 AND ace2 AND Children Sort by: Most Recent (“covid 19”[All Fields] OR “covid 19”[MeSH Terms] OR “covid 19 vaccines”[All Fields] OR “covid 19 vaccines”[MeSH Terms] OR “covid 19 serotherapy”[All Fields] OR “covid 19 serotherapy”[Supplementary Concept] OR “covid 19 nucleic acid testing”[All Fields] OR “covid 19 nucleic acid testing”[MeSH Terms] OR “covid 19 serological testing”[All Fields] OR “covid 19 serological testing”[MeSH Terms] OR “covid 19 testing”[All Fields] OR “covid 19 testing”[MeSH Terms] OR “sars cov 2”[All Fields] OR “sars cov 2”[MeSH Terms] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “ncov”[All Fields] OR “2019 ncov”[All Fields] OR ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields] OR “cov”[All Fields]) AND 2019/11/01:3000/12/31[Date—Publication])) AND “ace2”[All Fields] AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields] OR “child s”[All Fields] OR “children s”[All Fields] OR “childrens”[All Fields] OR “childs”[All Fields]) Translations covid 19: (“COVID-19” OR “COVID-19”[MeSH Terms] OR “COVID-19 Vaccines” OR “COVID-19 Vaccines”[MeSH Terms] OR “COVID-19 serotherapy” OR “COVID-19 serotherapy”[Supplementary Concept] OR “COVID-19 Nucleic Acid Testing” OR “covid-19 nucleic acid testing”[MeSH Terms] OR “COVID-19 Serological Testing” OR “covid-19 serological testing”[MeSH Terms] OR “COVID-19 Testing” OR “covid-19 testing”[MeSH Terms] OR “SARS-CoV-2” OR “sars-cov-2”[MeSH Terms] OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “NCOV” OR “2019 NCOV” OR ((“coronavirus”[MeSH Terms] OR “coronavirus” OR “COV”) AND 2019/11/01[PDAT]: 3000/12/31[PDAT])) Children: “child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields] OR “child’s”[All Fields] OR “children’s”[All Fields] OR “childrens”[All Fields] OR “childs”[All Fields] | 273 | ||||
| Pubmed | Search: covid 19 AND ace2 AND Pediatric Sort by: Most Recent (“covid 19”[All Fields] OR “covid 19”[MeSH Terms] OR “covid 19 vaccines”[All Fields] OR “covid 19 vaccines”[MeSH Terms] OR “covid 19 serotherapy”[All Fields] OR “covid 19 serotherapy”[Supplementary Concept] OR “covid 19 nucleic acid testing”[All Fields] OR “covid 19 nucleic acid testing”[MeSH Terms] OR “covid 19 serological testing”[All Fields] OR “covid 19 serological testing”[MeSH Terms] OR “covid 19 testing”[All Fields] OR “covid 19 testing”[MeSH Terms] OR “sars cov 2”[All Fields] OR “sars cov 2”[MeSH Terms] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “ncov”[All Fields] OR “2019 ncov”[All Fields] OR ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields] OR “cov”[All Fields]) AND 2019/11/01:3000/12/31[Date—Publication])) AND “ace2”[All Fields] AND (“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields] OR “paediatric”[All Fields] OR “pediatric”[All Fields]) Translations covid 19: (“COVID-19” OR “COVID-19”[MeSH Terms] OR “COVID-19 Vaccines” OR “COVID-19 Vaccines”[MeSH Terms] OR “COVID-19 serotherapy” OR “COVID-19 serotherapy”[Supplementary Concept] OR “COVID-19 Nucleic Acid Testing” OR “covid-19 nucleic acid testing”[MeSH Terms] OR “COVID-19 Serological Testing” OR “covid-19 serological testing”[MeSH Terms] OR “COVID-19 Testing” OR “covid-19 testing”[MeSH Terms] OR “SARS-CoV-2” OR “sars-cov-2”[MeSH Terms] OR “Severe Acute Respiratory Syndrome Coronavirus 2” OR “NCOV” OR “2019 NCOV” OR ((“coronavirus”[MeSH Terms] OR “coronavirus” OR “COV”) AND 2019/11/01[PDAT]: 3000/12/31[PDAT])) Pediatric: “paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields] OR “paediatric”[All Fields] OR “pediatric”[All Fields] | 249 | ||||
| Scopus | TITLE-ABS-KEY (covid 19 AND immunity AND innate AND children) | 94 | ||||
| Scopus | TITLE-ABS-KEY (covid 19 AND ace2 AND children) | 95 | ||||
| Scopus | TITLE-ABS-KEY (covid 19 AND ace2 AND pediatric) | 29 | ||||
| Total | 857 | 556 | 204 | 35 | 18 |
| First Author and Date | Patients | Number | Age, D.S. | Sample Type | Virus | Expression Receptor SARS-CoV-2 | Main Study Conclusions |
|---|---|---|---|---|---|---|---|
| Scagnolari et al. 2021 [32] | Children | 59 | 1.21 ± 2.45 | Nasopharyngeal washings | 14 respiratory viruses and SARS-CoV-2 | In vivo gene expression: ACE2, furin, GUS (beta-glucuronidase gene), and ISG15 (IFN-Stimulated Genes) | IFN (interferon) only increased the truncated ACE2 isoform; this activation would not increase the risk of SARS-CoV-2 infection in the respiratory tract. |
| Adults | 48 | 61.67 ± 16.91 | Nasopharyngeal swabs | ||||
| Sajuthi et al. 2020 [33] | Children | 695 | – | Nasal airway brushings | CoV species (OC43, JKU1, 229E, and NL63), rhinovirus species C (HRV-C), Influenza A, Influenza B, Orthopneumovirus, and metapneumovirus, Enterovirus, or parainfluenza | ACE2 and TMPRSS2 | The response of interferon to respiratory viruses highly upregulated the expression of ACE2. IL-13-mediated and virus infection effects on ACE2 expression at the protein level in the airway epithelium were also observed. |
| Adult | 1 | – | Nasal airway epithelium | ||||
| Somekh et al. 2020 [34] | Children | 31 | 5–17 | – | SARS COV 2 | ACE2 expression | The correlation between the two sets of values (sensory impairment scores and relative ACE2 expression) was 0.95 (p = 0.05). |
| Adults | 42 | +18 | |||||
| Bunyavanich et al. 2020 [35] | Children | 45 | Aged < 10 years, | Nasal epithelium | – | ACE2 expression | The age-dependent expression of ACE2 in the nasal epithelium. |
| 185 | older children (10–17 years) | ||||||
| Adults | 46 | young adults (18–24 years), | |||||
| 29 | and adults (≥25 years) | ||||||
| Zhang et al. 2021 [45] | Children | 173 | 0–1 years (n = 36), 1–5 years (n = 41) and 5–15 years (n = 96) | Nasopharyngeal swabs or sputum, biopsy samples (9 in each age group) | SARS-CoV-2 | ACE2 | Infants (<1-year-old) with SARS-CoV-2 infection were more vulnerable to lung injury. |
| Yonker et al. 2020 [29] | Children | 192 | 10.2 ± 7.0 | nasopharyngeal and oropharyngeal swabs and blood specimens | SARS-CoV-2 | ACE2 | Initial findings showed that although a low expression of ACE2 in younger children (<10 years of age) likely corresponds to reduced infection rates, children of all ages, once infected, can carry high SARS-CoV-2 viral loads. |
| Pavel et al. 2021 [43] | Children | 19 healthy 29 atopic dermatitis | Healthy infants and toddlers (≤5 years old, mean age: 2.1; 52.6% female) | Serum | – | ACE2 and CTSL1 (Cathepsin L1) | Data showed significantly higher ACE2 protein expression in the serum of adults compared with infants and toddlers and in adult males compared with adult females. These data suggest the potential systemic role of ACE2 protein levels in the differential clinical manifestations among various patient populations. |
| Adults | 17 healthy 55 atopic dermatitis | Healthy adults (age range: 24–55, mean age: 41; 35.3% female) | |||||
| Swärd et al. 2020 [36] | Children | Males and females: 824 | >18 | Serum | – | ACE2 | Subjects with a higher risk of severe COVID-19 had a higher sACE2 (adults > children and men > women). |
| Adults | Males and females: 241 | <18 | |||||
| Taglauer et al. 2020 [37] | Maternal-fetal dyads | 15, COVID-19 positive | Maternal age (years): Mean (SD) 31.8 (5.5), gestational age at birth (weeks): Mean (SD) 38.1 (1.7) | Placental tissue | SARS-CoV2 | ACE2 and TMPRSS2 | CoV2 SP (spike protein) and ACE2 expression were coherently localized mainly within the placental villi of the outer syncytiotrophoblastic layer. |
| 10 contemporary COVID-19 negative controls | Maternal age (years): mean (SD) 30.1 (5.5); Gestational age at birth (weeks): Mean (SD) 39.3 (1.6) | ||||||
| Vuille-Dit-Bille et al. 2020 [38] | Adults | 43 healthy | 60 (49–66) | Duodenal tissue | – | ACE2 | Increased intestinal ACE2 mRNA expression in elderly patients may affect their susceptibility to developing intestinal symptoms. |
| Zhang et al. 2021 [28] | Children | 173 | 0–16 years | Nasopharyngeal swabs | SARS-CoV-2 | ACE2 | Compared to children, ACE2-positive cells generally decreased in the elderly. |
| Adult | 126 | 16–80 | |||||
| Galván-Peña et al. 2020 [39] | Adults | 57 | 20–80 | SARS-CoV-2 | SARS-CoV-2 | Tregs and FOXP3 | Different identification of Treg lymphocytes in COVID-19 patients, which could impact the pathogenicity of COVID-19. |
| Ortiz Bezara et al. 2020[40] | Children Adult | 29 cases | 0.5–71 years | Tissues included nasal biopsies (n = 3), lung donors (n = 29), and autopsy tissues (control tissues such as small intestine and kidney) | – | ACE2 | The ACE2 protein was highest within regions of the sinonasal cavity and pulmonary alveoli. In the lung parenchyma, the ACE2 protein was found on the apical surface of a small subset of alveolar type II cells and colocalized with TMPRSS2, a cofactor for SARS-CoV-2 entry. The ACE2 protein did not increase with pulmonary risk factors for severe COVID-19. Additionally, the ACE2 protein was not reduced in children, a demographic with a lower incidence of severe COVID-19. |
| Sharif-Askari et al. 2020 [41] | Children | 4 datasets for children groups (healthy and asthmatics) | – | Blood, upper and lower respiratory tract tissue, and saliva | – | ACE2 and TMPRSS2 | The difference in COVID-19 severity between children and adults was, in part, attributed to the difference in ACE2 and TMPRSS2 airway tissue expression levels. |
| Adults | 15 datasets for adults with different comorbidities | ||||||
| Schweitzer et al. 2021 [42] | 100 | 4 months to 75 years of age. | Human lung tissue specimens | – | ACE2 and TMPRSS2 | Human small airway epithelial cells from healthy patients were subsequently infected with the influenza A virus, leading to an amplification of ACE2, sheddase ADAM17 (TACE), and TMPRSS2 expression, which are involved in the penetration of SARS-CoV-2 into cells. | |
| Inde et al. 2020 [44] | Children Adults | 9–75 years | Lung tissue specimens (n = 100) | – | ACE2 and TMRPRSS2 | ACE2 expression in distal lung epithelial cells generally increased with advancing age but exhibited extreme intraindividual and interindividual heterogeneity. ACE2 expression was also detected on neonatal airway epithelial cells and within the lung parenchyma. | |
| Koch et al. 2021 [30] | Children | 7 healthy | Curettage of nasal mucosa | SARS-CoV-2, respiratory syncytial virus (RSV), and influenza virus (IV) | ACE2 and TMPRSS2 | No difference in ACE2 or TMPRSS2 expression was observed between children and adults. No increase in ACE2 and TMPRSS2 expression was observed during SARS-CoV-2 or other active viral infections. | |
| 36 SARS-CoV-2 | 1.9 (0.4–15.0) | ||||||
| 24 RSV | 0.33 (0.16–0.44) | ||||||
| 9 IV | 1.7 (1.4–7.0) | ||||||
| Adults | 13 healthy | 37 (31–42) | |||||
| 16 SARS-CoV-2 | 31.5 (24.0–38.5) | ||||||
| Heinonen et al. 2021 [46] | Children (newborns) | 17 term | Gestational age: 40 + 0 ± 0.9 weeks | Nasal epithelium | – | ACE2, (TMPRSS2), neuropilin 1 (NRP1), neuropilin 2, (NRP2), and insulin-like growth factor 1 receptor (IGF1R) | Both term and preterm newborns, compared with adults, had lower expression of SARSCoV-2 entry receptors in the nasal epithelium. |
| 11 preterm | 30.1 ± 1.8 weeks | ||||||
| Adults | 10 | 30–60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dioguardi, M.; Cazzolla, A.P.; Arena, C.; Sovereto, D.; Caloro, G.A.; Dioguardi, A.; Crincoli, V.; Laino, L.; Troiano, G.; Lo Muzio, L. Innate Immunity in Children and the Role of ACE2 Expression in SARS-CoV-2 Infection. Pediatr. Rep. 2021, 13, 363-382. https://doi.org/10.3390/pediatric13030045
Dioguardi M, Cazzolla AP, Arena C, Sovereto D, Caloro GA, Dioguardi A, Crincoli V, Laino L, Troiano G, Lo Muzio L. Innate Immunity in Children and the Role of ACE2 Expression in SARS-CoV-2 Infection. Pediatric Reports. 2021; 13(3):363-382. https://doi.org/10.3390/pediatric13030045
Chicago/Turabian StyleDioguardi, Mario, Angela Pia Cazzolla, Claudia Arena, Diego Sovereto, Giorgia Apollonia Caloro, Antonio Dioguardi, Vito Crincoli, Luigi Laino, Giuseppe Troiano, and Lorenzo Lo Muzio. 2021. "Innate Immunity in Children and the Role of ACE2 Expression in SARS-CoV-2 Infection" Pediatric Reports 13, no. 3: 363-382. https://doi.org/10.3390/pediatric13030045
APA StyleDioguardi, M., Cazzolla, A. P., Arena, C., Sovereto, D., Caloro, G. A., Dioguardi, A., Crincoli, V., Laino, L., Troiano, G., & Lo Muzio, L. (2021). Innate Immunity in Children and the Role of ACE2 Expression in SARS-CoV-2 Infection. Pediatric Reports, 13(3), 363-382. https://doi.org/10.3390/pediatric13030045










