Extended-Spectrum β-Lactamase-/AmpC-Producing Escherichia coli and Associated Risk Factors in Shelter Dogs: A Baseline Study in North Macedonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Sample Collection
2.2. Fecal Sampling and Bacterial Isolation
2.3. Questionnaire and Data Collection
2.4. Antimicrobial Susceptibility Testing and ESBL/AmpC Phenotyping
2.5. Molecular Identification and Characterization of Antimicrobial Resistance Genes
2.6. Statistical Analysis
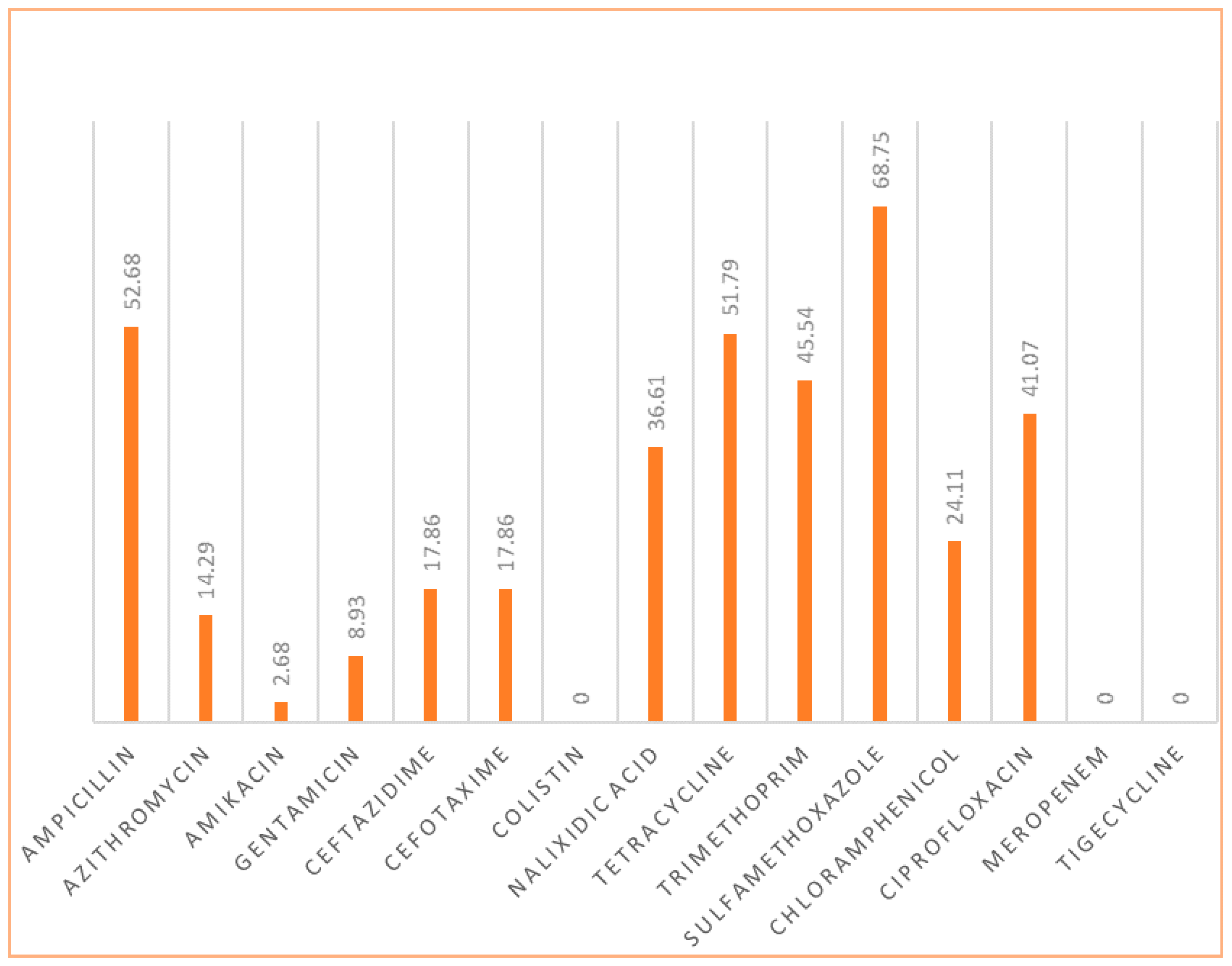
3. Results
3.1. Most Frequent Resistance Patterns
3.2. Correlation Analyses
3.3. Association Between Shelter Practices and AMR
3.4. Relationship Between Duration of Stay and Antibiotic Resistance
3.5. Shelter-Level Factors Associated with Antimicrobial Resistance
3.6. Cleaning Frequency
3.7. Rationale for Antibiotic Selection
3.8. Duration of Antibiotic Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Gortázar, C.; Herskin, M.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Antimicrobial-resistant Escherichia coli in dogs and cats, horses, swine, poultry, cattle, sheep and goats. EFSA J. 2022, 20, e07311. [Google Scholar] [CrossRef]
- EMA. Reflection Paper on the Risk of Antimicrobial Resistance Transfer from Companion Animals; EMA/CVMP/AWP/401740/2013; Committee for Medicinal Products for Veterinary Use (CVMP), European Medicines Agency: London, UK, 2013; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-risk-antimicrobial-resistance-transfer-companion-animals_en.pdf (accessed on 8 August 2025).
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing Escherichia coli in dogs and cats—A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef] [PubMed]
- Belas, A.; Salazar, A.S.; Da Gama, L.T.; Couto, N.; Pomba, C. Risk factors for faecal colonisation with Escherichia coli producing extended-spectrum and plasmid-mediated AmpC β-lactamases in dogs. Vet. Rec. 2014, 175, 202. [Google Scholar] [CrossRef] [PubMed]
- Shringi, S.; Shah, D.H.; Carney, K.; Verma, A. Pathogen detection and resistome analysis in healthy shelter dogs using whole metagenome sequencing. Pathogens 2025, 14, 33. [Google Scholar] [CrossRef]
- Bortolami, A.; Zendri, F.; Maciuca, E.I.; Wattret, A.; Ellis, C.; Schmidt, V.; Pinchbeck, G.; Timofte, D. Diversity, virulence, and clinical significance of extended-spectrum β-lactamase- and pAmpC-producing Escherichia coli from companion animals. Front. Microbiol. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Sun, L.; Meng, N.; Wang, Z.; Hong, J.; Jiao, X.; Dai, Y.; Wang, Z.; Wang, J.; Jiao, X. Genomic characterization of ESBL/AmpC-producing Escherichia coli in stray dogs sheltered in Yangzhou, China. Infect. Drug Resist. 2022, 15, 7741–7750. [Google Scholar] [CrossRef]
- Hansen, K.H.; Bortolaia, V.; Nielsen, C.A.; Nielsen, J.B.; Schønning, K.; Agersø, Y.; Guardabassi, L. Host-specific patterns of genetic diversity among IncI1-Iγ and IncK plasmids encoding CMY-2 β-lactamase in Escherichia coli isolates from humans, poultry meat, poultry, and dogs in Denmark. Appl. Environ. Microbiol. 2016, 82, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Buldain, D.; Castillo, L.G.; Buchamer, A.; Chirino-Trejo, M.; Mestorino, N. Pet and stray dogs as reservoirs of antimicrobial-resistant Escherichia coli. Int. J. Microbiol. 2021, 2021, 6664557. [Google Scholar] [CrossRef]
- Hur, B.A.; Hardefeldt, L.Y.; Verspoor, K.M.; Baldwin, T.; Gilkerson, J.R. Describing the antimicrobial usage patterns of companion animal veterinary practices: Free text analysis of more than 4.4 million consultation records. PLoS ONE 2020, 15, e0230049. [Google Scholar] [CrossRef]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial usage and resistance in companion animals: A cross-sectional study in three European countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef]
- Shikoska, I.; Cvetkovikj, A.; Nikolovski, M.; Cvetkovikj, I. Understanding antimicrobial prescription practices: Insights from small animal veterinarians in North Macedonia. Maced. Vet. Rev. 2024, 47, 77–86. [Google Scholar] [CrossRef]
- Karalliu, E.; Chung, K.Y.; MacKinnon, B.; Haile, B.; Beczkowski, P.M.; Barrs, V.R.; Elsohaby, I.; Nekouei, O. Risk factors for antimicrobial-resistant Enterobacterales in dogs: A systematic review. Front. Vet. Sci. 2024, 11, 1447707. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Murphy, B.G. Common and emerging infectious diseases in the animal shelter. Vet. Pathol. 2014, 51, 478–491. [Google Scholar] [CrossRef]
- Mader, R.; Muñoz Madero, C.; Aasmäe, B.; Bourély, C.; Broens, E.M.; Busani, L.; Callens, B.; Collineau, L.; Crespo-Robledo, P.; Damborg, P.; et al. Review and analysis of national monitoring systems for antimicrobial resistance in animal bacterial pathogens in Europe: A basis for the development of the European Antimicrobial Resistance Surveillance network in veterinary medicine (EARS-Vet). Front. Microbiol. 2022, 13, 838490. [Google Scholar] [CrossRef]
- EU Commission. Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria and repealing Implementing Decision 2013/652/EU. Off. J. Eur. Union. 2020, 387, 8–21. [Google Scholar]
- Olesen, I.; Hasman, H.; Aarestrup, F.M. Prevalence of beta-lactamases among ampicillin-resistant Escherichia coli and Salmonella isolated from food animals in Denmark. Microb. Drug Resist. 2004, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Moodley, A.; Guardabassi, L. Transmission of IncN plasmids carrying blaCTX-M-1 between commensal Escherichia coli in pigs and farm workers. Antimicrob. Agents Chemother. 2009, 53, 1709–1711. [Google Scholar] [CrossRef] [PubMed]
- Hasman, H.; Mevius, D.; Veldman, K.; Olesen, I.; Aarestrup, F.M. beta-Lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 2005, 56, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Mikoleit, M.; Kornschober, C.; Rickert, R.L.; Van Duyne, S.; Kjelsø, C.; Hasman, H.; Cormican, M.; Mevius, D.; Threlfall, J.; et al. Emergence of multidrug-resistant Salmonella Concord infections in Europe and the United States in children adopted from Ethiopia, 2003–2007. Pediatr. Infect. Dis. J. 2009, 28, 814–818. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, S.; Kim, Y.R.; Oh, E.J.; Woo, G.J.; Lee, K. Occurrence of extended-spectrum beta-lactamases and plasmid-mediated AmpC beta-lactamases among Korean isolates of Proteus mirabilis. J. Antimicrob. Chemother. 2006, 57, 156–158. [Google Scholar] [CrossRef]
- Briñas, L.; Lantero, M.; de Diego, I.; Alvarez, M.; Zarazaga, M.; Torres, C. Mechanisms of resistance to expanded-spectrum cephalosporins in Escherichia coli isolates recovered in a Spanish hospital. J. Antimicrob. Chemother. 2005, 56, 1107–1110. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Nucleo, E.; Luzzaro, F.; Giani, T.; Migliavacca, R.; Vailati, F.; Kroumova, V.; Pagani, L.; Rossolini, G.M. CMY-16, a novel acquired AmpC-type beta-lactamase of the CMY/LAT lineage in Proteus mirabilis. Antimicrob. Agents Chemother. 2006, 50, 618–624. [Google Scholar] [CrossRef]
- Arlet, G.; Rouveau, M.; Philippon, A. Substitution of alanine for aspartate at position 179 in the SHV-6 extended-spectrum β-lactamase. FEMS Microbiol. Lett. 1997, 152, 163–167. [Google Scholar] [CrossRef]
- Pai, H.; Lyu, S.; Lee, J.H.; Kim, J.; Kwon, Y.; Kim, J.W.; Choe, K.W. Survey of extended-spectrum β-lactamases in Escherichia coli and Klebsiella pneumoniae: Prevalence of TEM-52 in Korea. J. Clin. Microbiol. 1999, 37, 1758–1763. [Google Scholar] [CrossRef]
- Kruger, T.; Szabo, D.; Keddy, K.H.; Deeley, K.; Marsh, J.W.; Hujer, A.M.; Bonomo, R.A.; Paterson, D.L. Infections with nontyphoidal Salmonella species producing TEM-63 or a novel TEM enzyme, TEM-131, in South Africa. Antimicrob. Agents Chemother. 2004, 48, 4263–4270. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Bangtrakulnonth, A.; Pulsrikarn, C.; Pornreongwong, S.; Hasman, H.; Song, S.W.; Aarestrup, F.M. Antimicrobial resistance and molecular epidemiology of Salmonella Rissen from animals, food products and patients in Thailand and Denmark. Foodborne Pathog. Dis. 2008, 5, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.J. Enterobacteriaceae: Introduction and identification. In Manual of Clinical Microbiology, 7th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 442–458. [Google Scholar]
- Everett, M.J.; Jin, Y.F.; Ricci, V.; Piddock, L.J. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob. Agents Chemother. 1996, 40, 2380–2386. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Frimodt-Møller, N.; Hasman, H.; Guardabassi, L.; Nielsen, L.; Aarestrup, F.M. Prevalence of quinolone resistance mechanisms and associations to minimum inhibitory concentrations in quinolone-resistant Escherichia coli isolated from humans and swine in Denmark. Microb. Drug Resist. 2008, 14, 163–169. [Google Scholar] [CrossRef]
- Rahmani, M.; Peighambari, S.M.; Svendsen, C.A.; Cavaco, L.M.; Agersø, Y.; Hendriksen, R.S. Molecular clonality and antimicrobial resistance in Salmonella enterica serovars Enteritidis and Infantis from broilers in Iran. BMC Vet. Res. 2013, 9, 66. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Lertworapreecha, M.; Evans, M.C.; Bangtrakulnonth, A.; Chalermchaikit, T.; Hendriksen, R.S.; Wegener, H.C. Antimicrobial susceptibility and occurrence of resistance genes among Salmonella enterica serovar Weltevreden from different countries. J. Antimicrob. Chemother. 2003, 52, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Perreten, V.; Boerlin, P. A new sulphonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 2003, 47, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.H.; Rogowsky, P.; Grinsted, J.; Altenbuchner, J.; Schmitt, R. The tetracycline resistance determinants of RP1 and Tn1721: Nucleotide sequence analysis. Nucleic Acids Res. 1983, 11, 6089–6105. [Google Scholar] [CrossRef] [PubMed]
- Sengeløv, G.; Agersø, Y.; Halling-Sørensen, B.; Baloda, S.B.; Andersen, J.S.; Jensen, L.B. Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environ. Int. 2003, 28, 587–595. [Google Scholar] [CrossRef]
- Miranda, C.D.; Kehrenberg, C.; Ulep, C.; Schwarz, S.; Roberts, M.C. Diversity of tetracycline resistance genes in bacteria from Chilean salmon farms. Antimicrob. Agents Chemother. 2003, 47, 883–888. [Google Scholar] [CrossRef]
- Agersø, Y.; Sandvang, D. Class 1 integrons and tetracycline resistance genes in Alcaligenes, Arthrobacter, and Pseudomonas spp. isolated from pigsties and manured soil. Appl. Environ. Microbiol. 2005, 71, 7941–7947. [Google Scholar] [CrossRef]
- Cocco, A.; Alessiani, A.; Salini, R.; Iapaolo, F.; Averaimo, D.; Pompilii, C.; Foschi, G.; Bellucci, F.; Iannino, F.; Dalla Villa, P.; et al. Detection of potential zoonotic agents isolated in Italian shelters and the assessment of animal welfare correlation with antimicrobial resistance in Escherichia coli strains. Antibiotics 2023, 12, 863. [Google Scholar] [CrossRef]
- Johansson, V.; Nykäsenoja, S.; Myllyniemi, A.L.; Rossow, H.; Heikinheimo, A. Genomic characterization of ESBL/AmpC-producing and high-risk clonal lineages of Escherichia coli and Klebsiella pneumoniae in imported dogs with shelter and stray background. J. Glob. Antimicrob. Resist. 2022, 30, 183–190. [Google Scholar] [CrossRef]
- Black, C.A.; Benavides, R.; Bandy, S.M.; Dallas, S.D.; Gawrys, G.; So, W.; Moreira, A.G.; Aguilar, S.; Quidilla, K.; Smelter, D.F.; et al. Diverse role of blaCTX-Mand porins in mediating ertapenem resistance among carbapenem-resistant Enterobacterales. Antibiotics 2024, 13, 185. [Google Scholar] [CrossRef]
- Debergh, H.; Maex, M.; Garcia-Graells, C.; Boland, C.; Saulmont, M.; Van Hoorde, K.; Saegerman, C. First Belgian report of ertapenem resistance in an ST11 Klebsiella pneumoniae strain isolated from a dog carrying blaSCO-1 and blaDHA-1 combined with permeability defects. Antibiotics 2022, 11, 1253. [Google Scholar] [CrossRef]
- World Health Organization. WHO’s List of Medically Important Antimicrobials: A risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-008461-2. Available online: https://cdn.who.int/media/docs/default-source/gcp/who-mia-list-2024-lv.pdf?sfvrsn=3320dd3d_2 (accessed on 15 August 2025).
- World Organisation for Animal Health (WOAH). WOAH List of Antimicrobial Agents of Veterinary Importance; WOAH: Paris, France, 2024; Available online: https://www.woah.org/app/uploads/2021/03/en-amr-strategy-2022-final-single-pages.pdf (accessed on 15 September 2025).
- Biguenet, A.; Valot, B.; El Garch, F.; Bertrand, X.; Hocquet, D. Genomic epidemiology of third-generation cephalosporin-resistant Escherichia coli from companion animals and human infections across Europe. One Health 2025, 20, 100971. [Google Scholar] [CrossRef]
- Formenti, N.; Grassi, A.; Parisio, G.; Romeo, C.; Guarneri, F.; Birbes, L.; Pitozzi, A.; Scali, F.; Maisano, A.M.; Boniotti, M.B.; et al. Extended-spectrum-β-lactamase- and AmpC-producing Escherichia coli in domestic dogs: Spread, characterisation and associated risk factors. Antibiotics 2021, 10, 1251. [Google Scholar] [CrossRef]
- Hata, A.; Fujitani, N.; Ono, F.; Yoshikawa, Y. Surveillance of antimicrobial-resistant Escherichia coli in sheltered dogs in the Kanto region of Japan. Sci. Rep. 2022, 12, 14971. [Google Scholar] [CrossRef]
- Horsman, S.; Rynhoud, H.; Zhou, X.; Magalhães, R.J.S.; Gibson, J.S.; Meler, E. Environmental recovery of nosocomial bacteria in a companion animal shelter before and after infection control procedures. Front. Vet. Sci. 2021, 7, 608901. [Google Scholar] [CrossRef]
- Cozma, A.P.; Rimbu, C.M.; Zendri, F.; Maciuca, I.E.; Timofte, D. Clonal dissemination of extended-spectrum cephalosporin-resistant Enterobacterales between dogs and humans in households and animal shelters of Romania. Antibiotics 2022, 11, 1242. [Google Scholar] [CrossRef]
- Shikoska, I.; Hristovska, Z.P.; Matevski, I.; Pavlova, M.J.; Manovska, M.R.; Cvetkovikj, A.; Cvetkovikj, I. Phenotypic and molecular characterization of antimicrobial resistance in canine staphylococci from North Macedonia. Maced. Vet. Rev. 2025, 48, 22. [Google Scholar] [CrossRef]
- Farrell, S.; Bagcigil, A.F.; Chaintoutis, S.C.; Firth, C.; Aydin, F.G.; Hare, C.; Maaland, M.; Mateus, A.; Vale, A.P.; Windahl, U.; et al. A multinational survey of companion animal veterinary clinicians: How can antimicrobial stewardship guidelines be optimised for the target stakeholder? Vet. J. 2024, 303, 106045. [Google Scholar] [CrossRef]
- Hughes, L.A.; Williams, N.; Clegg, P.; Callaby, R.; Nuttall, T.; Coyne, K.; Pinchbeck, G.; Dawson, S. Cross-sectional survey of antimicrobial prescribing patterns in UK small animal veterinary practice. Prev. Vet. Med. 2012, 104, 309–316. [Google Scholar] [CrossRef]
- Food and Veterinary Agency of the Republic of North Macedonia. Rulebook on the Procedures for Capture, Interventions, and Treatment of Stray Dogs, the Method for Conducting the Socialization Test, the Manner and Content of Data Recording, Supervision Procedures. 2021. Available online: https://drive.google.com/file/d/1NlvdCjffRUZS253tSo_O650obbiywNbZ/view (accessed on 15 August 2025). (In Macedonian)
- Rossow, H.; Joutsen, S.; Tuominen, P.; Pelkola, K. Zoonotic Pathogens in Imported Dogs; Finnish Food Authority Research Reports; Finnish Food Authority: Seinäjoki, Finland, 2019; Volume 2, 41p. [Google Scholar]
- Weese, J.S.; O’Brien, T.; Bateman, S. Fecal shedding of extended-spectrum beta-lactamase-producing Enterobacterales in cats admitted to an animal shelter. J. Feline Med. Surg. 2022, 24, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.; Datta, S.; Zawar, A.; Kafle, P. Evaluation of extended-spectrum β-lactamase-producing bacteria in feces of shelter dogs as a biomarker for altered gut microbial taxa and functional profiles. Front. Microbiol. 2025, 16, 1556442. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef] [PubMed]
- Tillotson, G.S. Trojan Horse Antibiotics—A Novel Way to Circumvent Gram-Negative Bacterial Resistance? Infect. Dis. 2016, 9, 45–52. [Google Scholar] [CrossRef]
| EUVSEC3 | EUVSEC2 | ||
|---|---|---|---|
| Antibiotic | ECOFF | Antibiotic | ECOFF |
| Amikacin | 8 | Cefepime | 0.125 |
| Ampicillin | 8 | Cefotaxime | 0.25 |
| Azithromycin | 16 | Cefotaxime/clavulanic acid | 0.25 |
| Cefotaxime | 0.25 | Cefoxitin | 16 |
| Ceftazidime | 1 | Ceftazidime | 1 |
| Chloramphenicol | 16 | Ceftazidime/clavulanic acid | 1 |
| Ciprofloxacin | 0.06 | Ertapenem | 0.03 |
| Colistin | 2 | Imipenem | 0.5 |
| Gentamicin | 2 | Meropenem | 0.06 |
| Meropenem | 0.125 | Temocillin | 16 |
| Nalidixic acid | 8 | ||
| Sulfamethoxazole | 64 | ||
| Tetracycline | 8 | ||
| Tigecycline | 0.5 | ||
| Trimethoprim | 2 | ||
| Antibiotic Class | Gene | n | % |
|---|---|---|---|
| β-lactams (ESBL/AmpC) | blaTEM | 16 | 88.9 |
| blaCTX-M | 17 | 94.4 | |
| blaCTX-M-1 | 17 | 94.4 | |
| blaCMY-1 | 18 | 100 | |
| blaCMY-2 | 16 | 88.9 | |
| blaACC | 18 | 100 | |
| blaFOX | 18 | 100 | |
| Tetracyclines | tetA | 15 | 83.3 |
| tetB | 2 | 11.1 | |
| Sulfonamides | sul1 | 1 | 5.6 |
| sul2 | 16 | 88.9 | |
| sul3 | 1 | 5.6 | |
| Phenicols | cmlA | 13 | 72.2 |
| catA1 | 2 | 11.1 |
| Shelter | Total Isolates | MDR Isolates | MDR Prevalence (%) |
|---|---|---|---|
| Shelter 1 | 24 | 18 | 75.00 |
| Shelter 2 | 36 | 16 | 41.67 |
| Shelter 3 | 13 | 6 | 38.46 |
| Shelter 4 | 22 | 5 | 13.64 |
| Shelter 5 | 4 | 2 | 50.00 |
| Shelter 6 | 13 | 9 | 53.85 |
| Shelter | Cap. (Dogs) | Dogs (Visit Day) | Shared Area | Clean. Freq. | Hyg. Prot. | Avg. Stay (Days) | Outd. Acc. |
|---|---|---|---|---|---|---|---|
| 1 | 48 | 35 | Yes | 2×/day | Yes | 30 | Yes |
| 2 | 150 | 132 | Yes | 3×/day | Yes | 10–15 | Yes |
| 3 | 35 | 34 | No | 2×/day | Yes | ∞ | No |
| 4 | 34 | 25 | No | 1×/day | Yes | 7–10 | Yes |
| 5 | 28 | 6 | No | 1×/day | No | 3–4 | No |
| 6 | 19 | 15 | No | 1×/day | No | 10 | No |
| Shelter | Guidelines for AMU | Post-Op Therapy Duration | Antibiotics Used | Basis of Choice | AST Performed |
|---|---|---|---|---|---|
| 1 | No | 3 days | ENR, CRO, SXT, AMP | Sci. lit. + Pers. exp. + Pharma info | No |
| 2 | No | 3 days | AMX, PEN, STR | Sci. lit. + Pers. exp. | No |
| 3 | No | 1–2 days | AMX | Sci. lit. + Pers. exp. | No |
| 4 | No | None | None | Pers. exp. | No |
| 5 | No | 3 days | PEN, STR | Pers. exp. | No |
| 6 | No | >3 days | PEN, AMX | Pers. exp. | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikoska, I.; Duvnjak, S.; Koritnik, T.; Chapkunovska, B.; Vlahov, J.; Ratkova Manovska, M.; Cvetkovikj, A.; Cvetkovikj, I. Extended-Spectrum β-Lactamase-/AmpC-Producing Escherichia coli and Associated Risk Factors in Shelter Dogs: A Baseline Study in North Macedonia. Microbiol. Res. 2025, 16, 206. https://doi.org/10.3390/microbiolres16090206
Shikoska I, Duvnjak S, Koritnik T, Chapkunovska B, Vlahov J, Ratkova Manovska M, Cvetkovikj A, Cvetkovikj I. Extended-Spectrum β-Lactamase-/AmpC-Producing Escherichia coli and Associated Risk Factors in Shelter Dogs: A Baseline Study in North Macedonia. Microbiology Research. 2025; 16(9):206. https://doi.org/10.3390/microbiolres16090206
Chicago/Turabian StyleShikoska, Ivana, Sanja Duvnjak, Tom Koritnik, Bojana Chapkunovska, Jane Vlahov, Marija Ratkova Manovska, Aleksandar Cvetkovikj, and Iskra Cvetkovikj. 2025. "Extended-Spectrum β-Lactamase-/AmpC-Producing Escherichia coli and Associated Risk Factors in Shelter Dogs: A Baseline Study in North Macedonia" Microbiology Research 16, no. 9: 206. https://doi.org/10.3390/microbiolres16090206
APA StyleShikoska, I., Duvnjak, S., Koritnik, T., Chapkunovska, B., Vlahov, J., Ratkova Manovska, M., Cvetkovikj, A., & Cvetkovikj, I. (2025). Extended-Spectrum β-Lactamase-/AmpC-Producing Escherichia coli and Associated Risk Factors in Shelter Dogs: A Baseline Study in North Macedonia. Microbiology Research, 16(9), 206. https://doi.org/10.3390/microbiolres16090206






