Heat Tolerance of Wildtype Salmonella Tennessee and Its Knock-Off Mutants in Peanut Butter and Peanut Spread
Abstract
1. Introduction
2. Materials and Methods
2.1. Salmonella Culture and Growth Conditions
2.2. Peanut Products
2.3. Inoculation of Peanut Products with Salmonella
2.4. Heat Treatment
2.5. Data Analysis
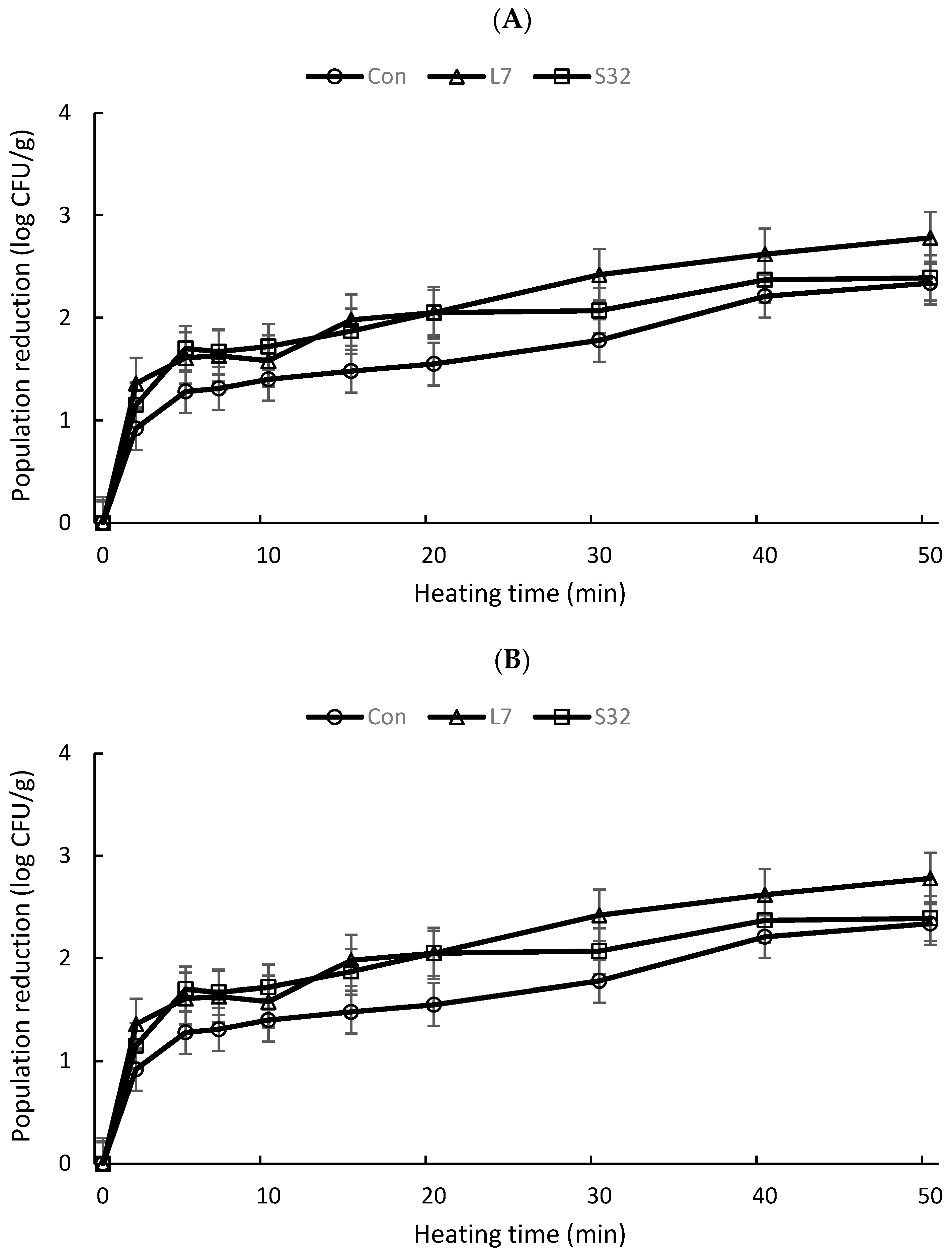
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acuff, J.; Dickson, J.S.; Farber, J.M.; Grasso-Kelley, E.M.; Hedberg, C.; Lee, A.; Zhu, M.-J. Practice and progress: Updates on outbreaks, advances in research, and processing technologies for low-moisture food safety. J. Food Prot. 2023, 86, 100018. [Google Scholar] [CrossRef]
- Burnett, S.L.; Gehm, E.R.; Weissinger, W.R.; Beuchat, L.R. Survival of Salmonella in peanut butter and peanut butter spread. J. Appl. Microbiol. 2000, 89, 472–477. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, G.; Gerner-Smidt, P.; Mantripragada, V.; Ezeoke, I.; Doyle, M.P. Thermal Inactivation of Salmonella in peanut butter. J. Food Prot. 2009, 72, 1596–1601. [Google Scholar] [CrossRef]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef] [PubMed]
- Sheth, A.N.; Hoekstra, M.; Patel, N.; Ewald, G.; Lord, C.; Clarke, C.; Villamil, E.; Niksich, K.; Bopp, C.; Nguyen, T.-A.; et al. A national outbreak of Salmonella serotype Tennessee infections from contaminated peanut butter: A new food vehicle for salmonellosis in the United States. Clin. Infect. Dis. 2011, 53, 356–362. [Google Scholar] [CrossRef]
- U.S. FDA. Outbreak Investigation of Salmonella: Peanut Butter (May 2022). 2024. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-peanut-butter-may-2022#:~:text=of%20new%20recalls.-,As%20of%20May%2025%2C%202022%2C%20CDC%20reports%20that%20of%20the,illnesses%20in%20this%20current%20outbreak (accessed on 15 September 2025).
- Hvizdzak, A.L.; Beamer, S.; Jaczynski, J.; Matak, K.E. Use of electron beam radiation for the reduction of Salmonella enterica serovars Typhimurium and Tennessee in peanut butter. J. Food Prot. 2010, 73, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Enache, E.; Black, D.G.; Elliott, P.H.; Napier, C.D.; Podolak, R.; Hayman, M.M. Survival of Salmonella Tennessee, Salmonella Typhimurium DT104, and Enterococcus faecium in peanut paste formulations at two different levels of water activity and fat. J. Food Prot. 2014, 77, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Gorrepati, K.; Balasubramanian, S.; Chandra, P. Plant based butters. J. Food Sci. Technol. 2015, 52, 3965–3976. [Google Scholar] [CrossRef]
- Holečková, N.; Doubravová, L.; Massidda, O.; Molle, V.; Buriánková, K.; Benada, O.; Kofroňová, O.; Ulrych, A.; Branny, P. LocZ Is a new cell division protein involved in proper septum placement in Streptococcus pneumoniae. mBio 2015, 6, e01700-14. [Google Scholar] [CrossRef]
- CDC. Multistate outbreak of Salmonella infections associated with peanut butter and peanut butter-containing products—United States, 2008–2009. Morb. Mortal. Wkly. Rep. 2009, 58, 85–90. [Google Scholar]
- Li, C.; Huang, L.; Chen, J. Comparative study of thermal inactivation kinetics of Salmonella spp. in peanut butter and peanut butter spread. Food Control 2014, 45, 143–149. [Google Scholar] [CrossRef]
- Shachar, D.; Yaron, S. Heat tolerance of Salmonella enterica serovars Agona, Enteritidis, and Typhimurium in peanut butter. J. Food Prot. 2006, 69, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Salazar, J.K.; Yang, J.; Tortorello, M.L.; Zhang, W. Increased water activity reduces the thermal resistance of Salmonella enterica in peanut butter. Appl. Environ. Microbiol. 2013, 79, 4763–4767. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, D.; Yang, J.; Tortorello, M.L.; Zhang, W. Survival and heat resistance of Salmonella enterica and Escherichia coli O157:H7 in peanut butter. Appl. Environ. Microbiol. 2011, 77, 8434–8438. [Google Scholar] [CrossRef]
- Juneja, V.K.; Eblen, B.S. Heat inactivation of Salmonella typhimurium DT104 in beef as affected by fat content. Lett. Appl. Microbiol. 2000, 30, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.L.; Board, R.G. The fate of Salmonella enteritidis PT4 in deliberately infected commercial mayonnaise. Food Microbiol. 1994, 11, 499–504. [Google Scholar] [CrossRef]
- Sirsat, S.A.; Burkholder, K.M.; Muthaiyan, A.; Dowd, S.E.; Bhunia, A.K.; Ricke, S.C. Effect of sublethal heat stress on Salmonella Typhimurium virulence. J. Appl. Microbiol. 2011, 110, 813–822. [Google Scholar] [CrossRef]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of desiccation on tolerance of Salmonella enterica to multiple stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef]
- Mao, Y.; Doyle, M.P.; Chen, J. Insertion mutagenesis of wca reduces acid and heat tolerance of enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 2001, 183, 3811–3815. [Google Scholar] [CrossRef]
- Villa-Rojas, R.; Zhu, M.-J.; Paul, N.C.; Gray, P.; Xu, J.; Shah, D.H.; Tang, J. Biofilm forming Salmonella strains exhibit enhanced thermal resistance in wheat flour. Food Control 2017, 73, 689–695. [Google Scholar] [CrossRef]
- Dhir, V.K.; Dodd, C.E. Susceptibility of suspended and surface-attached Salmonella enteritidis to biocides and elevated temperatures. Appl. Environ. Microbiol. 1995, 61, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, J. Identification of the genetic elements involved in biofilm formation by Salmonella enterica serovar Tennessee using mini-Tn10 mutagenesis and DNA sequencing. Food Microbiol. 2022, 106, 104043. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Food Standard Innovations: Peanut Butter’s Sticky Standard. 2022. Available online: https://www.fda.gov/about-fda/histories-product-regulation/food-standard-innovations-peanut-butters-sticky-standard#:~:text=FDA%20proposed%20a%20standard%20for,did%20prevail%20as%20the%20US (accessed on 15 September 2025).
- Code of Federal Regulation. Part 102—Common or Usual Name for Nonstandaridized Foods. 2025. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-102 (accessed on 15 September 2025).
- Cui, Y.; Liu, D.; Chen, J. Fate of Salmonella enterica and enterohemorrhagic Escherichia coli on vegetable seeds contaminated by direct contact with artificially inoculated soil during germination. J. Food Prot. 2020, 83, 1218–1226. [Google Scholar] [CrossRef]
- Nakayama, H.; Kurokawa, K.; Lee, B.L. Lipoproteins in bacteria: Structures and biosynthetic pathways. FEBS J. 2012, 279, 4247–4268. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Hantke, K. Lipoproteins: Structure, Function, Biosynthesis. In Bacterial Cell Walls and Membranes, Subcellular Biochemistry; Kuhn, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 39–77. [Google Scholar] [CrossRef]
- Kovacs-Simon, A.; Titball, R.W.; Michell, S.L. Lipoproteins of bacterial pathogens. Infect. Immun. 2011, 79, 548–561. [Google Scholar] [CrossRef]
- Narita, S.; Matsuyama, S.; Tokuda, H. Lipoprotein trafficking in Escherichia coli. Arch. Microbiol. 2004, 182, 1–6. [Google Scholar] [CrossRef]
- Liao, C.-T.; Li, C.-E.; Chang, H.-C.; Hsu, C.-H.; Chiang, Y.-C.; Hsiao, Y.-M. The lolB gene in Xanthomonas campestris pv. campestris is required for bacterial attachment, stress tolerance, and virulence. BMC Microbiol. 2022, 22, 17. [Google Scholar] [CrossRef]
- Vedyaykin, A.D.; Ponomareva, E.V.; Khodorkovskii, M.A.; Borchsenius, S.N.; Vishnyakov, I.E. Mechanisms of bacterial cell division. Microbiology 2019, 88, 245–260. [Google Scholar] [CrossRef]
- Durand-Heredia, J.M.; Yu, H.H.; De Carlo, S.; Lesser, C.F.; Janakiraman, A. Identification and characterization of ZapC, a stabilizer of the FtsZ ring in Escherichia coli. J. Bacteriol. 2011, 193, 1405–1413. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ray, S.; Singh, D.; Dhaked, H.P.S.; Panda, D. ZapC promotes assembly and stability of FtsZ filaments by binding at a different site on FtsZ than ZipA. Int. J. Biol. Macromol. 2015, 81, 435–442. [Google Scholar] [CrossRef]
- Hale, C.A.; Shiomi, D.; Liu, B.; Bernhardt, T.G.; Margolin, W.; Niki, H.; de Boer, P.A.J. Identification of Escherichia coli ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. J. Bacteriol. 2011, 193, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.M.; Prestes, F.S.; Silva, A.C.M.; Nascimento, M.S. Evaluation of the thermal resistance of Salmonella Typhimurium ATCC 14028 after long-term blanched peanut kernel storage. LWT 2020, 117, 108701. [Google Scholar] [CrossRef]
- Mañas, P.; Pagan, R.; Leguérinel, I.; Condon, S.; Mafart, P.; Sala, F. Effect of sodium chloride concentration on the heat resistance and recovery of Salmonella typhimurium. Int. J. Food Microbiol. 2001, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
| Strains | Features | Source | |
|---|---|---|---|
| Wildtype | S. Tennessee | The peanut butter associated outbreak strain: amps, kans, nalr | [6] |
| Mutants | ST-L7 | With a mini Tn10 insertion: amps, kanr, nalr, defective in lpa | [25] |
| ST-S32 | With a mini Tn10 insertion: amps, kanr, nalr, defective in zapC | [25] |
| Product | Nutrition Facts (% Daily Value) | |||
|---|---|---|---|---|
| Protein | Lipid | Carbohydrate | Sodium | |
| Regular | 7 | 21 | 3 | 6 |
| Reduced-fat | 7 | 16 | 5 | 8 |
| Natural | 7 | 20 | 3 | 3 |
| Main Effects | Reduced Populations (log CFU/g) | ||
|---|---|---|---|
| Tryptic Soy Agar | Bismuth Sulfite Agar | ||
| Culture | Wildtype | 1.43 C | 1.60 B |
| L7 | 1.80 A | 1.96 A | |
| S32 | 1.70 B | 1.92 A | |
| Product | Regular | 1.83 A | 2.09 A |
| Reduced-fat | 1.41 C | 1.62 C | |
| Natural | 1.69 B | 1.76 B | |
| Heating time (min) | 0 | 0.00 F | 0.00 I |
| 2.5 | 1.14 E | 1.25 H | |
| 5 | 1.52 D | 1.63 G | |
| 7.5 | 1.54 D | 1.77 F | |
| 10 | 1.57 D | 1.82 EF | |
| 15 | 1.77 C | 2.01 D | |
| 20 | 1.88 C | 1.94 DE | |
| 30 | 2.09 B | 2.25 C | |
| 40 | 2.40 A | 2.68 B | |
| 50 | 2.50 A | 2.87 A | |
| Mean Population (log CFU/g) | ||||||
|---|---|---|---|---|---|---|
| Tryptic Soy Agar | Bismuth Sulfite Agar | |||||
| Culture Product | Wild Type | L7 | S32 | Wild Type | L7 | S32 |
| Regular | 1.63 Ab | 1.98 Aa | 1.88 Aa | 1.71 Ac | 2.38 Aa | 2.17 Ab |
| Reduced-fat | 1.13 Bb | 1.55 Ba | 1.54 Ca | 1.42 Bc | 1.57 Cb | 1.88 Ba |
| Natural | 1.53 Ac | 1.87 Aa | 1.68 Bb | 1.66 Ab | 1.91 Ba | 1.72 Cb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lee, S.; Kong, F.; Chen, J. Heat Tolerance of Wildtype Salmonella Tennessee and Its Knock-Off Mutants in Peanut Butter and Peanut Spread. Microbiol. Res. 2026, 17, 13. https://doi.org/10.3390/microbiolres17010013
Lee S, Kong F, Chen J. Heat Tolerance of Wildtype Salmonella Tennessee and Its Knock-Off Mutants in Peanut Butter and Peanut Spread. Microbiology Research. 2026; 17(1):13. https://doi.org/10.3390/microbiolres17010013
Chicago/Turabian StyleLee, Seulgi, Fanbin Kong, and Jinru Chen. 2026. "Heat Tolerance of Wildtype Salmonella Tennessee and Its Knock-Off Mutants in Peanut Butter and Peanut Spread" Microbiology Research 17, no. 1: 13. https://doi.org/10.3390/microbiolres17010013
APA StyleLee, S., Kong, F., & Chen, J. (2026). Heat Tolerance of Wildtype Salmonella Tennessee and Its Knock-Off Mutants in Peanut Butter and Peanut Spread. Microbiology Research, 17(1), 13. https://doi.org/10.3390/microbiolres17010013







