Genetic Interactions with Intrauterine Diabetes Exposure in Relation to Obesity: The EPOCH and Project Viva Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study-Specific Information
2.1.1. EPOCH
Exposure and Control Variables
Outcomes
Genetic Data
2.1.2. Project Viva
Exposure and Control Variables
Outcomes
Genetic Data
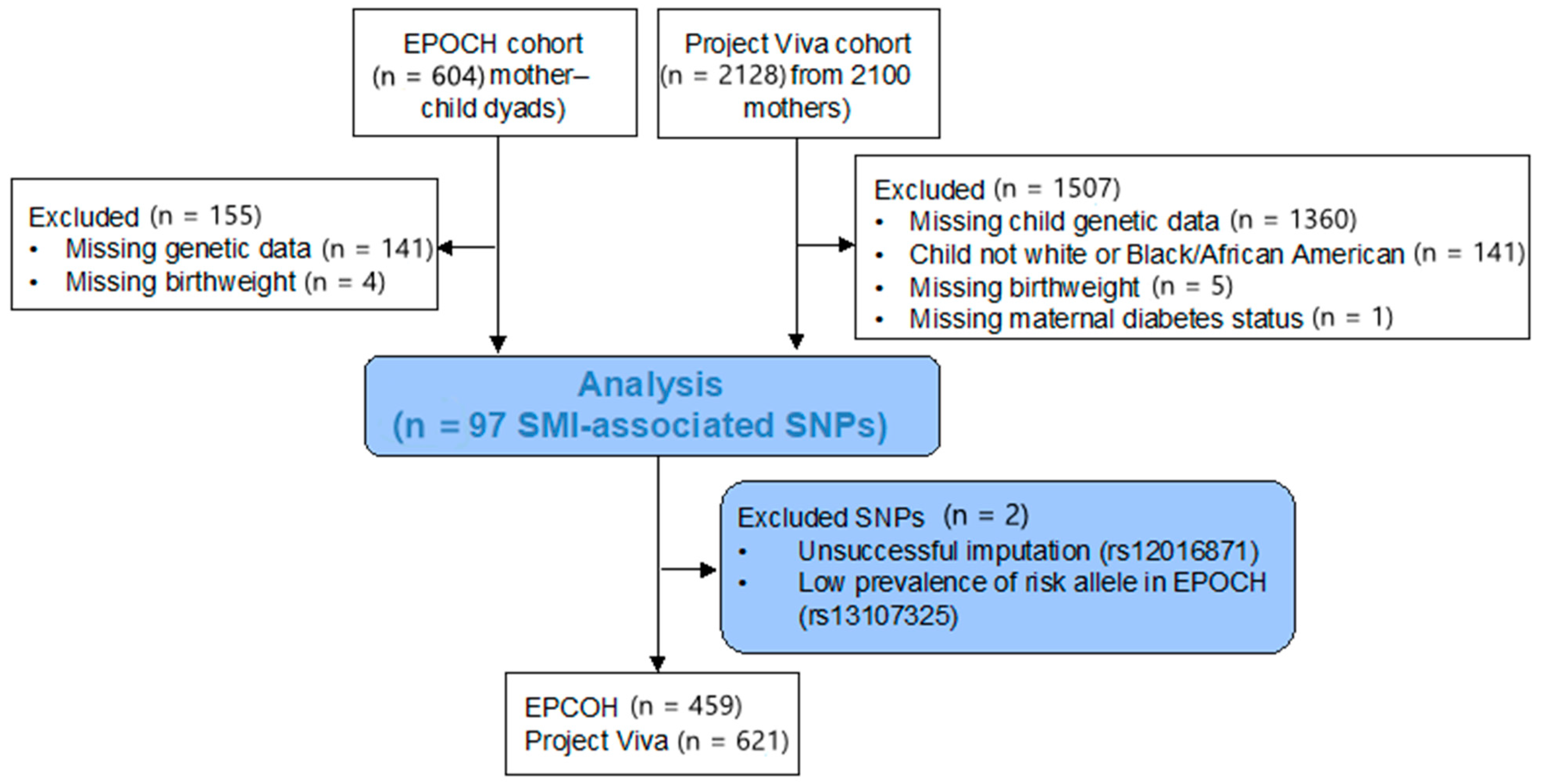
2.2. Variant Selection
2.3. Statistical Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkins, S.S.; Oken, E.; Gillman, M.W. Early in the Life Course: Time for Obesity Prevention. In Handbook of Life Course Health Development; Halfon, N., Forrest, C.B., Lerner, R.M., Faustman, E.M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Sovio, U.; Kaakinen, M.; Tzoulaki, I.; Das, S.; Ruokonen, A.; Pouta, A.; Hartikainen, A.-L.; Molitor, J.; Järvelin, M.-R. How do changes in body mass index in infancy and childhood associate with cardiometabolic profile in adulthood? Findings from the Northern Finland Birth Cohort 1966 Study. Int. J. Obes. 2014, 38, 53–59. [Google Scholar] [CrossRef]
- Aris, I.M.; Rifas-Shiman, S.L.; Li, L.-J.; Kleinman, K.P.; Coull, B.A.; Gold, D.R.; Hivert, M.-F.; Kramer, M.S.; Oken, E. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. Int. J. Epidemiol. 2019, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Hockett, C.W.; Sauder, K.A.; Dabelea, D. Influence of genetic variants associated with body mass index on eating behavior in childhood. Pediatr. Obes. 2020, 15, e12611. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Strauss, R. Risks and consequences of childhood and adolescent obesity. Int. J. Obes. 1999, 23, S2–S11. [Google Scholar] [CrossRef]
- Chesi, A.; Grant, S.F.A. The Genetics of Pediatric Obesity. Trends Endocrinol. Metab. 2015, 26, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Harrod, C.S. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr. Rev. 2013, 71 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Crume, T.L.; Ogden, L.; West, N.A.; Vehik, K.S.; Scherzinger, A.; Daniels, S.; McDuffie, R.; Bischoff, K.; Hamman, R.F.; Norris, J.M.; et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: The Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 2011, 54, 87–92. [Google Scholar] [CrossRef]
- Khoury, M.J.; Wacholder, S. Invited Commentary: From Genome-Wide Association Studies to Gene-Environment-Wide Interaction Studies--Challenges and Opportunities. Am. J. Epidemiol. 2009, 169, 227–230. [Google Scholar] [CrossRef]
- Raghavan, S.; Zhang, W.; Yang, I.V.; Lange, L.A.; Lange, E.M.; Fingerlin, T.E.; Dabelea, D. Association between gestational diabetes mellitus exposure and childhood adiposity is not substantially explained by offspring genetic risk of obesity. Diabet. Med. 2017, 34, 1696–1700. [Google Scholar] [CrossRef]
- Riedel, C.; Von Kries, R.; Fenske, N.; Strauch, K.; Ness, A.R.; Beyerlein, A.; Sobotzki, C. Interactions of genetic and environmental risk factors with respect to body fat mass in children: Results from the ALSPAC study. Obesity 2013, 21, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Data Group Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [CrossRef] [PubMed]
- CDC. SAS Program (Ages 0 to <20 years). In Growth Chart Training. 2016. Available online: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 6 January 2017).
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Wrayner W Strand Home. Available online: https://www.well.ox.ac.uk/~wrayner/strand/index.html (accessed on 16 August 2019).
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Sudmant, P.H.; Rausch, T.; Gardner, E.J.; Handsaker, R.E.; Abyzov, A.; Huddleston, J.; Zhang, Y.; Ye, K.; Jun, G.; Fritz, M.H.-Y.; et al. An integrated map of structural variation in 2504 human genomes. Nature 2015, 526, 75–81. [Google Scholar] [CrossRef]
- Verma, S.S.; Andrade, M.E.; Etromp, G.; Ekuivaniemi, H.; Epugh, E.; Namjou-Khales, B.; Emukherjee, S.; Jarvik, G.P.; Kottyan, L.C.; Eburt, A.; et al. Imputation and quality control steps for combining multiple genome-wide datasets. Front. Genet. 2014, 5, 370. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Baccarelli, A.A.; Gold, D.R.; Kleinman, K.P.; Litonjua, A.A.; De Meo, D.; Rich-Edwards, J.W.; Rifas-Shiman, S.L.; Sagiv, S.; Taveras, E.M.; et al. Cohort Profile: Project Viva. Int. J. Epidemiol. 2015, 44, 37–48. [Google Scholar] [CrossRef]
- Regnault, N.; Gillman, M.W.; Rifas-Shiman, S.L.; Eggleston, E.; Oken, E. Sex-Specific Associations of Gestational Glucose Tolerance With Childhood Body Composition. Diabetes Care 2013, 36, 3045–3053. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- R Core Team. R: The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Willer, C.J.; Li, Y.; Abecasis, G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010, 26, 2190–2191. [Google Scholar] [CrossRef]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A.; et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell 2019, 177, 587–596.e9. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Taveras, E.M.; Gillman, M.W.; Oken, E. Intrauterine Exposure to Gestational Diabetes, Child Adiposity, and Blood Pressure. Am. J. Hypertens. 2009, 22, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef]
- Josey, M.J.; McCullough, L.E.; Hoyo, C.; Williams-DeVane, C. Overall gestational weight gain mediates the relationship between maternal and child obesity. BMC Public Health 2019, 19, 1062. [Google Scholar] [CrossRef]
- Tam, C.H.T.; Ma, R.C.W.; Yuen, L.Y.; Ozaki, R.; Li, A.M.; Hou, Y.; Chan, M.H.M.; Ho, C.S.; Yang, X.; Chan, J.C.N.; et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia 2018, 61, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Byberg, K.K.; Øymar, K.; Eide, G.E.; Forman, M.R.; Júlíusson, P.B. Exposure to preeclampsia in utero affects growth from birth to late childhood dependent on child’s sex and severity of exposure: Follow-up of a nested case-control study. PLoS ONE 2017, 12, e0176627. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gennings, C.; Wright, R.J.; Wilson, A.; Burris, H.H.; Just, A.C.; Braun, J.M.; Svensson, K.; Zhong, J.; Brennan, K.J.; et al. Prenatal Stress, Methylation in Inflammation-Related Genes, and Adiposity Measures in Early Childhood: The Programming Research in Obesity, Growth Environment and Social Stress Cohort Study. Psychosom. Med. 2018, 80, 34–41. [Google Scholar] [CrossRef]
- Aerts, L.; Holemans, K.; Van Assche, F.A. Maternal diabetes during pregnancy: Consequences for the offspring. Diabetes Metab. Rev. 1990, 6, 147–167. [Google Scholar] [CrossRef]
- Crume, T.L.; Ogden, L.; Daniels, S.; Hamman, R.F.; Norris, J.M.; Dabelea, D. The Impact of In Utero Exposure to Diabetes on Childhood Body Mass Index Growth Trajectories: The EPOCH Study. J. Pediatr. 2011, 158, 941–946. [Google Scholar] [CrossRef]
- Hockett, C.W.; Bedrick, E.J.; Zeitler, P.; Crume, T.L.; Daniels, S.; Dabelea, D. Exposure to Diabetes in Utero Is Associated with Earlier Pubertal Timing and Faster Pubertal Growth in the Offspring: The EPOCH Study. J. Pediatr. 2019, 206, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yuen, L.; Saeedi, P.; Riaz, M.; Karuranga, S.; Divakar, H.; Levitt, N.; Yang, X.; Simmons, D. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pr. 2019, 157, 107841. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus: A public health perspective. Diabetes Care 2007, 30 (Suppl. 2), S141–S146. [Google Scholar] [CrossRef] [PubMed]
- Dunston, J.A.; Hamlington, J.D.; Zaveri, J.; Sweeney, E.; Sibbring, J.; Tran, C.; Malbroux, M.; O’Neill, J.P.; Mountford, R.; McIntosh, I. The human LMX1B gene: Transcription unit, promoter, and pathogenic mutations. Genomics 2004, 84, 565–576. [Google Scholar] [CrossRef]
- Zhu, Q.; Xue, K.; Guo, H.W.; Yang, Y.H. LMX1B rs10733682 Polymorphism Interacts with Macronutrients, Dietary Patterns on the Risk of Obesity in Han Chinese Girls. Nutrients 2020, 12, 1227. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Allen, H.L.; Lindgren, C.M.; Luan, J.; Mägi, R.; et al. Association analyses of 249,796 individuals reveal eighteen new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef]
- Krishnan, M.; Thompson, J.M.D.; Mitchell, E.A.; Murphy, R.; McCowan, L.M.E.; Shelling, A.N.; on behalf of the Children of SCOPE Study Group. Analysis of association of gene variants with obesity traits in New Zealand European children at 6 years of age. Mol. BioSyst. 2017, 13, 1524–1533. [Google Scholar] [CrossRef]
- Young, K.L.; Graff, M.; Fernandez-Rhodes, L.; North, K.E. Genetics of Obesity in Diverse Populations. Curr. Diabetes Rep. 2018, 18, 145. [Google Scholar] [CrossRef]
- Martin, A.R.; Gignoux, C.R.; Walters, R.K.; Wojcik, G.L.; Neale, B.M.; Gravel, S.; Daly, M.J.; Bustamante, C.D.; Kenny, E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2017, 100, 635–649. [Google Scholar] [CrossRef]
| EPOCH | Project Viva | |||||||
|---|---|---|---|---|---|---|---|---|
| Intrauterine Diabetes Exposure Status | Intrauterine Diabetes Exposure Status | |||||||
| Overall | No | Yes | p-Value | Overall | No | Yes | p-Value | |
| n | 459 | 373 (81.3) | 86 (18.7) | 621 | 588 (94.7) | 33 (5.3) | ||
| Age, years | 10.3 (1.5) | 10.5 (1.4) | 9.6 (1.7) | <0.001 | 7.9 (0.8) | 7.9 (0.8) | 7.9 (0.8) | 0.81 |
| Sex: Male (%) | 228 (49.7) | 183 (49.1) | 45 (52.3) | 0.67 | 312 (50.2) | 293 (49.8) | 19 (57.6) | 0.49 |
| Race/Ethnicity (%) | 0.15 | 0.76 | ||||||
| Non-Hispanic White | 248 (54.0) | 193 (51.7) | 55 (64.0) | 511 (82.3) | 485 (82.5) | 26 (78.8) | ||
| Black/African American | 30 (6.5) | 26 (7.0) | 4 (4.7) | 110 (17.7) | 103 (17.5) | 7 (21.2) | ||
| Hispanic | 161 (35.1) | 135 (36.2) | 26 (30.2) | |||||
| Other | 20 (4.4) | 19 (5.1) | 1 (1.2) | |||||
| Birthweight (g) | 3223 (561) | 3197 (560) | 3333 (554) | 0.04 | 3548 (528) | 3547 (533) | 3564 (449) | 0.86 |
| BMI z-score * | 0.23 (1.24) | 0.18 (1.21) | 0.43 (1.33) | 0.09 | 0.34 (0.98) | 0.33 (0.97) | 0.45 (1.02) | 0.49 |
| EPOCH | Project Viva | Meta-Analysis | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interaction | Interaction | Interaction | Heterogeneity | ||||||||||||||||
| SNP | Effect Allele | Other Allele | Chr. | Position (bp) | Nearest Gene | Effect Frequency | Beta | SE | p | Effect Frequency | Beta | SE | p | Beta | SE | p | FDR | I² | p |
| rs17203016 | G | A | 2 | 207963763 | CREB1 | 0.19 | −0.57 | 0.26 | 0.028 | 0.19 | 0.50 | 0.38 | 0.19 | −0.23 | 0.21 | 0.274 | 0.27 | 81.4 | 0.02 |
| rs2176040 | A | G | 2 | 226801046 | LOC646736 | 0.36 | −0.52 | 0.22 | 0.018 | 0.34 | 0.30 | 0.26 | 0.25 | −0.19 | 0.17 | 0.272 | 0.27 | 82.7 | 0.02 |
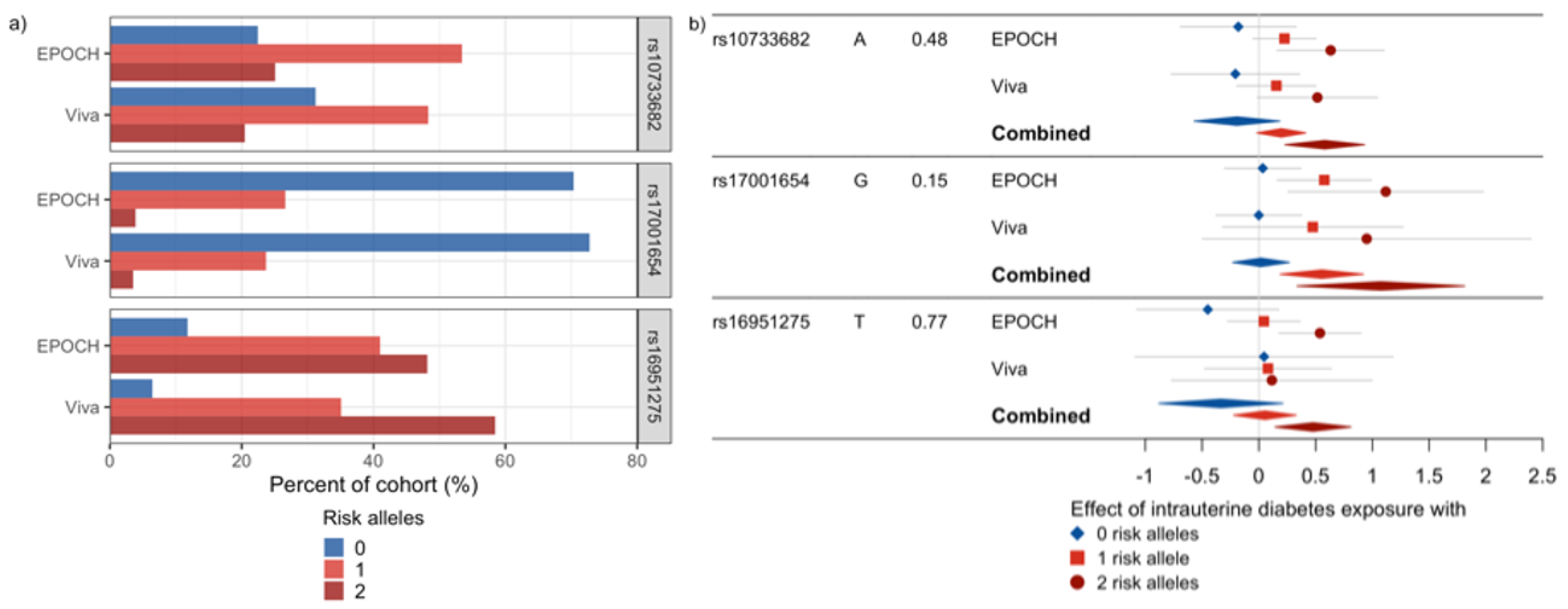
| rs17001654 | G | C | 4 | 77348592 | SCARB2 | 0.15 | 0.54 | 0.26 | 0.035 | 0.15 | 0.48 | 0.45 | 0.29 | 0.53 | 0.22 | 0.019 | 0.065 | 0 | 0.90 |
| rs10733682 | A | G | 9 | 128500735 | LMX1B | 0.48 | 0.41 | 0.21 | 0.050 | 0.45 | 0.36 | 0.28 | 0.19 | 0.39 | 0.17 | 0.018 | 0.065 | 0 | 0.90 |
| rs16951275 | T | C | 15 | 65864222 | MAP2K5 | 0.77 | 0.49 | 0.20 | 0.016 | 0.76 | 0.03 | 0.33 | 0.92 | 0.37 | 0.17 | 0.034 | 0.080 | 29.7 | 0.23 |
| rs1558902 | A | T | 16 | 52361075 | FTO | 0.41 | 0.42 | 0.21 | 0.044 | 0.36 | 0.12 | 0.30 | 0.69 | 0.32 | 0.17 | 0.061 | 0.086 | 0 | 0.40 |
| rs9914578 | G | C | 17 | 1951886 | SMG6 | 0.23 | −0.54 | 0.22 | 0.016 | 0.27 | −0.03 | 0.26 | 0.91 | −0.33 | 0.17 | 0.056 | 0.086 | 54.5 | 0.14 |
| EPOCH | |||||
|---|---|---|---|---|---|
| Mean (SD) | Interaction | ||||
| Component SNPs | GRS | Beta | Standard Error | p | |
| 95 BMI-associated SNPs | Weighted | 2.25 (0.15) | 0.60 | 0.97 | 0.53 |
| Unweighted | 89.66 (5.88) | −0.002 | 0.03 | 0.93 | |
| 8 SNPs showing interactions with intrauterine exposure to diabetes | Weighted | 0.16 (0.07) | 3.43 | 2.06 | 0.10 |
| Unweighted | 5.02 (1.72) | 0.12 | 0.10 | 0.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanislawski, M.A.; Litkowski, E.; Fore, R.; Rifas-Shiman, S.L.; Oken, E.; Hivert, M.-F.; Lange, E.M.; Lange, L.A.; Dabelea, D.; Raghavan, S. Genetic Interactions with Intrauterine Diabetes Exposure in Relation to Obesity: The EPOCH and Project Viva Studies. Pediatr. Rep. 2021, 13, 279-288. https://doi.org/10.3390/pediatric13020036
Stanislawski MA, Litkowski E, Fore R, Rifas-Shiman SL, Oken E, Hivert M-F, Lange EM, Lange LA, Dabelea D, Raghavan S. Genetic Interactions with Intrauterine Diabetes Exposure in Relation to Obesity: The EPOCH and Project Viva Studies. Pediatric Reports. 2021; 13(2):279-288. https://doi.org/10.3390/pediatric13020036
Chicago/Turabian StyleStanislawski, Maggie A., Elizabeth Litkowski, Ruby Fore, Sheryl L. Rifas-Shiman, Emily Oken, Marie-France Hivert, Ethan M. Lange, Leslie A. Lange, Dana Dabelea, and Sridharan Raghavan. 2021. "Genetic Interactions with Intrauterine Diabetes Exposure in Relation to Obesity: The EPOCH and Project Viva Studies" Pediatric Reports 13, no. 2: 279-288. https://doi.org/10.3390/pediatric13020036
APA StyleStanislawski, M. A., Litkowski, E., Fore, R., Rifas-Shiman, S. L., Oken, E., Hivert, M.-F., Lange, E. M., Lange, L. A., Dabelea, D., & Raghavan, S. (2021). Genetic Interactions with Intrauterine Diabetes Exposure in Relation to Obesity: The EPOCH and Project Viva Studies. Pediatric Reports, 13(2), 279-288. https://doi.org/10.3390/pediatric13020036






