14-3-3η Protein as a Potential Biomarker in Juvenile Idiopathic Arthritis
Abstract
1. Introduction
2. Experimental Section
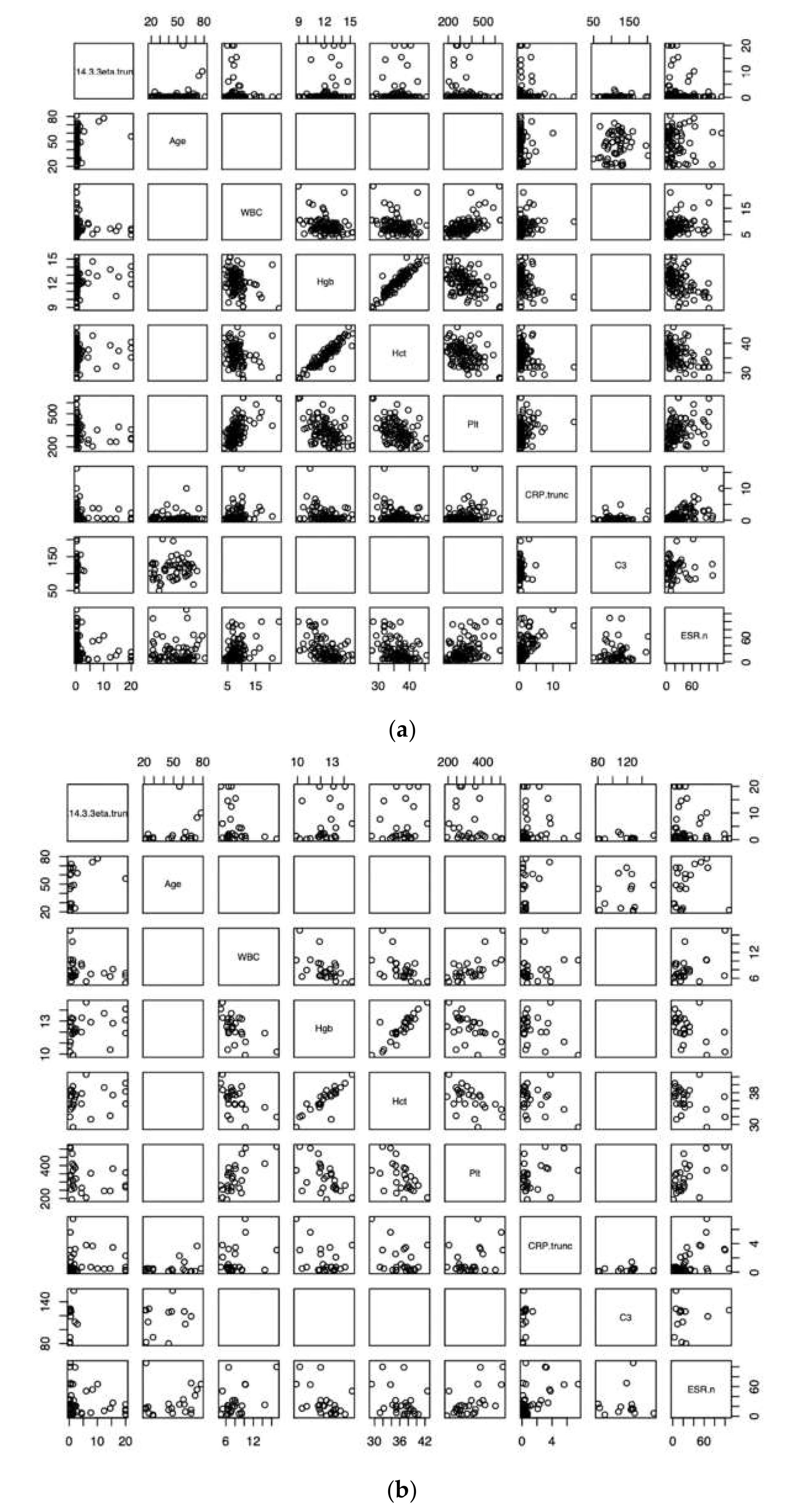
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petty, R.E.; Laxer, R.M.; Wedderburn, L.R. Juvenile idiopathic arthritis. In Textbook of Pediatric Rheumatology, 7th ed.; Petty, R.E., Laxer, R.M., Lindsley, C.B., Wedderburn, L.R., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 188–203. [Google Scholar]
- Syed, R.H.; Gilliam, B.E.; Moore, T.L. Rheumatoid factors and anti-cyclic citrullinated peptide antibodies in pediatric rheumatology. Curr. Rheumatol. Rep. 2008, 10, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.H.; Gilliam, B.E.; Moore, T.L. Prevalence and significance of isotypes of anti-cyclic citrullinated peptide antibodies in juvenile idiopathic arthritis. Ann. Rheum. Dis. 2008, 67, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, B.E.; Chauhan, A.K.; Low, J.M.; Moore, T.L. Measurement of biomarkers in juvenile idiopathic arthritis patients and their predication of disease severity: A comparative study. Clin. Exp. Rheumatol. 2008, 26, 492–497. [Google Scholar] [PubMed]
- Gilliam, B.E.; Reed, M.R.; Chauhan, A.K.; Dehlendorf, A.B.; Moore, T.L. Evidence of fibrinogen as a target of citrullination in IgM rheumatoid factor positive polyarticular juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2011, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, B.E.; Chauhan, A.K.; Moore, T.L. Evaluation of anti-cyclic citrullinated fibrinogen and anti-citrullinated α-enolase antibodies in juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2013, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Gilliam, B.E.; Crespo-Pagnussat, S.; Feller, L.; Chauhan, A.K. Measurement and evaluation of isotypes of anti-citrullinated fibrinogen and anti-citrullinated α-enolase antibodies in juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 2014, 32, 740–746. [Google Scholar] [PubMed]
- Moore, T.L.; Bhardwaj, P.; Pepmueller, P.H. Anti-carbamylated protein antibodies in juvenile idiopathic arthritis: A new biomarker. Autoimmun. Rev. 2017, 4, 1022–1029. [Google Scholar]
- Feller, L.S.; Tuttle, P.V.; Dalrymple, A.M.; Bathula, S.; Temmprano, K.; Syed, R.H.; Bandlamudi, R.; Coulson, K.; Pepmueller, P.; Moore, T. Single academic center experience with 14-3-3η in the evaluation of inflammatory arthritis. Arthritis Rheumatol. 2015, 67, 3155–3156. [Google Scholar]
- Feller, L.S.; Dalrymple, A.M.; Tuttle, P.V.; Syed, R.; Pepmueller, P.; Moore, T. Examination of the clinical significance of 14-3-3η in juvenile idiopathic arthritis. Arthritis Rheumatol. 2015, 67, 2969. [Google Scholar]
- Dalrymple, A.; Tuttle, P.I.V.; Feller, L.; Zhukov, O.S.; Lagier, R.J.; Bridgforth, R.; Williams, G.J.; Popov, J.M.; Naides, S.J.; Moore, T. 14-3-3η protein in juvenile idiopathic arthritis. Arthritis Rheumatol. 2017, 69, 3327–3328. [Google Scholar]
- Carrier, N.; Marotta, A.; de Brum-Fernandes, A.J.; Liang, P.; Masetto, A.; Ménard, H.A.; Maksymowych, W.P.; Boire, G. Serum levels of 14-3-3η protein supplement C-reactive protein and rheumatoid arthritis-associated antibodies to predict clinical and radiographic outcomes in a prospective cohort of patients with recent-onset inflammatory polyarthritis. Arthritis Res. Ther. 2016, 18, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, W.P.; Naides, S.J.; Bykerk, V.; Siminovitch, K.A.; van Schaardenburg, D.; Boers, M.; Landewé, R.; van der Heijde, D.; Tak, P.P.; Genovese, M.C.; et al. Serum 14-3-3η is a novel marker that complements current serological measurements to enhance detection of patients with rheumatoid arthritis. J. Rheumatol. 2014, 41, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, W.P.; van der Heijde, D.; Allaart, C.F.; Landewé, R.; Boire, G.; Tak, P.P.; Gui, Y.; Ghahary, A.; Kilani, R.; Marotta, A. 14-3-3η is a novel mediator associated with the pathogenesis of rheumatoid arthritis and joint damage. Arthritis Res. Ther. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Group | Female/Male, N | Average Age * (SD) |
|---|---|---|
| RF+ polyarticular | 25/4 | 15 (+/−8) |
| RF- polyarticular | 23/6 | 12 (+/−7) |
| Oligoarticular | 32/2 | 9 (+/−7) |
| Systemic Onset | 8/4 | 9 (+/−5) |
| Adult SLE | 53/7 | 46 (+/−15) |
| Adult RA | 11/8 | 55 (+/−17) |
| Healthy Controls | 17/3 | 7 (+/−5) |
| Group | ≥0.2 ng/mL (% [95% CI]) | ≥0.5 ng/mL (% [95% CI]) | Neg | N |
|---|---|---|---|---|
| RF+ polyarticular | 10 (34 [18,54]) | 8 (28 [13,47]) | 11 | 29 |
| RF- polyarticular | 9 (31 [15,51]) | 8 (28 [13,47]) | 12 | 29 |
| Oligoarticular | 6 (18 [7,34]) | 5 (15 [5,31]) | 23 | 34 |
| Systemic Onset | 2 (17 [2,48]) | 1 (8 [0,38]) | 9 | 12 |
| Disease Controls | ||||
| SLE | 14 (23 [13,36]) | 7 (12 [5,23]) | 39 | 60 |
| RA | 7 (37 [16,62]) | 5 (26 [9,51]) | 7 | 19 |
| Healthy Controls | 3 (15 [3,38]) | 1 (5 [0,25]) | 16 | 20 |
| Test Group | Reference Group | OR (95% CI) | p Value |
|---|---|---|---|
| RF+ polyarticular | Systemic Onset | 2.6 (0.4,28.7) | 0.452 |
| Oligoarticular | 2.4 (0.7,9.6) | 0.154 | |
| Adult SLE | 1.7 (0.6,5.0) | 0.312 | |
| Adult RA | 0.9 (0.2,3.6) | 1.000 | |
| Healthy Controls | 2.9 (0.6,19.3) | 0.191 | |
| RF- polyarticular | Systemic Onset | 2.2 (0.4,24.8) | 0.457 |
| Oligoarticular | 2.1 (0.6,8.3) | 0.247 | |
| Adult SLE | 1.5 (0.5,4.4) | 0.450 | |
| Adult RA | 0.8 (0.2,3.2) | 0.759 | |
| Healthy Controls | 2.5 (0.5,16.7) | 0.313 | |
| RF all polyarticular | Systemic Onset | 2.4 (0.4,24.8) | 0.325 |
| Oligoarticular | 2.3 (0.7,7.8) | 0.148 | |
| Adult SLE | 1.6 (0.7,3.9) | 0.307 | |
| Adult RA | 0.8 (0.3,2.9) | 0.784 | |
| Healthy Controls | 2.7 (0.7,16.3) | 0.158 |
| Test Group | Reference Group | OR (95% CI) | p Value |
|---|---|---|---|
| RF+ polyarticular | Systemic Onset | 4.1 (0.4,202.4) | 0.240 |
| Oligoarticular | 2.2 (0.5,9.8) | 0.230 | |
| Adult SLE | 2.8 (0.8,10.5) | 0.075 | |
| Adult RA | 1.1 (0.2,5.0) | 1.000 | |
| Healthy Controls | 7.0 (0.8,337.1) | 0.064 | |
| RF- polyarticular | Systemic Onset | 4.1 (0.4,202.4) | 0.240 |
| Oligoarticular | 2.2 (0.5,9.8) | 0.230 | |
| Adult SLE | 2.8 (0.8,10.5) | 0.075 | |
| Adult RA | 1.1 (0.2,5.0) | 1.000 | |
| Healthy Controls | 7.0 (0.8,337.1) | 0.064 | |
| RF all polyarticular | Systemic Onset | 4.1 (0.5,191.3) | 0.269 |
| Oligoarticular | 2.2 (0.7,8.5) | 0.202 | |
| Adult SLE | 2.9 (1.0,9.0) | 0.037 | |
| Adult RA | 1.1 (0.3,4.4) | 1.000 | |
| Healthy Controls | 7.1 (1.0,318.8) | 0.056 |
| 14-3-3η | Age | WBC | Hgb | Hct | Plt | CRP | C3 | ESR | |
|---|---|---|---|---|---|---|---|---|---|
| 14-3-3η | 1 | 0.05 (p = 0.5) | −0.10 (0.3) | 0.13 (0.2) | 0.06 (0.6) | −0.10 (0.3) | 0.02 (0.8) | 0.11 (0.4) | 0.04 (0.6) |
| Age | 1 | −0.41 | 0.38 | 0.44 | −0.46 | −0.37 | 0.15 | −0.20 | |
| WBC | 1 | −0.30 | −0.28 | 0.55 | 0.26 | NA | 0.30 | ||
| Hgb | 1 | 0.92 | −0.44 | −0.32 | NA | −0.46 | |||
| Hct | 1 | −0.43 | −0.32 | NA | −0.38 | ||||
| Plt | 1 | 0.31 | NA | 0.41 | |||||
| CRP | 1 | 0.09 | 0.59 | ||||||
| C3 | 1 | 0.04 | |||||||
| ESR | 1 |
| 14-3-3η | Age | WBC | Hgb | Hct | Plt | CRP | C3 | ESR | |
|---|---|---|---|---|---|---|---|---|---|
| 14-3-3η | 1 | −0.06 (p = 0.7) | −0.34 (0.09) | 0.36 (0.07) | 0.22 (0.3) | −0.31 (0.1) | 0.27 (0.07) | 0.33 (0.3) | −0.12 (0.4) |
| Age | 1 | −0.45 | 0.28 | 0.36 | −0.68 | −0.37 | −0.06 | 0.01 | |
| WBC | 1 | −0.55 | −0.43 | 0.62 | 0.01 | NA | 0.38 | ||
| Hgb | 1 | 0.86 | −0.54 | −0.14 | NA | −0.49 | |||
| Hct | 1 | −0.53 | −0.25 | NA | −0.34 | ||||
| Plt | 1 | 0.15 | NA | 0.56 | |||||
| CRP | 1 | 0.39 | 0.30 | ||||||
| C3 | 1 | −0.18 | |||||||
| ESR | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalrymple, A.; Tuttle, P.; Feller, L.; Zhukov, O.; Lagier, R.; Popov, J.; Naides, S.; Moore, T. 14-3-3η Protein as a Potential Biomarker in Juvenile Idiopathic Arthritis. Pediatr. Rep. 2021, 13, 65-71. https://doi.org/10.3390/pediatric13010008
Dalrymple A, Tuttle P, Feller L, Zhukov O, Lagier R, Popov J, Naides S, Moore T. 14-3-3η Protein as a Potential Biomarker in Juvenile Idiopathic Arthritis. Pediatric Reports. 2021; 13(1):65-71. https://doi.org/10.3390/pediatric13010008
Chicago/Turabian StyleDalrymple, Austin, Paul Tuttle, Lance Feller, Olga Zhukov, Robert Lagier, Joanna Popov, Stanley Naides, and Terry Moore. 2021. "14-3-3η Protein as a Potential Biomarker in Juvenile Idiopathic Arthritis" Pediatric Reports 13, no. 1: 65-71. https://doi.org/10.3390/pediatric13010008
APA StyleDalrymple, A., Tuttle, P., Feller, L., Zhukov, O., Lagier, R., Popov, J., Naides, S., & Moore, T. (2021). 14-3-3η Protein as a Potential Biomarker in Juvenile Idiopathic Arthritis. Pediatric Reports, 13(1), 65-71. https://doi.org/10.3390/pediatric13010008





