Abstract
This study evaluates the efficacy of commercial clean-label additives, specifically fermentates, in inhibiting mold growth in vitro and extending the shelf life of preservative-free bread. The mold growth on selected bread was modeled using the time-to-growth approach. The pH, aw, and moisture content of fresh bread were determined. In addition, selected fermentates were characterized physicochemically. Fermentates, defined as liquid or powdered preparations containing microorganisms, their metabolites, and culture supernatants, were tested at varying concentrations (1% to 12%) to assess their antimicrobial performance and impact on bread quality parameters, including moisture content, water activity, and pH. The results showed significant differences in fermentate efficacy, with Product A as the best mold growth inhibitor in vitro and a clear dose-dependent response. For Penicillium corylophilum, inhibition increased from 51.90% at 1% to 62.60% at 4%, while P. chrysogenum had an inhibition ranging from 32.26% to 34.49%. Product F exhibited moderate activity on both molds at 4%, inhibiting between 28.48% and 46.27%. The two molds exhibited differing sensitivities to the fermentates, with P. corylophilum consistently more susceptible to inhibition. Product A displayed a low pH (2.61) and high levels of lactic acid (1053.6 mmol/L) and acetic acid (1061.3 mmol/L). Product F presented a similar pH but lower levels of lactic and acetic acid. A time-to-growth model, validated by significant coefficients (p < 0.05) and high predictive accuracy (R2 > 0.95), was employed to predict the appearance of mold on bread loaves. The model revealed that higher concentrations of fermentates A and F delayed mold growth, with fermentate A demonstrating superior efficacy. At 2% concentration, fermentate A delayed mold growth for 8 days, compared to 6 days for fermentate F. At 8% concentration, fermentate A prevented mold growth for over 25 days, significantly outperforming the control (4 days). Additionally, fermentates influenced bread quality parameters, with fermentate A improving crust moisture retention and reducing water activity at higher concentrations. These findings highlight the potential of fermentates as sustainable, consumer-friendly alternatives to synthetic preservatives, offering a viable solution to the challenge of bread spoilage while maintaining product quality.
1. Introduction
Among bakery products, white bread is a staple food worldwide. Fungal contamination is a significant issue for bread producers, as it results in economic losses and safety hazards, ultimately impacting sensory quality. The short shelf life of white bread is primarily due to microbial spoilage, particularly by molds such as Penicillium and Aspergillus, posing a significant challenge [1,2]. Synthetic preservatives, such as calcium propionate, have been widely used to inhibit mold growth and extend shelf life. However, consumer concerns about the potential health risks associated with synthetic additives have induced the search for natural antimicrobial agents [3,4,5]. Thus, a global demand for clean-label foods, free from synthetic additives, has driven the food industry to explore natural alternatives that ensure safety and quality [6].
In this context, lactic acid bacteria (LAB) have been widely used for centuries in fermented foods because most are Generally Recognized as Safe or Qualified Presumption of Safety [7,8,9]. Thus, they have potential application for food bio-preservation to control fungal growth by secreting antimicrobial compounds that destroy bacterial and fungal cells [5,10]. LAB historically prevent spoilage and extend food shelf life [11], highlighting the importance of microbial metabolites in bio-preservation, which is widely used in the dairy and bakery industries. LAB produce a range of antimicrobial compounds, including organic acids, bacteriocins, bioactive peptides, reuterin, and fatty acids, which have demonstrated effectiveness against spoilage microorganisms in food systems [4,5,12,13]. These natural compounds inhibit microbial growth and align with the clean-label trend, making them attractive for bakery products [14]. The term ‘fermentate’ refers to a liquid or powdered preparation from a fermented product containing microorganisms, their components, culture supernatants, substrates, and various metabolites with potential benefits [5,15]. The antimicrobial activity observed is associated with the bioactive metabolites found in culture supernatants, rather than originating directly from the in situ activity of the lactic acid bacteria strains. Sourdough can be considered a type of fermentate under the given definition, as it is a fermented product containing a complex ecosystem of microorganisms, primarily lactic acid bacteria and yeasts, along with their metabolic byproducts, such as organic acids, exopolysaccharides, and enzymes [5,16]. These components contribute to the unique flavor, texture, and preservation properties of sourdough bread. The microbial activity in sourdough produces culture supernatants and metabolites, such as lactic and acetic acids, which lower pH and inhibit spoilage organisms, aligning with the functional characteristics of fermentates [17]. Additionally, sourdough’s ability to enhance nutritional quality and shelf life further supports its classification as a fermentate [18]. Thus, sourdough exemplifies a natural and traditional form of fermentate, offering technological benefits.
Currently, commercial fermentates are available for foods targeting specific microorganisms. Nisaplin and Natamax are well-known fermentates for combating bacteria and fungi in food applications, respectively. Other commercial fermentates are available on the market for food applications, such as mold and pathogen control [19], mold inhibition [20], flavor enhancement and mold inhibition [21], and controlling the growth of Gram-negative bacteria, yeast, and molds [22], or offering a sourdough line to improve the flavor, texture, and consistent quality of baked products [23]. On the other hand, various researchers have investigated the formulation and application of fermentates or sourdoughs against mold in bread or bread slices [24,25,26,27,28,29,30,31,32,33], with promising results regarding the delay of mold growth. Commercial fermentates (MicroGard, Dupont Danisco, Copenhagen, Denmark) have also been assessed on selected foods as antifungals in sorghum-malt-based fermented milk (MicroGard 100 [34]), cottage cheese (MicroGard 400 [35]), dressings (MicroGard 200 [36]), pound cake (fermentate from cultured dextrose [37]), and bread (four commercial fermentates [38]) with satisfactory results in fungal delaying. Despite this, few studies have evaluated more than one fermentate and assessed their impact on physicochemical properties. Therefore, an approach combining microbiological and physicochemical analyses is essential to assess the effectiveness and feasibility of natural additives in extending the shelf life of preservative-free bread. This study aims to evaluate the efficacy of twelve commercial clean-label additives, particularly fermentates, in inhibiting mold growth in vitro and extending the shelf life of preservative-free bread. The mold growth on selected bread was modeled using the time-to-growth model. The pH, aw, and moisture content of fresh bread were determined. In addition, selected fermentates were characterized physicochemically. By comparing the antimicrobial performance of these commercial natural agents and assessing their impact on bread quality, this research seeks to provide a sustainable and consumer-friendly solution to the challenge of bread spoilage.
2. Materials and Methods
2.1. Materials and Mold Culture Conditions
For this study, commercial products or ingredients (fermentate or extract) were selected based on their technical data sheets, which indicated that they might contain metabolites from LAB and were suitable for use in cereal-based foods. Bioprotectants and cultured food products are pasteurized mixtures or powders designed to improve flavor, extend shelf life, and inhibit microbial growth in various food applications. For each product, its technical data sheet was reviewed, along with product descriptions, usage levels, applications, and potential improvements in bread, to determine the recommended and possible usage doses (Tables S1 and S2). Table 1 shows the products tested in the various experiments, including controls and calcium propionate. Five recommended products for enhancing bread flavor, texture, and stability were selected from Puratos (Pennsauken, NJ, USA): Aroldo (A), Fidelio (F), Panarome (P), Rigoletto (R), and Traviata (T). Additionally, seven products from the DuPont Danisco MicroGard (Copenhagen, Denmark) line were included, namely 100, 200, 300, 520, 730, CS1-50 CO, and 910F.

Table 1.
Selected tested fermentates, application levels, and tests performed.
The bread formulation was based on bakers’ percentages, using wheat flour as the reference (100%). The dough included water (60%), skim milk (2%), sugar (7.79%), salt (1.5%), vegetable oil (1.85%), bread improver (1.925%), and fresh yeast (4%). For each product tested, its addition to the bread was expressed as a percentage, with flour serving as the base (Table 1). All ingredients for the bread formulation were acquired in Puebla, Puebla, Mexico.
Ten pieces of bread were prepared for each commercial ferment or extract, and in some cases, they were evaluated at different concentrations. For breadmaking, the straight dough method was followed; the ingredients were kneaded for 15 min (Legacy HL200 mixer/kneader, Hobart, Troy, OH, USA), and then the dough was weighed (650 g), shaped, placed in suitable casts, and fermented at 35 °C for 60 min. Afterward, they were baked at 200 °C for 21 min (Mini Combo Oven, Zucchelli Alpha, Trevenzuolo, Verona, Italy) and left to cool for 60 min before being packaged in polyethylene bags and stored at room temperature (23 ± 2 °C). Control bread, prepared without any additives, and bread with calcium propionate (0.3%, a typical antifungal agent) were also prepared under the same conditions. For bread formulated with fermentates A and F, a higher quantity of yeast was added for every 4% of fermentate added to the bread formulation (the yeast percentage increase was selected from previous tests.).
2.2. Antifungal Effect of Selected Commercial Fermentates In Vitro
Penicillium corylophilum and P. chrysogenum from the Food Microbiology Laboratory culture collection at Universidad de las Americas Puebla were selected as testing strains due to their common appearance in spoiled bread. The molds were cultured in potato dextrose agar (PDA, Bioxon, BD, Estado de Mexico, Mexico) slants for 7 days at 25 °C. When the surface of the slant was entirely covered with mycelia and spores, 5 mL of sterile 1% Tween 80 (Sigma-Aldrich, St. Louis, MO, USA) aqueous solution was used to recover the spores. The spore count in the suspension was performed using a hematocytometer and adjusted to a concentration of 106 spores/mL.
PDA was prepared, and before pouring, it was added with different concentrations (Aroldo (A) and Fidelio (F) at 1, 2, and 4%, and Panerome (P) at 1.5, 3, and 6%) of fermentates (Table 1). After solidifying the agar, the plates were inoculated (in the center) with 3 μL of a suspension of P. corylophilum or P. chrysogenum spores (~103 spores per dish). For controls, plates without additives were prepared in parallel and inoculated in a similar manner. The inoculated plates were incubated at 27 °C for 4 days. These procedures were carried out in triplicate, and three plates were prepared for each case. The colony diameter was measured with a vernier caliper at right angles in two directions. From these values, the percentage inhibition was calculated by comparing the mold colony diameters exposed to the different treatments with the colony diameter in the control (without fermentate) as follows:
where DC is the diameter of the colony in the control, and DT is the diameter of the colony under treatment.
To identify the antifungal compounds in the commercial fermentates, 50 g of fermentates A and F was neutralized with a 40% (w/v) NaOH solution to a pH of 6.5. The PDA was formulated with 6% and 8% of fermentates A and F, and the neutralized fermentates (pH 6.5) were poured into plates and allowed to solidify. Afterward, 100 μL of a suspension of P. corylophilum spores (~102 spores per dish) was spread on the agar surface, and inoculated Petri dishes were incubated for 5 days at 27 °C. The colonies were then counted, and the result is expressed as Log (N/N0), where N is the count in the tested media and N0 is the count of the initial inoculum.
2.3. Physicochemical Properties of Selected Commercial Fermentates
Titratable acidity (TA), pH, and acetic and lactic acids were analyzed in selected fermentates (A and F). For TA, 5 g of fermentate was weighed, mixed with 10 mL of water, and titrated with 0.1 N NaOH, using phenolphthalein as an indicator. TA was expressed as the primary organic acid, chosen from high-performance liquid chromatography (HPLC) analysis. The pH was measured (in triplicate) using a pH-meter by electrode immersion (Oakton Instruments pH 700, Vernon Hills, IL, USA). The analysis and quantification of acetic and lactic acids were performed using HPLC, with an Agilent 1260 chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a diode-array detector (DAD) set to a wavelength of 210 nm. A mass of 15 g of fermentates were centrifuged at 8000× g for 5 min at 4 °C (Marathon 21K/R, Fisher Scientific, Schwerte, Germany) and filtered through a cellulose nitrate filter with a 0.45 µm pore size (Advantec, MFS, Dublin, CA, USA). The injection volume was 20 µL, and samples were taken by an Agilent G1329 autosampler (Agilent Technologies, Santa Clara, CA, USA). The separation of compounds was performed in an Aminex HPX-87H column (300 × 7.8 mm) (BIO-RAD, Hercules, CA, USA) using a monobasic potassium phosphate buffer (20 mmol/L) solution (pH 2.4, adjusted with phosphoric acid) as the isocratic mobile phase at 0.6 mL/min at room temperature. Acetic and lactic acid standard solutions (30–400 mmol/L) were prepared to quantify the concentrations of these acids. The peak area for each solution was correlated with the concentration using a linear fit, yielding correlation coefficients (R2) greater than 0.99.
2.4. Antimicrobial Effect of Commercial Extracts on Bread
Packaged bread was stored at room temperature (23 ± 2 °C) and observed daily until mold growth was visually detected on the surface to determine the antifungal activity of the fermentate or calcium propionate. Ten pieces of bread without fermentate or extract were also prepared as controls to evaluate the time of visible mold growth. The initial mold contamination in the crust of bread loaves was determined using 10 g of bread crust, diluted with 90 mL of peptone water (0.1 g/100 mL). Three 10-fold dilutions were performed and cultured using acidified PDA (1.4 mL/100 mL agar from a 10 g tartaric acid/100 g solution), and the plates were incubated at 25 °C for 72 h and counted.
2.5. Modeling the Time to Growth
Survival analysis, or more generally, time-to-event analysis, refers to a set of methods for analyzing the length of time until a well-defined endpoint of interest, in this case, the appearance of mold colonies in the bread during storage. A unique feature of survival data is that not all bread pieces typically experience the event by the end of the observation period, so the actual survival times for some cases are unknown. This phenomenon, known as censoring, must be taken into account in the analysis to ensure valid interpretations. The regression model used for time to growth (TTG) was as follows:
where βi represents the model coefficients, T is the type of fermentate (1 for fermentate A and 2 for fermentate F), and C is the concentration tested. The variables were previously normalized for the regression analysis. An actual survival time (when the mold was observed) was classified as a failure, while cases where the mold was not observed were classified as censored. The cut-off time was 25 days of observation during the storage of the loaves of bread. Only significant variables and interactions (p < 0.05) were included in the model construction using Minitab 20 software (Minitab LLC, State College, PA, USA).
2.6. Bread Physicochemical Properties
The crumb and crust from the loaves of bread were carefully separated for moisture content and aw tests, and they were analyzed individually. The bread’s moisture content (crust and crumb) was determined using the AOAC [39] 930.15 method. The bread’s crust and crumb’s water activity (aw) was measured with AquaLab 4TEV Series equipment (Meter Food, Pullman, WA, USA). The pH was determined using the method 945.42 AOAC [39] using the previously mentioned pH meter (Section 2.3).
2.7. Statistical Analysis
Microbial counts and physicochemical analysis data are presented as mean values and standard deviations and were statistically analyzed with one-way ANOVA. Pair comparisons for mean values were performed with Tukey’s test at p < 0.05 to determine whether there are significant differences between the tested products using Minitab 20 software. Principal component analysis (PCA) was conducted to identify correlations between fermentate type, concentration, shelf life, and the physicochemical properties of bread. The data were standardized before PCA to ensure equal weighting of variables. A hierarchical cluster analysis was performed using Euclidean distances to group bread samples based on similarities in shelf life and physicochemical characteristics. These analyses were performed using Minitab 20 software (Minitab LLC, State College, PA, USA).
3. Results and Discussion
3.1. Physicochemical Properties and Antifungal Activity of Selected Fermentates In Vitro
The physicochemical properties of selected fermentates are presented in Table 2. According to Table 2, the selected fermentates and extracts are characterized by acidic pH. This was expected because fermentates are derived from the fermentation of LAB, which produce lactic and acetic acids at high levels (1–3%) [5,40,41]. The active compounds in 0′ sourdough and sponge products likely include organic acids (lactic and acetic acids), fermentation metabolites (such as peptides and exopolysaccharides), and antimicrobial compounds derived from microbial activity, which collectively enhance bread quality and delay mold growth. The pH and TA values observed in the commercial additives align with the manufacturer’s specifications; therefore, the products are of adequate quality. Fermentate A contains higher amounts of acetic and lactic acids (6.07 and 7.87%, respectively) than fermentate F or extract P. Similar values of acetic acid (7.6%) were reported by Samapundo et al. [37,38] for a commercial fermentate derived from cultured wheat solids, whereas lactic acid was at an insignificant level (<0.10%).

Table 2.
Physicochemical properties of selected fermentates.
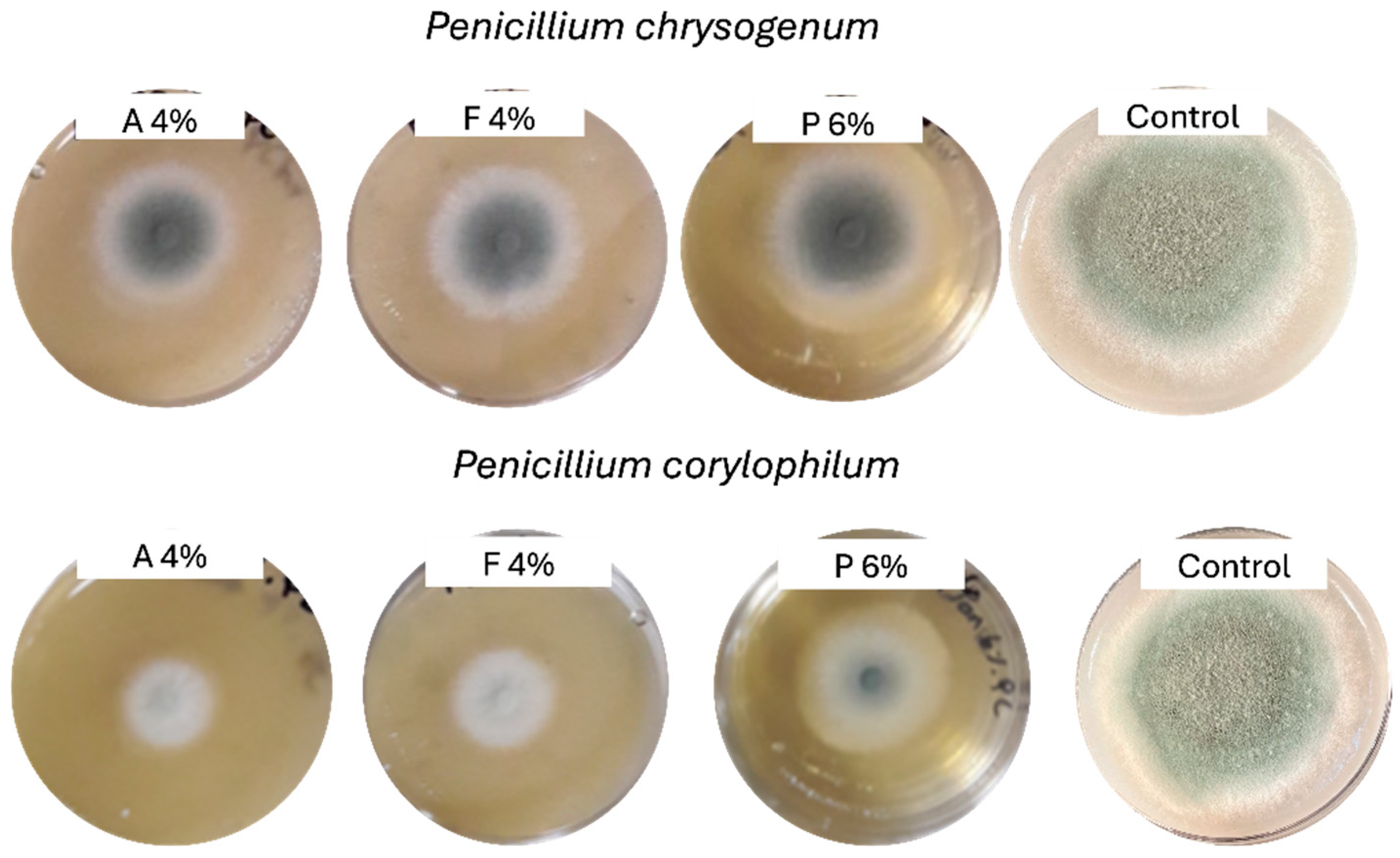
Table 3 shows the radial mold growth inhibition of two molds in vitro after 4 days of exposure to varying concentrations of three products (A, F, and P). Figure 1 displays the mold colonies after 4 days for the highest concentration of tested fermentates. Significant differences (p < 0.05) in the efficacy of the fermentate products were observed, and varying sensitivities to the two molds were identified. Product A was the most effective mold growth inhibitor, exhibiting a clear dose-dependent response. For P. corylophilum, inhibition increased from 51.90% at 1% concentration to 62.60% at 4% concentration, while for P. chrysogenum, inhibition varied from 32.26% to 34.49% over the same concentration range. This suggests that fermentate A has strong antifungal properties and maintains its effectiveness across higher concentrations. In contrast, fermentate F showed moderate inhibitory activity, with its effects being more pronounced against P. corylophilum than P. chrysogenum. For P. corylophilum, inhibition increased from 41.33% (1% concentration) to 46.27% at 4% concentration. However, for P. chrysogenum, inhibition slightly increased from 25.62% to 28.48% over the same concentration range. Extract P, on the other hand, was the least effective of the three products; for P. corylophilum, inhibition increased as the concentration of extract P increased, from 25.22% (1.5% concentration) to 38.81% at 6% concentration. The two molds exhibited differing sensitivities to the fermentates. P. corylophilum was consistently more susceptible to inhibition than P. chrysogenum, as evidenced by higher inhibition percentages across all products and concentrations. For instance, at 1% concentration of fermentate A, P. corylophilum showed 51.90% inhibition, while P. chrysogenum showed only 32.26%. This difference in sensitivity highlights the importance of considering species-specific responses when developing antifungal treatments. Concentration-dependent antifungal activity was previously reported for commercial fermentates against P. chrysogenum and Penicillium paneum [37,38].

Table 3.
Mold growth inhibition (%) in vitro at different concentrations of commercial additives.
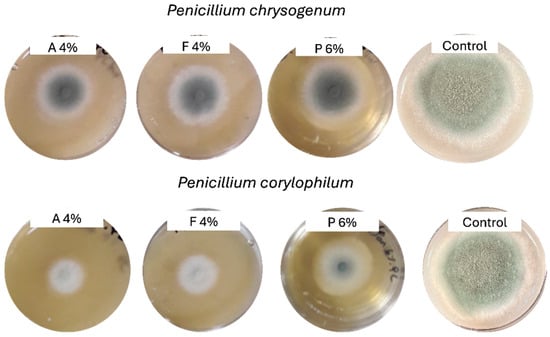
Figure 1.
Radial mold growth in potato dextrose agar supplemented with fermentate A (4%), fermentate F (4%), extract P (6%), and control after 4 days at 27 °C.
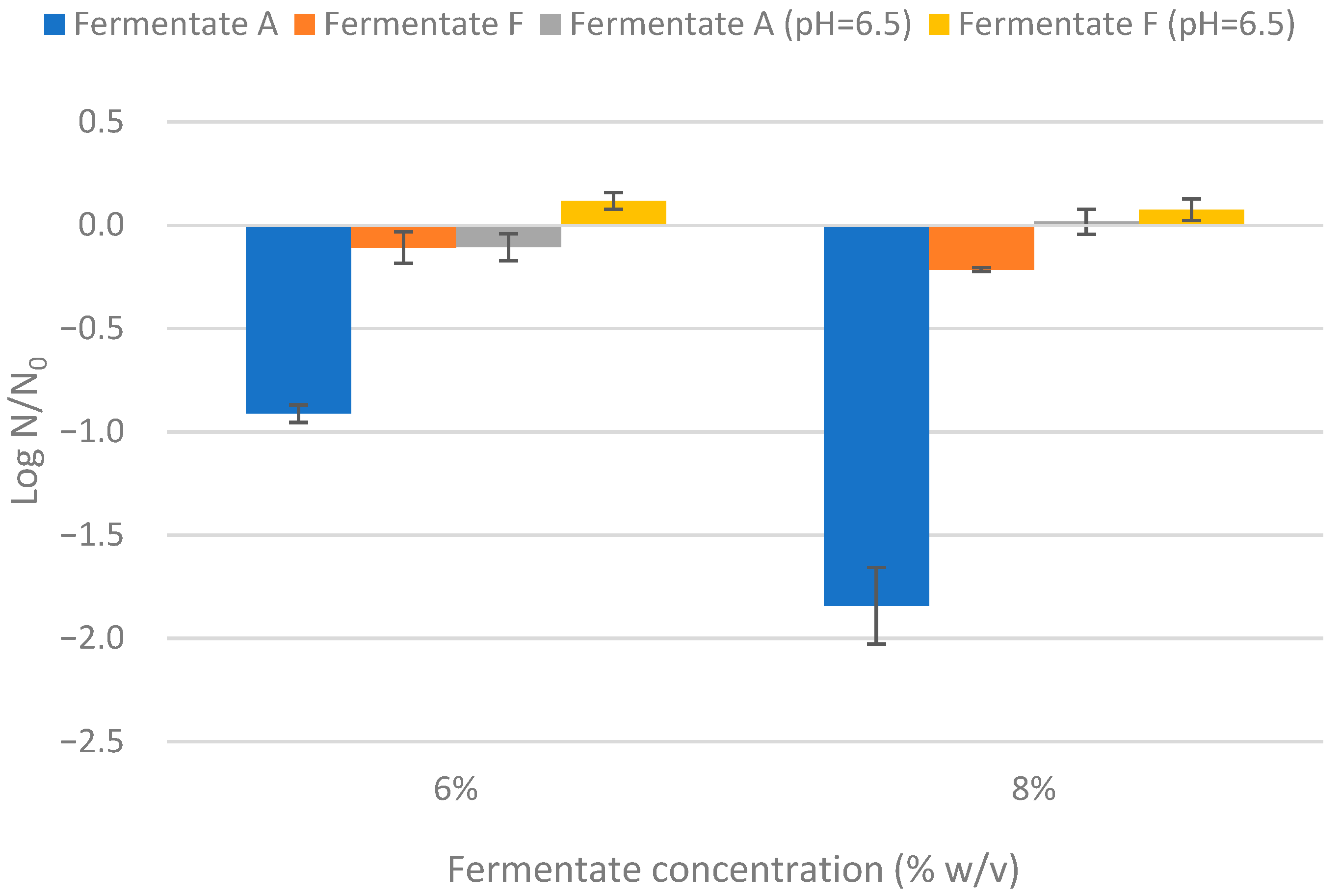
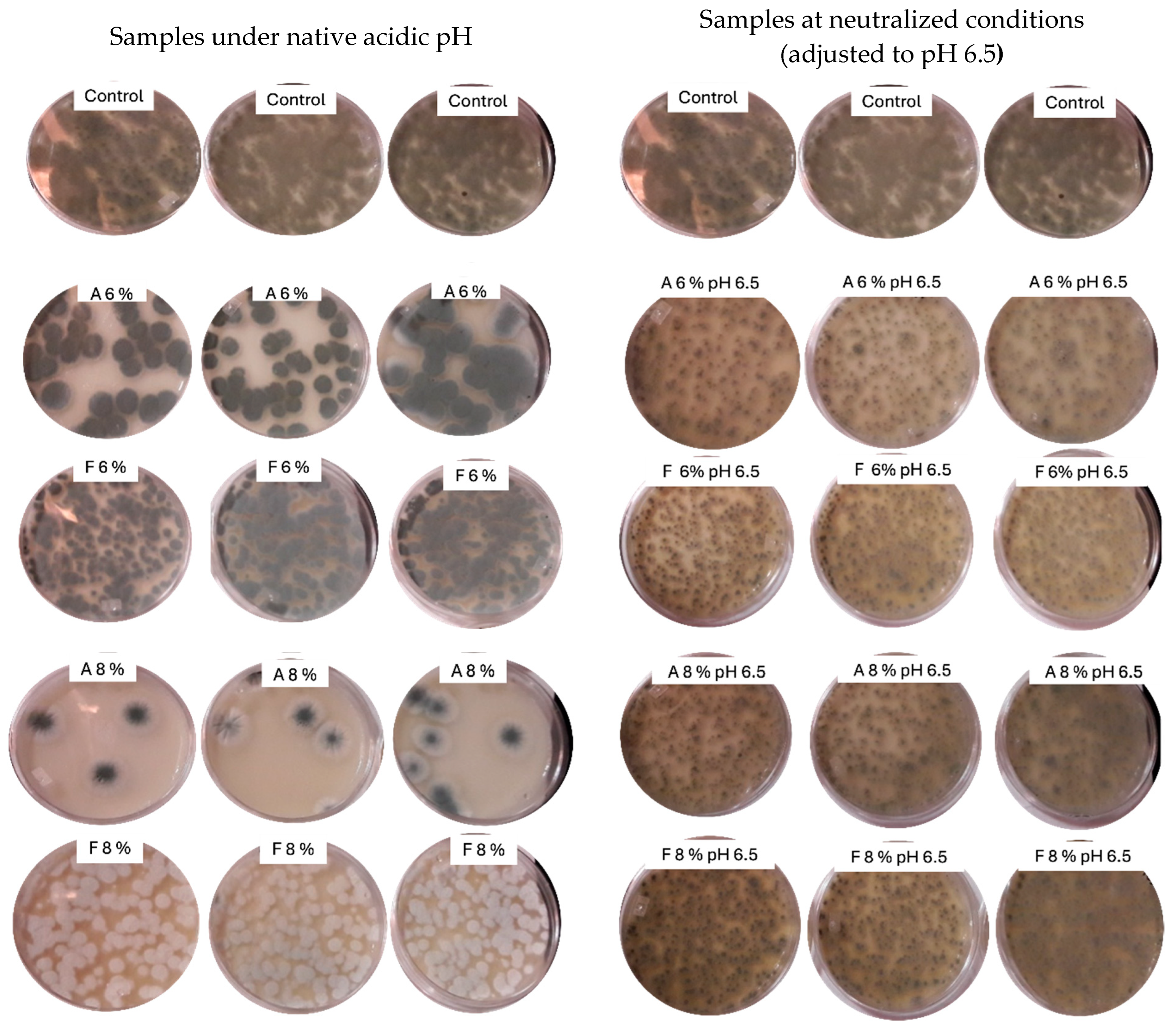
Mold reduction counts for fermentates A and F at two concentrations (6% and 8%) and under native and neutralized pH conditions (pH 6.5) are shown in Figure 2. Fermentate A at 8% achieved the highest reduction in P. corylophilum counts (>1.5 log), while fermentate F showed only a moderate effect (~0.2 log). These findings are consistent with previous radial inhibition assays. As illustrated in Figure 3, the antifungal activity of fermentate A was markedly diminished after pH neutralization, supporting the role of organic acids—primarily lactic and acetic acids—as the main inhibitory agents. Under native acidic conditions, visible inhibition of fungal growth was evident for fermentate A at both concentrations, but this effect was almost completely lost at pH 6.5. In contrast, fermentate F retained limited antifungal activity after neutralization. It is probable that other antimicrobial compounds such as proteinaceous compounds, peptides, reuterin, and esters contribute to the antifungal activity [41].
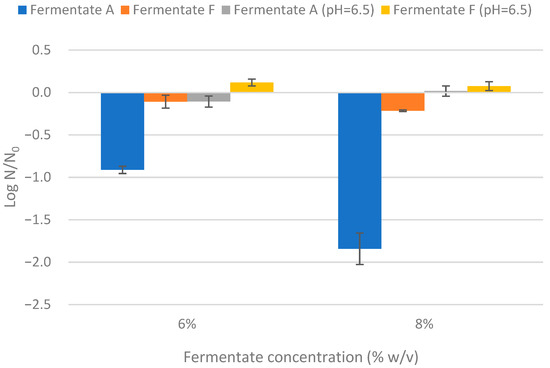
Figure 2.
Effect of pH on the antifungal activity of two commercial fermentates against Penicillium corylophilum.
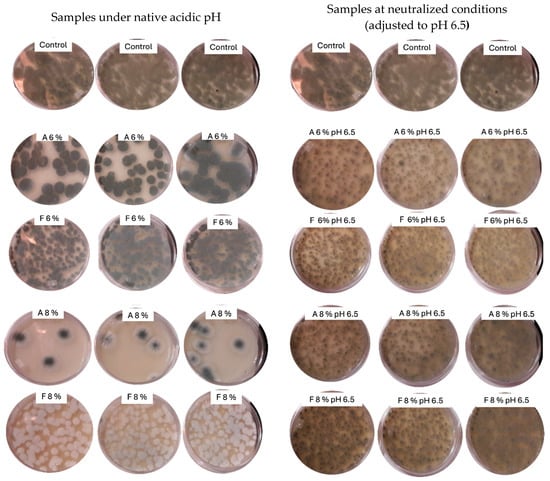
Figure 3.
Growth of Penicillium corylophilum on potato dextrose agar (PDA) supplemented with two commercial fermentates, A and F, at concentrations of 6% and 8% (w/v), under native acidic pH and neutralized conditions (adjusted to pH 6.5).
The effect of pH and antifungal activity in commercial fermentates was previously investigated by Samapundo et al. [37,38]. They also recorded decreases in antifungal activity at the three concentrations tested, at pH 6.5, for four fermentates (ranging from 80 to 91% less) compared with pH 4.5, highlighting the importance of organic acids in antimicrobial activity.
3.2. Antifungal Activity of Fermentates or Extracts on Bread
Initial mold contamination on the bread’s crust was analyzed and found to be low (10 CFU/g). Table 4 displays the time for mold to become visible in bread supplemented with commercial fermentates, extract, or calcium propionate. According to Table 4, only three fermentates (910F, A, and F) delayed the mold growth in bread during storage. In the control bread, mold growth appears within 3 to 5 days. This study revealed significant differences in mold-free shelf life between breads treated with MicroGard fermentates and those treated with calcium propionate. Calcium propionate (0.3%) delayed mold growth until day 19 at 23 ± 2 °C. A shorter time was previously reported for propionate mold growth inhibition on white bread (8 days) [38]. In contrast, 910F (1%) extended the shelf life to 13 days, an improvement over the control (5 days), but still shorter than calcium propionate. Other fermentates like 200 (1.5%), 520 (0.5%), CS1-50 CO (1%), 730 (1.5%), and F (4%) showed moderate extensions (6–8 days), while P (4%), T (4%), R (4%), 100, and 300 (1.5%) displayed marginal benefits (6 days). These results suggest that selected fermentates can delay mold growth, but calcium propionate is the most effective preservative. Likewise, Samapundo et al. [38] recorded variable times of visible mold growth (8–20 days) when using four commercial fermentates at different concentrations to formulate white bread. On the other hand, fermentates A and F at concentrations ≥ 6% inhibited mold growth (18–20 days), similar to calcium propionate, providing an alternative for bread preservation. These fermentates at 8 or 12% retarded mold growth for more than 25 days. Higher concentrations of fermentates generally correlate with more extended periods before mold growth is observed. This implies that elevated concentrations of the fermentates may inhibit mold growth or delay its onset. Although some of the natural fermentates from Puratos were tested at concentrations above the commonly recommended usage levels (>4%), this decision was part of an approach to identify their antifungal potential. The aim was to determine whether these natural ingredients could extend the mold-free shelf life of bread beyond that achieved by conventional additives such as calcium propionate. While some of the fermentates (A and F) were effective only at higher concentrations, the results provide important insight into their dose–response behavior. Such levels may lead to stronger acidic notes and may not yet be cost-effective; however, this work serves as a screening tool to guide future efforts in optimizing formulations for both sensory quality and economic viability.

Table 4.
Storage time to observe mold growth on bread formulated with different fermentates and calcium propionate during storage at 23 °C.
Although higher levels of selected fermentates (>4%) slowed down the yeast activity during dough fermentation, this was counteracted by increasing the yeast amount in the bread formulation (see Section 2.1) to maintain the bread’s volume. However, fermentation intensity may contribute indirectly to mold inhibition by modifying dough acidification or gas production. These changes during fermentation may lead to a drier bread crust and/or a moister bread crumb, which impacts mold growth. In Section 3.4, the physicochemical properties of the bread were described for each of the fermentates tested. For F (≥6%), a lower aw of bread crust was observed, which could favor the mold growth inhibition. In contrast, A (≥6%) slightly reduced aw in bread crust while retarding mold growth on bread. Further research on the microstructural changes in bread could explain the impact of fermentation on mold inhibition.
Axel et al. [42] reported that increasing concentrations of LAB fermentates significantly delayed mold growth in bread, with some formulations extending shelf life by up to 25 days, as observed in this study with fermentate A at 8%. Fermentate A is more effective than fermentate F at delaying mold growth at similar concentrations. This observation aligns with the findings of Arendt et al. [43] and Gerez et al. [1], who highlighted that the efficacy of LAB fermentates is influenced by the specific strains, type of substrate, fermentation duration, and microbial composition utilized in fermentation. Longer periods for visible mold growth (33 to 54 days) were observed on cakes when increasing concentrations of a commercial fermentate (0.5–2%) were added to the formulation [37]. These authors showed that higher concentrations of fermentates more effectively delayed mold growth, as their inhibitory activity increased with a decrease in pH and an increase in concentration. This corresponds with the anticipated dose-dependent effects of active compounds. However, the tested fermentates did not reach the required inhibitory concentration to significantly delay mold growth at lower levels [37].
Fermentates A and F contain acids (Table 2), specifically acetic (1061.3 and 384.7 mmol/L, respectively) and lactic (1053.6 and 1002.1 mmol/L, respectively), which inhibit mold growth, and probably have selected antimicrobial peptides that contribute to their activity. The role of organic acids, such as lactic and acetic acids, in inhibiting mold growth is well documented in the literature. Lactic and acetic acids lower the pH of bread, creating an unfavorable environment for mold growth and thereby inhibiting it [44]. Lower pH levels disrupt the cell membranes of mold and inhibit enzymatic activity, slowing down spore germination and growth. Fermentate A presents high amounts of acetic and lactic acids and slightly more acetic acid than lactic, which is effective on mold spores or mycelial growth as an antifungal [45], leading to longer delays in observable mold growth compared with fermentate F. Similarly, as Gálvez et al. [12] highlighted, acetic acid exhibits strong antifungal properties. This enhances the shelf life of bread. The antimicrobial properties of these fermentates can reduce the need for synthetic preservatives. The results of this study add to the growing body of evidence supporting the use of LAB-derived fermentates as natural alternatives to synthetic preservatives, providing a sustainable and clean-label solution to bread spoilage.
3.3. Time-to-Growth Model for Fermentates A and F
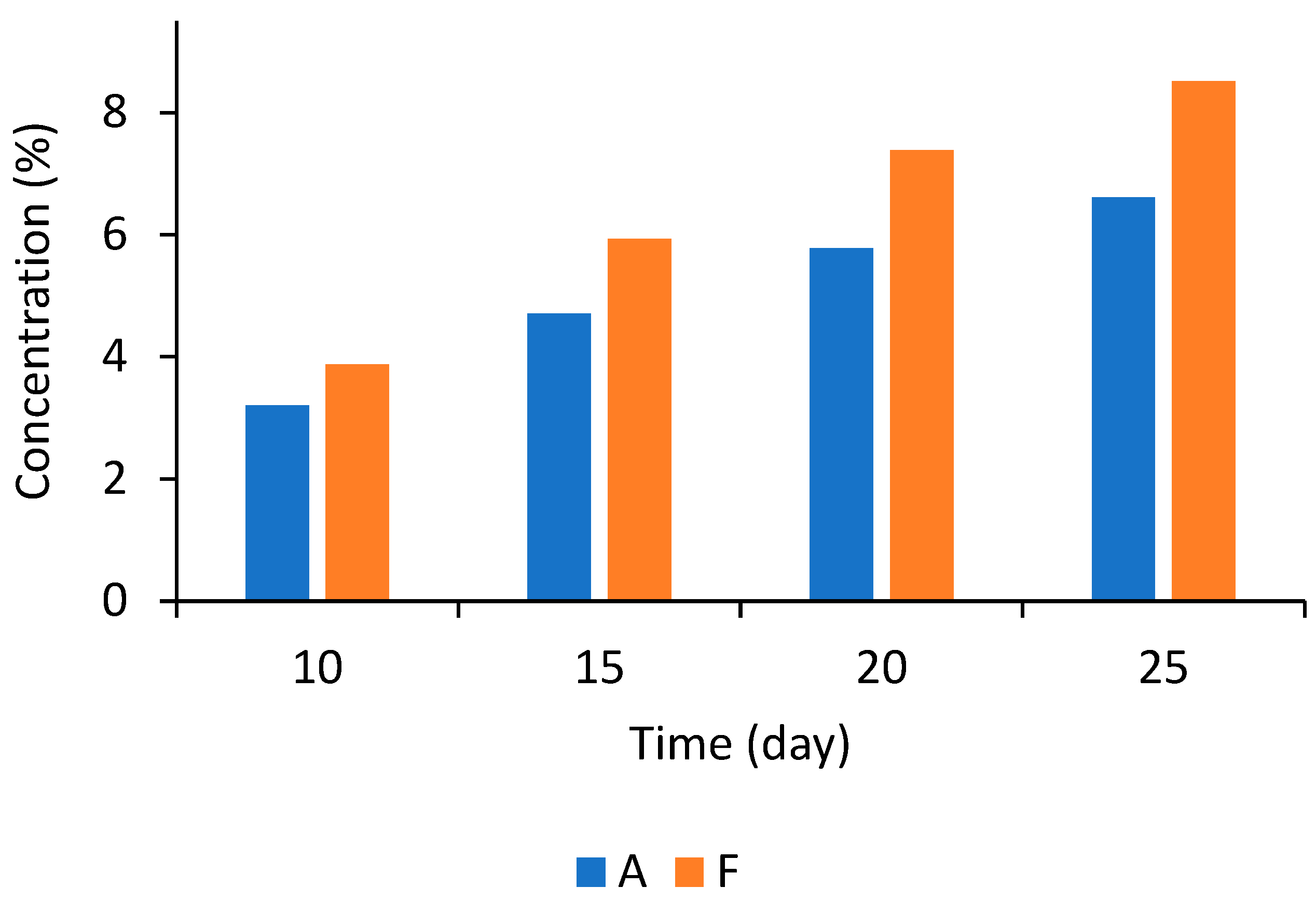
The mold growth rate is not the primary biological response in food products since visible growth is more relevant [46]. The use of time for visible growth models is a choice because they are related to the practical world [47]. The visible growth in a product potentially spoiled by mold depends on processing and formulation characteristics [48]. Therefore, a time-to-growth (TTG) model was employed to determine the storage time for bread to remain mold-free. The TTG model coefficients are found in Table S3. The coefficients were significant (p < 0.05), and the model provided an adequate fit to the experimental data (R2 > 0.95). Therefore, it was used to predict TTG under various conditions, as shown in Figure 4.
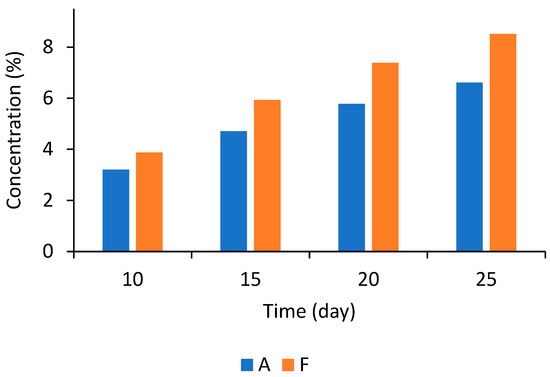
Figure 4.
Time-to-growth mold predictions for bread pieces formulated with fermentates A and F at different concentrations during storage at 23 °C.
Figure 4 shows the predicted time for the appearance of mold colonies on the surface of bread loaves as a function of the concentration of two fermentate products, A and F, using the TTG model. The data indicate that fermentate A, at lower concentrations, shows a longer delay in the appearance of mold compared to fermentate F. As the concentrations of both fermentates increase, the time until mold colonies appear also extends, demonstrating that higher levels of A and F effectively delay mold growth. This positive relationship suggests a dose-dependent response, where greater concentrations of the fermentates enhance their antifungal properties. Likewise, fermentate concentration was significant (p < 0.05) in the time to visible growth for all tested fermentates and molds on malt extract agar supplemented with or without 20% sucrose [37,38]. However, fermentate A exhibits superior efficacy relative to fermentate F. For example, at a concentration of approximately 3%, fermentate A delays mold growth by about 10 days, whereas fermentate F achieves this delay at approximately 4%. This trend continues across the estimated concentrations, highlighting that fermentate A presents a more effective inhibitory effect, likely due to higher organic acid concentrations, primarily acetic acid.
Choosing between the two fermentates depends on the desired shelf life extension and acceptable concentration levels. The data suggest that increasing the concentration of fermentate can significantly delay mold appearance in bread. However, effectiveness varies, with fermentate A showing a stronger response to concentration increases.
3.4. Physicochemical Characteristics of Bread Formulated with Commercial Fermentates
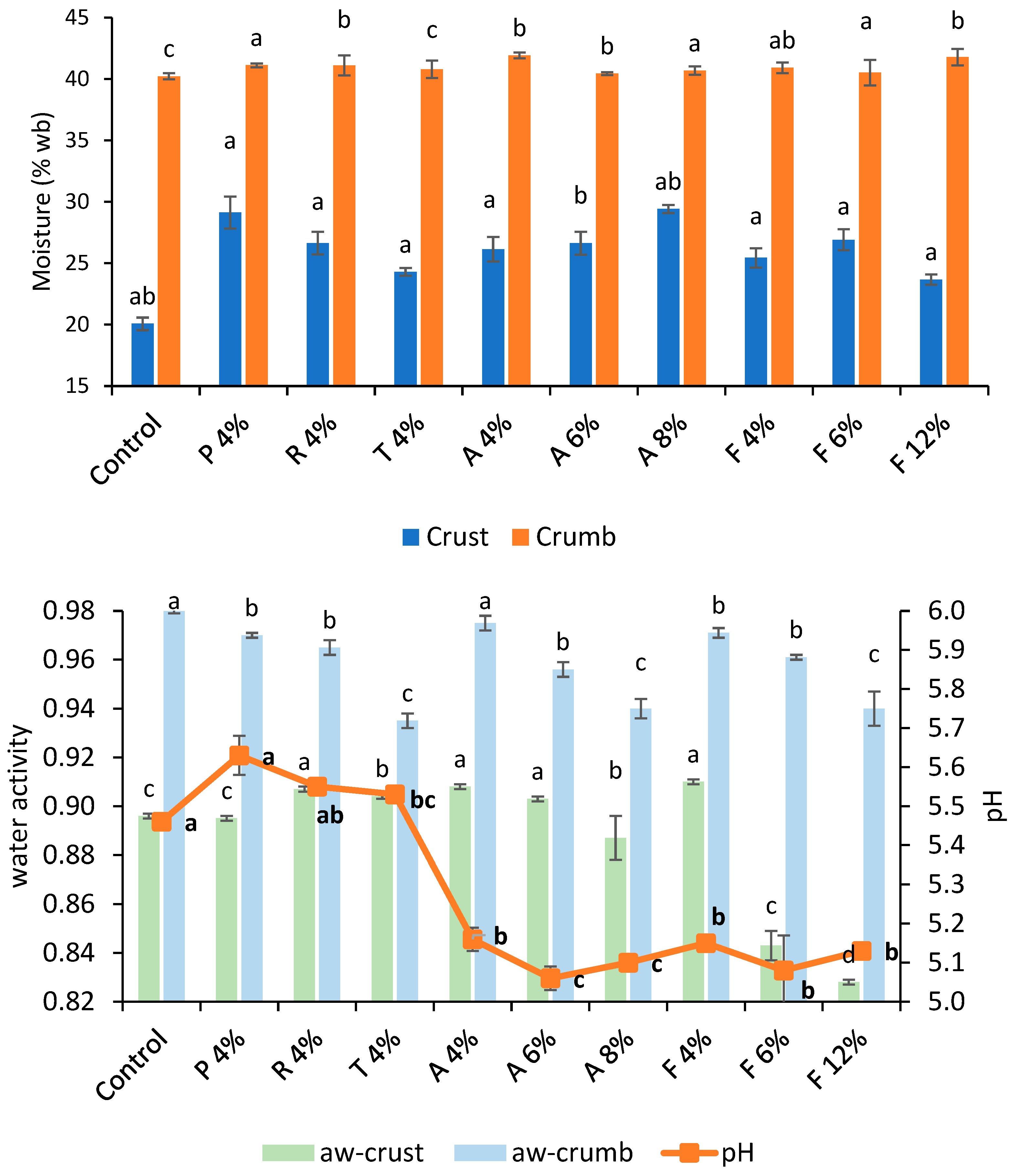
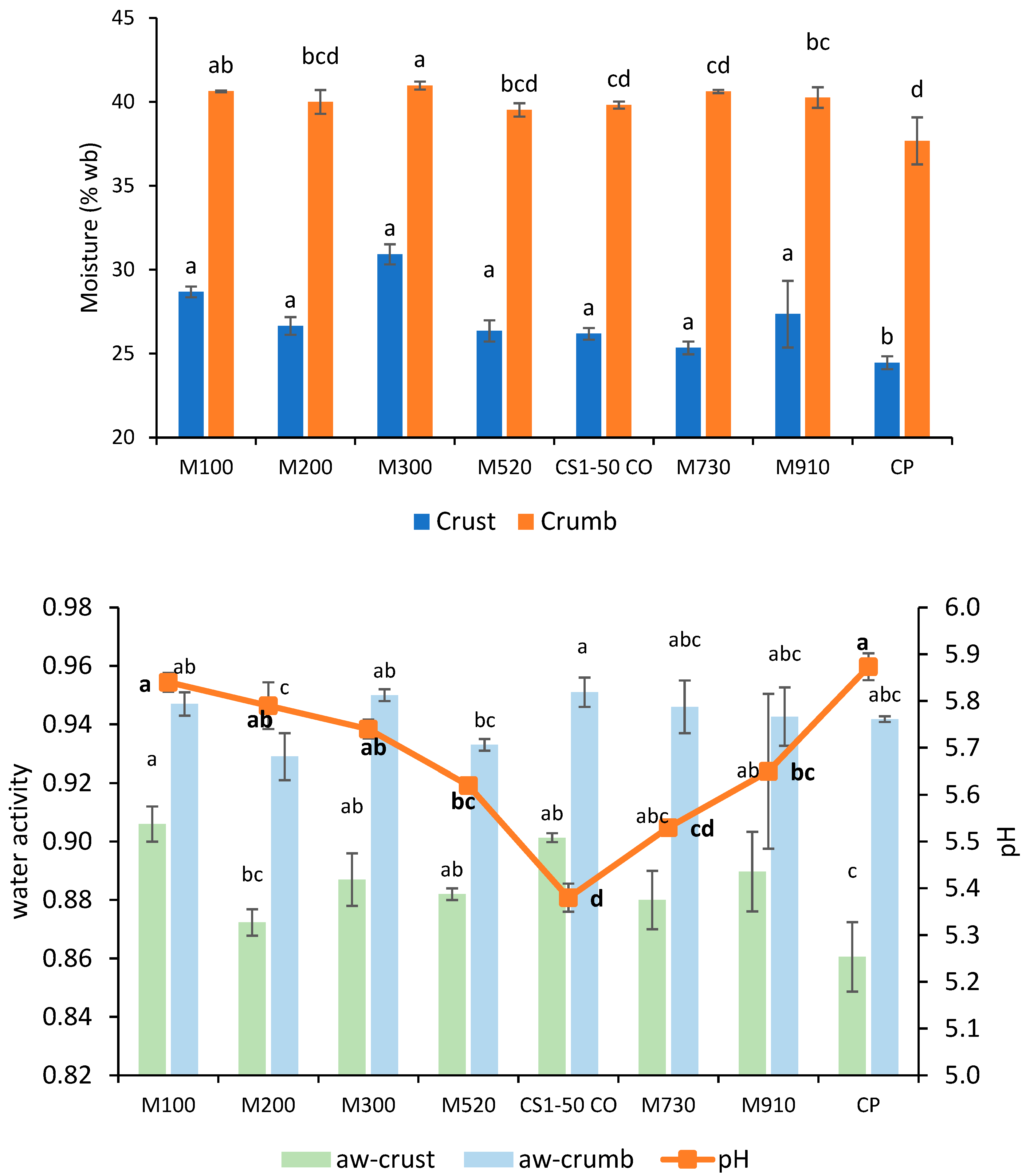
Moisture content and water activity measurements showed the performance differences of additives when incorporated into bread formulations. Figure 5 and Figure 6 exhibit the moisture content, water activity, and pH of bread formulated with commercial fermentates. Bread with fermentates had higher crust moisture levels (increases > 15%), whereas the crumb remained similar to those of the control bread. Bread with the addition of 300 (1.5%) had the highest crust moisture (30.9%), while the control produced the driest crust. Water activity presented a similar trend. pH did not demonstrate a clear relationship with mold inhibition. Calcium-propionate-treated bread had the highest pH (5.87), despite the typical pH-lowering effect of propionic acid, which may be attributed to the formulation’s buffering properties. Bread with fermentates A and F (6–12%), which had the lowest pH (~5.10), delayed mold growth ≥ 18 days, whereas 910F (pH 5.65) performed best among MicroGard’s fermentates, lasting until day 13. This suggests that pH alone does not determine mold inhibition; rather, specific antimicrobial metabolites, present in 910F’s wheat starch base, likely play a role.
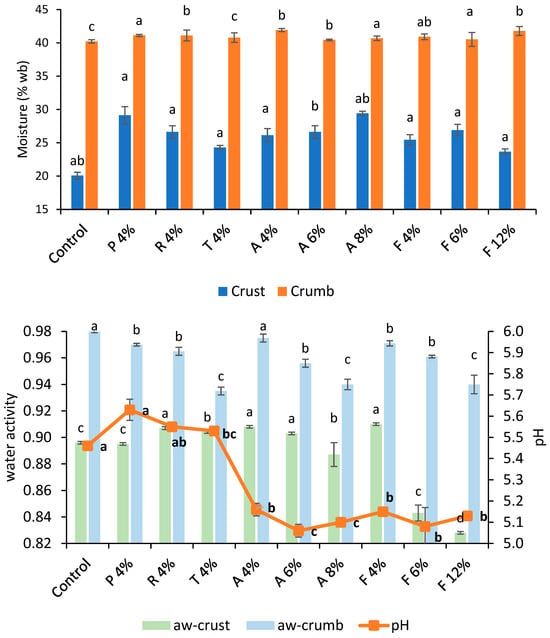
Figure 5.
Moisture content (crumb and crust) (top) of control bread and those formulated with different fermentates at selected concentrations (%); (bottom) water activity (crust and crumb) and pH. Lowercase letters indicate a significant difference (p < 0.05) between breads. Lowercase bold letters indicate a significant difference (p < 0.05) in pH.
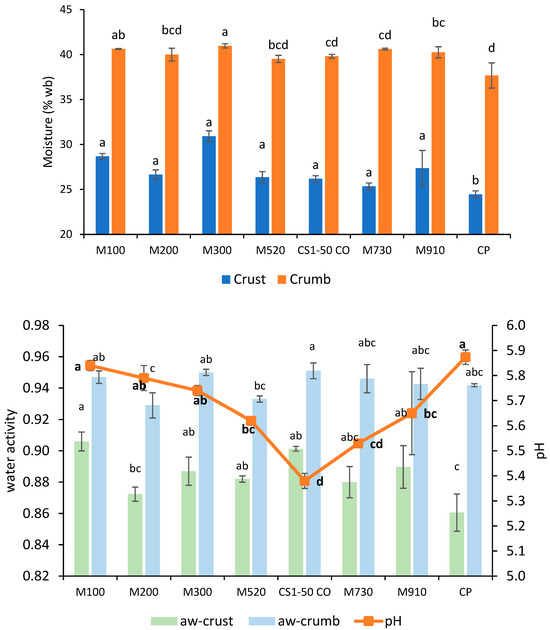
Figure 6.
Moisture content (crumb and crust) (top) of bread formulated with different fermentates and calcium propionate (CP); (bottom) water activity (crust and crumb) and pH. Lowercase letters indicate a significant difference (p < 0.05) between breads. Lowercase bold letters indicate a significant difference (p < 0.05) in pH.
Fermentate A shows an increasing trend in crust moisture content with rising concentration (4% to 8%), ranging from 25.14% to 29.74%. This indicates that higher concentrations of fermentate A may help retain more moisture in the crust during baking. Additionally, several studies have reported that a decrease in pH (Figure 6) activates endogenous wheat proteases, resulting in modifications to the gluten network. This structural change reduces the crumb’s ability to hold water during baking. As a result, the moisture that is not retained migrates toward the crust, increasing its moisture content [32]. Fermentate F at 12% concentration shows a slight decrease in crust moisture compared to at 4% concentration, suggesting that higher concentrations may reduce crust moisture. Fermentates P and R at 4% concentration exhibit higher crust moisture (27.82–30.42%) than fermentate T (23.96–24.6%), indicating variability in moisture retention among fermentates. Among MicroGard fermentates, 300 at a 1.5% concentration resulted in bread with the highest crust moisture (30.32–31.52%), indicating that it retains more moisture than the others. Fermentate 730 at 1.5% exhibits the lowest moisture content (24.96–25.72%), resulting in a drier crust. Crumb moisture content remains relatively stable across all fermentates and concentrations, ranging from 39.52% to 41.91%. This indicates that the crumb is less affected by fermentate type or concentration than the crust. The control bread (0% concentration) has a crumb moisture content similar to bread with fermentates, suggesting that the crumb is less sensitive to the presence or absence of the tested additives.
Fermentate A decreases crust water activity with increasing concentration (0.907–0.909 at 4% to 0.878–0.896 at 8%), indicating that higher concentrations reduce water availability in the crust. The control bread (0% concentration) has the lowest crust water activity (0.845–0.847), consistent with its low moisture content. Fermentate F at 12% concentration shows a significant reduction in crust aw (0.827–0.829) compared to 4% concentration (0.909–0.911), suggesting that higher concentrations of fermentate F reduce aw. Fermentates P, R, and T at 4% concentration show similar crust aw (0.899–0.908), indicating comparable effects. Crumb water activity is generally higher than crust aw, ranging from 0.932 to 0.983, indicating that the crumb retains more free water. Control bread (0% concentration) has the highest crumb aw (0.981–0.983), while fermentate F at 12% concentration shows a reduction in crumb aw (0.933–0.947), suggesting that higher concentrations of fermentate F reduce water availability in the crumb. Fermentate 200 at 1.5% shows low crust aw (0.868–0.877), indicating reduced water availability. In contrast, 100 at 1.5% has a higher crust aw (0.9–0.912), suggesting greater water availability. Fermentate 200 at 1.5% has a slightly lower crumb aw (0.921–0.937), indicating reduced water availability. The crumb is less affected by fermentate type and concentration than the crust, which shows significant moisture content and aw variability. Lower water activity in the crust (fermentate F at 12%) may extend the shelf life by reducing microbial growth.
The pH of bread samples ranges from 5.06 to 5.87, indicating a slightly acidic environment. Fermentates 100 and 200 at 1.5% concentration show higher pH values (5.84–5.79) than other tested fermentates, indicating that these fermentates may produce less acidic bread. Fermentate A exhibits a slight decrease in pH with increasing concentration, from 5.16 at 4% to 5.10 at 8%, indicating that higher concentrations may increase acidity. The data show that the most affected parameter is pH, which decreased by approximately 0.4 units in bread formulated with A, F, 100, and 200. The pH differences among fermentates may influence the flavor profile, with a lower pH (fermentate A) potentially contributing to a more flavorful taste. The pH reduction in bread samples formulated with fermentates A and F (5.03 to 5.68) is consistent with the antimicrobial effects of LAB fermentates, as reported by Gálvez et al. [12]. However, the higher pH values observed with fermentates P and R (5.54–5.68) suggest variability in acid production among fermentates, a finding confirmed by Arendt et al. [43], who noted that the metabolic activity of LAB strains can differ significantly.
Bread formulated with MicroGard fermentates has similar physicochemical properties (moisture content, aw, and pH) or varies slightly compared with the control, except for 200 (aw crust) and CS1-50 CO (pH). Therefore, the general quality was uniform in these bread formulations. The P, R, T, A (4%), and F (4%) fermentates obtained higher crust moisture and aw values than the control, while the pH decreased for A (4%) and F (4%) compared with the control. The lower pH improves the bread’s appeal. Fermentates A (8%) and F (12%) yielded similar crust aw or lower than the control, whereas the pH declined drastically. Also, compact crumb, acidity odor, and flavor were recorded. Future research should include a sensory analysis of formulated bread to evaluate its quality and acceptability when containing fermentates.
The results of this study align with and diverge from existing findings in the current literature on the use of fermentates, particularly those derived from lactic acid bacteria, in bread preservation and quality enhancement. The observed increase in crust moisture content (30–45%) with higher concentrations of fermentate A is consistent with studies demonstrating that certain fermentates can improve moisture retention in baked goods. For instance, Axel et al. [42] reported that LABs fermentates enhanced water-binding capacity in bread, leading to a softer crust and extended shelf life. However, the slight decrease in crust moisture observed with fermentate F at higher concentrations contrasts with some studies, such as those by Gerez et al. [1], which reported that LAB fermentates generally increase moisture retention. This discrepancy may be attributed to differences in the composition and metabolic activity of the specific fermentates. The stability of crumb moisture content across all fermentates and concentrations (39.52% to 41.91%) is consistent with the review by Zhang et al. [49], which states that the crumb is less sensitive to external additives due to its inherent structure and water distribution. Similarly, the higher crumb water activity (0.932 to 0.983) compared to the crust aligns with research by Guynot et al. [50], which highlighted the crumb’s ability to retain free water, making it less susceptible to drying. The reduction in crumb aw with higher concentrations of fermentate F (0.933–0.947) suggests its potential to modulate water availability, a finding supported by Dal Bello et al. [51], who observed that LAB fermentates can alter water activity and inhibit mold growth.
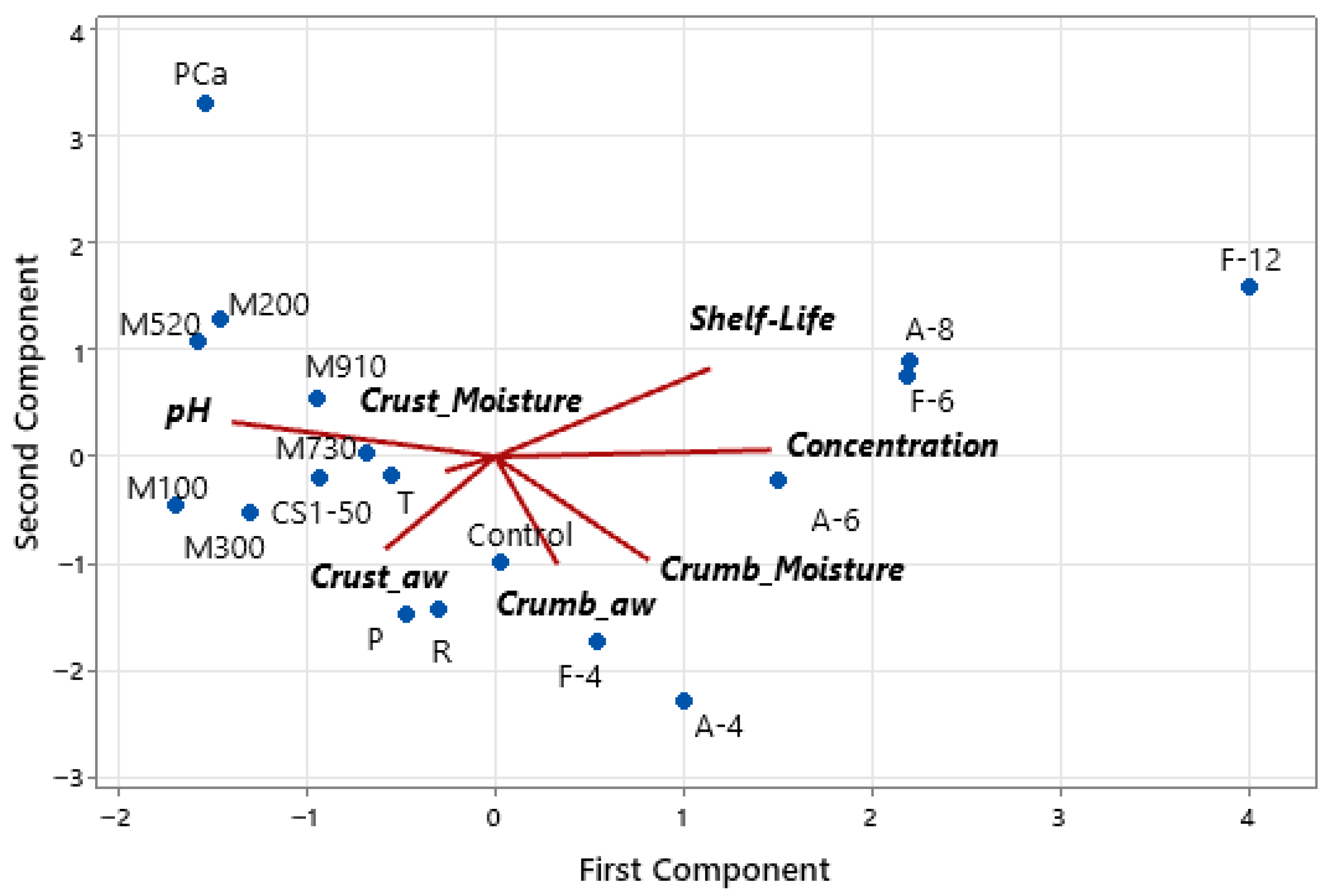
Principal component analysis reveals that the first two principal components account for 82% of the variance, with PC1 accounting for 46% and PC2 accounting for 36%. Figure 7 shows the biplot from the PCA, where it can be observed that the shelf life is positively associated with fermentate concentration, indicating a dose-dependent antifungal effect. Lower crust and crumb aw, as well as reduced crust moisture, are also positively correlated with extended shelf life, reinforcing the role of moisture control in fungal inhibition. pH is inversely related to shelf life, suggesting that lower pH contributes to the antimicrobial efficacy of the formulations.
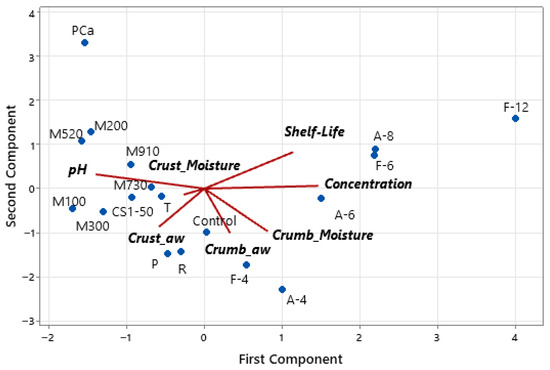
Figure 7.
Biplot of the principal component analysis (PCA) with the distribution of bread samples and the contribution of variables to the first two principal components. The lines indicate the direction and strength of each variable’s contribution to variability among the samples.
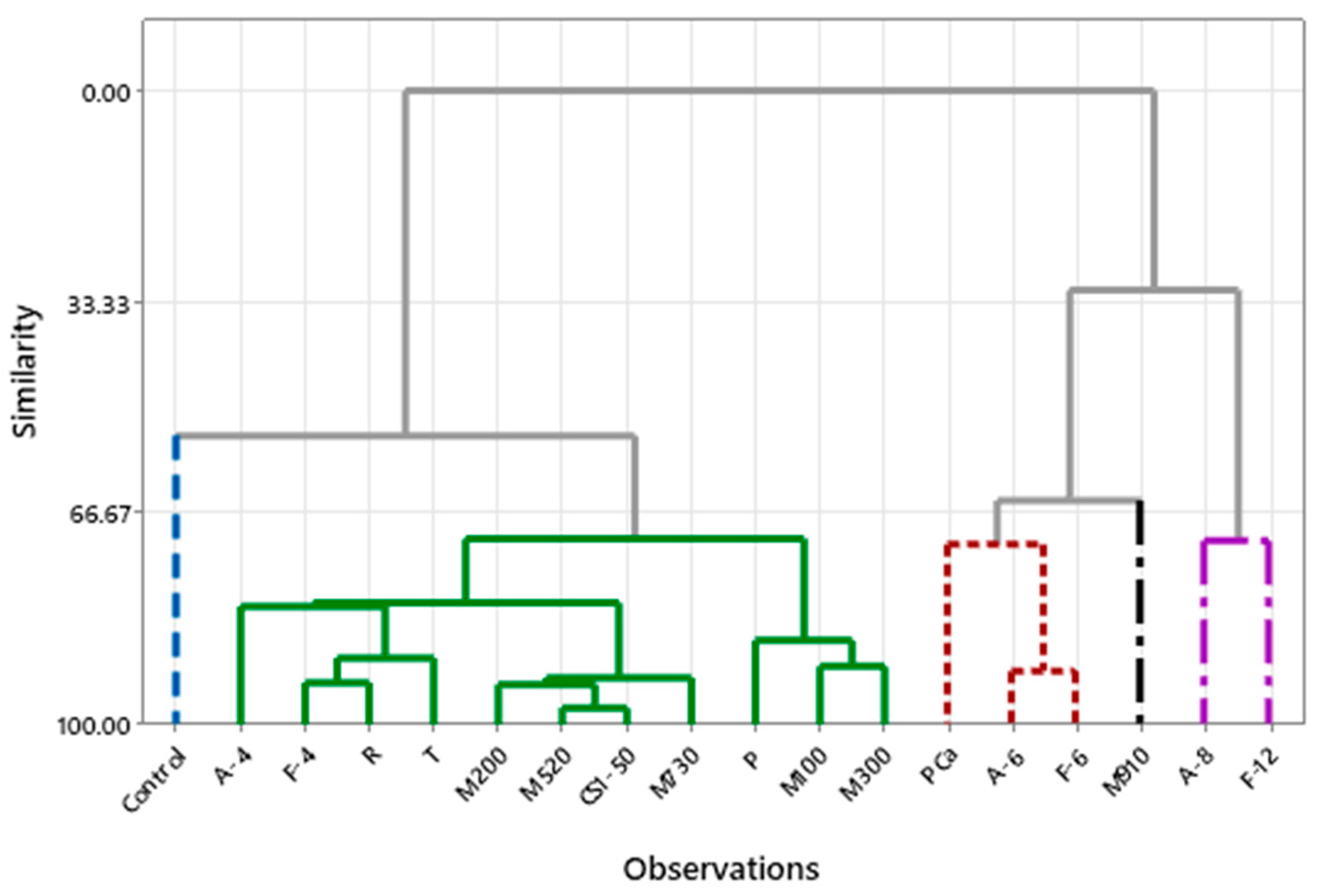
Hierarchical cluster analysis (Figure 8) revealed differentiated groups. One cluster (on the right side of the dendrogram) comprises the most effective samples in terms of mold inhibition, characterized by fermentate A (8%) and fermentate F (12%), which exhibit high additive concentrations, reduced crust aw, and lower pH values. The other cluster includes the control. The remaining fermentates have shelf lives ranging from 6 to 9 days, with formulations that have a lower impact on microbial growth. Bread formulated with calcium propionate (PCa) and fermentates A and F at 6% shares similar characteristics, while fermentate M910 forms another cluster.
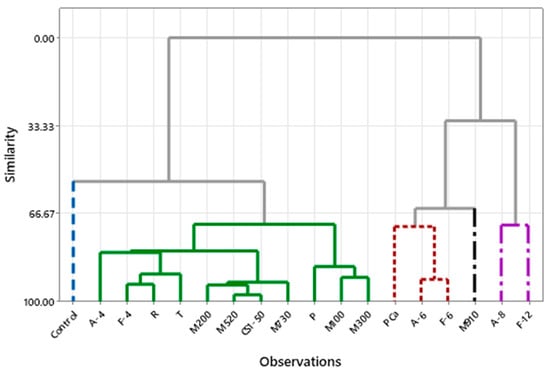
Figure 8.
Hierarchical cluster analysis. Dendrogram of bread samples grouped by similarity in physicochemical characteristics and shelf life. Clusters represent sample groups with similar responses to the type and concentration of added fermentates.
The multivariate approach allowed for a comprehensive understanding of the complex interactions between formulation variables and shelf life extension in bread. Fermentates, especially types F and A, at higher concentrations proved effective in extending mold-free shelf life through mechanisms involving moisture reduction, acidification, and reduced water activity. Overall, the results of this study are consistent with the existing literature on the use of fermentates in bread preservation, particularly their ability to modulate moisture, water activity, and pH. However, the variability observed among different fermentates underscores the importance of strain-specific effects and the need for optimized formulation.
Rahimi et al. [52] evaluate the use of 10% sourdough fermented with Fructilactobacillus sanfranciscensis in bread formulations with an aw of 0.96 and 0.92, either with or without the addition of propionic acid. The authors concluded that using sourdough alone is insufficient to achieve a mold-free shelf life of more than 10 days; however, sourdough can effectively complement preservative systems that extend shelf life. Integrating dried sourdough fermented with LAB Pediococcus pentosaceus TI6 into the production process prolonged the product’s shelf life by as much as 4 days when A. flavus and P. verrucosum were intentionally inoculated [53]. Additionally, using sourdough powder in bread significantly enhances shelf life and minimizes fungal contamination [53,54]. Sourdoughs as bio-preservatives in baking, using L. plantarum and F. sanfranciscensis, showed reduced mold growth in bread, highlighting the effectiveness of these lactic acid bacteria for bio-preservation [55]. The findings contribute to the growing body of evidence supporting the use of LAB-derived fermentates as sustainable, consumer-friendly alternatives to chemically preserved bread.
4. Conclusions
This study demonstrated the antifungal effectiveness of selected LAB-derived fermentates in bread preservation, highlighting their physicochemical properties, in vitro antifungal activity, and practical performance in a food matrix. Fermentates A (4%) and 910F moderately delayed the mold growth by 9–13 days. Other fermentates (200, 520, CS1-50 CO, 730, and F (4%) inhibited mold by 7–8 days, while 100, 300, and T (4%) exhibited a marginal reduction in mold growth. The P (4%) and R (4%) fermentates obtained similar growth to the control. Among the evaluated products, fermentate A exhibited the highest concentrations of lactic and acetic acids and showed superior mold inhibition both in vitro and in bread, with a clear dose-dependent response. The time-to-growth model confirmed that increasing concentrations of fermentate A significantly delayed mold appearance more effectively than fermentate F. Bread formulated with fermentates A and F at concentrations of 6% or higher achieved mold-free shelf lives comparable to those of calcium propionate, extending beyond 25 days at higher concentrations. Moisture content and water activity analyses revealed that the crust characteristics are less affected by the addition of fermentates than the crumb, and lower crust water activity can be associated with extended mold inhibition. While pH alone did not fully explain antifungal efficacy, the combined effect of organic acids and potentially bioactive peptides contributed to the observed preservative action. These findings support the use of LAB fermentates, particularly fermentate A, as natural, clean-label alternatives to synthetic preservatives in bread, aligning with current industry and consumer demands for safer and more sustainable food preservation strategies. Future research should explore these interactions under various storage conditions and evaluate sensory effects to inform commercial decisions. This work serves as a screening to guide future efforts in optimizing formulations for both sensory quality and economic feasibility benefits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16080179/s1.
Author Contributions
Conceptualization, R.H.H.-F., B.M.-G., N.R.-C., A.L.-M. and E.M.-L.; methodology, R.H.H.-F., B.M.-G. and E.M.-L.; formal analysis, R.H.H.-F., B.M.-G., N.R.-C., A.L.-M. and E.M.-L.; investigation, R.H.H.-F., B.M.-G., N.R.-C., A.L.-M. and E.M.-L.; resources, A.L.-M. and N.R.-C.; data curation, R.H.H.-F., B.M.-G., N.R.-C. and E.M.-L.; writing—original draft preparation, R.H.H.-F., B.M.-G., N.R.-C., A.L.-M. and E.M.-L.; writing—review and editing, R.H.H.-F., B.M.-G., N.R.-C., A.L.-M. and E.M.-L.; visualization, R.H.H.-F., B.M.-G., A.L.-M. and E.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request.
Acknowledgments
The authors thank Universidad de las Américas Puebla for its support in carrying out this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gerez, C.L.; Torino, M.I.; Rollán, G.; de Valdez, G.F. Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 2009, 20, 144–148. [Google Scholar] [CrossRef]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Figueroa, R.H.; López-Malo, A.; Mani-López, E. Antimicrobial activity and applications of fermentates from lactic acid bacteria–a review. Sustain. Food Technol. 2024, 2, 292–306. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Statement on the Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, 6174. [Google Scholar] [CrossRef]
- Ayiyi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; da Silva, R.C.; Ibrahim, S.A. Lactic acid bacteria. Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional applications of lactic acid bacteria: Enhancing safety, quality, and nutritional value in foods and fermentes beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Sharma, N.; Kumar, M.; Ozogul, F.; Purewal, S.S.; Trif, M. Recent developments in applications of lactic acid bacteria against mycotoxin production and fungal contamination. Food Biosci. 2021, 44, 101444. [Google Scholar] [CrossRef]
- Ponzio, A.; Rebecchi, A.; Zivoli, R.; Morelli, L. Reuterin, phenyllactic acid, and exopolysaccharides as main antifungal molecules produced by lactic acid bacteria: A scoping review. Foods 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Mokhtari, S.; Khomeiri, M.; Saris, P.E. Antifungal preservation of food by lactic acid bacteria. Foods 2022, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Galle, S. Sourdough: A tool to improve bread structure. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer: New York, NY, USA, 2012; pp. 217–228. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kerry Group PLC. Ingredients for Clean-Label Preservation, Fermentate-Based Ingredients. Available online: https://www.kerry.com/products/food-protection-and-preservation/clean-label-preservation (accessed on 2 April 2025).
- AB Mauri. Mild Inhibitors–Nabitor® 2025. Available online: https://abmauri.ae/product/mold-inhibitors-nabitor/ (accessed on 2 April 2025).
- IFF, International Flavors & Fragances Inc. Bakery 2025. Available online: https://www.iff.com/food-beverage/food-ingredients/food-protection/ (accessed on 2 April 2025).
- Dairy Connection Inc, Fermentates. 2025. Available online: https://dairyconnection.com/antimicrobial/fermentates/?mode=1 (accessed on 2 April 2025).
- Puratos. Sapore 2025. Available online: https://www.puratos.com/products/sapore (accessed on 2 April 2025).
- Coda, R.; Cassone, A.; Rizzello, C.G.; Nionelli, L.; Cardinali, G.; Gobbetti, M. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: Identification of novel compounds and long-term effect during storage of wheat bread. Appl. Environ. Microbiol. 2011, 77, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Cassone, A.; Coda, R.; Gobbetti, M. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 2011, 127, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.A.M.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Black, B.A.; Zannini, E.; Curtis, J.M.; Gänzle, M.G. Antifungal hydroxy fatty acids produced during sourdough fermentation: Microbial and enzymatic pathways, and antifungal activity in bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Fornaguera, M.J.; Obregozo, M.D.; Font De Valdez, G.; Torino, M.I. Antifungal starter culture for packed bread: Influence of two storage conditions. Rev. Argent. Microbiol. 2015, 47, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int. J. Food Microbiol. 2016, 239, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, S.M.; Aly, M.H.; Attia, A.A.; Osman, D.B. Effect of sourdough on shelf life, freshness and sensory characteristics of Egyptian Balady bread. J. Appl. Environ. Microbiol. 2016, 4, 39–45. [Google Scholar]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal capacity of poolish-type sourdough supplemented with Lactiplantibacillus plantarum and its aqueous extracts in vitro and bread. Antibiotics 2022, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal activity of wheat-flour sourdough (Type II) from two different Lactobacillus in vitro and bread. Appl. Food Res. 2023, 3, 100319. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Optimizing lactic acid bacteria proportions in sourdough to enhance antifungal activity and quality of partially and fully baked bread. Foods 2024, 13, 2318. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Garg, F.C.; Pal, D. Effect of different preservative treatments on the shelf-life of sorghum malt based fermented milk beverage. J. Food Sci. Technol. 2014, 51, 1582–1587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makhal, S.; Kanawjia, S.K.; Giri, A. Effect of MicroGARD on keeping quality of direct acidified cottage cheese. J. Food Sci. Technol. 2015, 52, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; De Baenst, I.; Eeckhout, M.; Devlieghere, F. Inhibitory activity of fermentates towards Zygosaccharomyces bailii and their potential to replace potassium sorbate in dressings. LWT—Food Sci. Technol. 2017, 79, 309–315. [Google Scholar] [CrossRef]
- Samapundo, S.; Devlieghere, F.; Vroman, A.; Eeckhout, M. Antifungal properties of fermentates and their potential to replace sorbate and propionate in pound cake. Int. J. Food Microbiol. 2016, 237, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; Devlieghere, F.; Vroman, A.; Eeckhout, M. Antifungal activity of fermentates and their potential to replace propionate in bread. LWT—Food Sci. Technol. 2017, 76, 101–107. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 19th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Sneath, P.H.A.; Mair, N.S.; Sharpe, M.E.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: New York, NY, USA, 1986; Volume 2. [Google Scholar]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Gobbetti, M.; Rossi, J.; Damiani, P. Antimould activity of sourdough lactic acid bacteria: Identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 1998, 50, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Rogawansamy, S.; Gaskin, S.; Taylor, M.; Pisaniello, D. An evaluation of antifungal agents for the treatment of fungal contamination of indoor air environments. Int. J. Environ. Res. Public Health 2015, 12, 6319–6332. [Google Scholar] [CrossRef] [PubMed]
- Dantigny, P. Applications of predictive modeling techniques to fungal growth in foods. Curr. Opin. Food Sci. 2021, 38, 86–90. [Google Scholar] [CrossRef]
- Marín, S.; Freire, L.; Femenias, A.; Sant’Ana, A.S. Use of predictive modelling as tool for prevention of fungal spoilage at different points of the food chain. Curr. Opin. Food Sci. 2021, 41, 1–7. [Google Scholar] [CrossRef]
- Dagnas, S.; Membré, J.-M. Predicting and preventing mold spoilage of food products. J. Food Prot. 2013, 76, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Zhang, Z.; Guan, H.; Zhang, Y.; Xu, D.; Xu, X.; Li, D. Improvement on wheat bread quality by in situ produced dextran—A comprehensive review from the viewpoint of starch and gluten. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13353. [Google Scholar] [CrossRef] [PubMed]
- Guynot, M.E.; Marín, S.; Sanchis, V.; Ramos, A.J. Modified atmosphere packaging for prevention of mold spoilage of bakery products with different pH and water activity levels. J. Food Prot. 2003, 66, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Rahimi, D.; Auld, K.; Sadeghi, A.; Kashaninejad, M.; Ebrahimi, M.; Zhang, J.; Gänzle, M.G. Optimising bread preservation: Use of sourdough in combination with other clean label approaches for enhanced mould-free shelf life of bread. Eur. Food Res. Technol. 2025, 251, 1269–1278. [Google Scholar] [CrossRef]
- Lafuente, C.; Nazareth, T.M.; Dopazo, V.; Meca, G.; Luz, C. Enhancing bread quality and extending shelf life using dried sourdough. LWT—Food Sci. Technol. 2024, 203, 116379. [Google Scholar] [CrossRef]
- Lafuente, C.; Calpe, J.; Musto, L.; Nazareth, T.D.; Dopazo, V.; Meca, G.; Luz, C. Preparation of sourdoughs fermented with isolated lactic acid bacteria and characterization of their antifungal properties. Foods 2023, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.B.; Campos, J.Z.; Kothe, C.I.; Welke, J.E.; Rodrigues, E.; Frazzon, J.; Thys, R.C.S. Type III sourdough: Evaluation of biopreservative potential in bakery products with enhanced antifungal activity. Food Res. Int. 2024, 189, 114482. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).