Abstract
The increasing demand for fresh fruits and vegetables has been accompanied by a rise in foodborne illness outbreaks linked to fresh produce. Traditional antimicrobial washing treatments, such as chlorine and peroxyacetic acid, have limitations in efficacy and pose environmental and worker health concerns. This study evaluated the effectiveness of organic acids (citric, malic, and lactic acid) and power ultrasound, individually and in combination, for the reduction in Salmonella enterica and Listeria monocytogenes on four fresh produce types: romaine lettuce, cucumber, tomato, and strawberry. Produce samples were inoculated with bacterial cocktails at 8–9 log CFU/unit and treated with organic acids at 2 or 5% for 2 or 5 min, with or without power ultrasound (40 kHz). Results showed that pathogen reductions varied based on the produce matrix with smoother surfaces such as tomato, exhibiting greater reductions than rougher surfaces (e.g., romaine lettuce and strawberry). Lactic and malic acids were the most effective treatments, with 5% lactic acid achieving a reduction of >5 log CFU/unit for S. enterica and 4.53 ± 0.71 log CFU/unit for L. monocytogenes on tomatoes. The combination of organic acids and power ultrasound demonstrated synergistic effects, further enhancing pathogen reduction by <1.87 log CFU/unit. For example, S. enterica on cucumbers was reduced by an additional 1.87 log CFU/unit when treated with 2% malic acid and power ultrasound for 2 min compared to malic acid alone. Similarly, L. monocytogenes on strawberries was further reduced by 1.84 log CFU/unit when treated with 5% malic acid and power ultrasound for 2 min. These findings suggest that organic acids, particularly malic and lactic acids, combined with power ultrasound, may serve as an effective hurdle technology for enhancing the microbial safety of fresh produce. Future research can include validating these treatments in an industrial processing environment.
1. Introduction
Consumer demand for fresh fruits and vegetables has increased in recent years due to the desire for a healthier diet with more healthy foods. With the increased consumption of fresh produce, there has also been an increase in the number of foodborne illness outbreaks [1,2]. From 2010 to 2017, a total of 85 multistate foodborne outbreaks associated with fresh produce occurred in the U.S., resulting in a combined total of 4658 illnesses, 1187 hospitalizations, and 55 deaths [2]. A total of 83 of these multistate outbreaks were linked to bacterial pathogens; Salmonella enterica accounted for the majority of these illnesses (81.1%) and hospitalizations (67.5%), while Listeria monocytogenes was associated with the highest proportion of deaths (67.3%). Fresh produce are susceptible to contamination with foodborne bacterial pathogens during cultivation, harvesting, and post-harvesting activities and serve as vectors for pathogens, often providing an environment conducive to their survival, persistence, and even multiplication [3].
Some types of fresh produce, such as leafy greens, tomatoes, and cucumbers, may undergo minimal processing, which includes washing with water supplemented with antimicrobials, such as chlorine, peroxyacetic acid, or trisodium phosphate to remove soil and debris. Antimicrobials are added to the wash water to limit cross-contamination if a pathogen is present on the produce. While washing with chlorine or peroxyacetic acid is generally effective at reducing the microbial population on certain produce items, the efficacy of these antimicrobials may be reduced in the presence of organic material or when used to treat select produce items or pathogens [4,5,6,7,8,9]. Some antimicrobials also produce residues and volatile by-products which can be harmful to both workers and the environment [8,9]. Chlorine and chlorine-based compounds, for example, produce halogenated trihalomethanes and haloacetic acids, which are carcinogenic and can cause skin and respiratory tract irritation; some environmental groups have suggested terminating use of these compounds in food processing [8]. Consequently, there is a need to develop and evaluate alternative technologies to ensure the microbial safety of fresh produce, while also maintaining quality and ensuring the safety of workers and the environment.
One such technology, power ultrasound, is a non-thermal decontamination method which has gained attention in recent years [10,11]. Power ultrasound utilizes frequencies between 20 and 100 kHz and causes damage to bacterial cell wall structures via the phenomenon of acoustic cavitation: the high intensity ultrasound waves cause the generation of gas bubbles which collapse, causing pressure changes, and thus bacterial detachment and/or inactivation [10,12]. Power ultrasound technology is currently used in the medical field as a way to decontaminate equipment and may have applicability to food processing, particularly during the minimal processing of fresh produce [13]. This technology has demonstrated effectiveness for the detachment and inactivation of both native microbiota and foodborne bacterial pathogens on fresh produce items, including leafy greens and tomatoes [14,15,16,17,18,19,20]. Synergistic treatment effects have also been observed when combining power ultrasound with antimicrobials, such as organic acids. For example, treatment of romaine lettuce with 2% lactic acid for 5 min resulted in L. monocytogenes and S. enterica population reductions of 1.30 and 1.73 log CFU/g, respectively; when power ultrasound was also incorporated, populations were further reduced by an additional 1.20 and 0.98 log CFU/g [20].
Organic acids, such as citric, lactic, and malic acid, are generally recognized as safe (GRAS) and have been historically used for the preservation and shelf-life extension of foods [21,22]. Organic acids also have antimicrobial properties, causing a reduction in bacterial populations, growth inhibition via cellular acidification, and membrane permeabilization [22]. In addition to their known antimicrobial properties, their use results in no harmful by-products [22]. The combination of organic acids and power ultrasound may be a viable hurdle technology for the reduction in harmful bacterial pathogens on fresh produce. The objective of this study was therefore to evaluate the efficacy of combined organic acid and power ultrasound hurdle treatments for the reduction of two foodborne pathogens, L. monocytogenes and S. enterica, on different fresh produce matrices.
2. Materials and Methods
2.1. Produce Selection and Preparation
Fresh mini snack cucumbers, romaine lettuce heads, Roma tomatoes and red strawberries were acquired from local retail grocers in the Illinois (IL), USA, area. All produce were stored at 4 °C for up to 24 h prior to use. Cucumbers, tomatoes, and strawberries were placed individually onto weight boats and their weights were recorded. Romaine lettuce was chopped into pieces of approximately 3 cm × 6 cm and 25 g portions were placed into weight boats. Samples each consisted of one unit of each of the produce (one whole cucumber, tomato, or strawberry, or 25 g of romaine lettuce).
2.2. Strains and Culture Conditions
Four-strain cocktails of Salmonella enterica and Listeria monocytogenes were used in this study. The S. enterica strains used were S. Poona (8785, isolated from cucumbers [23]), S. Alachua (isolated from peach tree leaf), S. Agona (447967, isolated from roasted oats cereal), and S. Enteritidis (PT30, isolated from almonds). The L. monocytogenes strains used were ScottA (clinical isolate), LS3132 (isolated from avocado), LS810 (isolated form cantaloupe [24]) and 573-035 (isolated from caramel apple [25]). All strains used were resistant to rifampicin (100 µg/mL). All strains were cultured individually in 25 mL of Tryptic Soy Broth (TSB; Becton, Dickinson and Company, Sparks, MD, USA) at 37 °C for 16–18 h. Cultures were centrifuged at 6000× g for 10 min and cell pellets were resuspended in 2.5 mL Butterfield’s Phosphate Buffer (BPB, pH 7.2). The four strains of L. monocytogenes or S. enterica were combined to create a four-strain cocktail (10 mL total) of approximately 10 log CFU/mL. The cocktails were serially diluted and plated onto Brain Heart Infusion Agar (BHIA) to verify initial population levels.
2.3. Inoculation of Produce
The produce samples were each inoculated with 100 µL of the L. monocytogenes or the S. enterica cocktail by placing 10 dots of 10 µL each onto the surface, avoiding the pedicel juncture. For romaine lettuce, both cut and uncut surfaces were inoculated. The inoculum was then allowed to dry on the produce surfaces for 1 h in a biosafety cabinet.
2.4. Treatment of Produce
2.4.1. Treatment with Organic Acids
Malic, citric, and lactic acid (Fisher Scientific, Walthman, MA, USA) were used in this study. Treatment solutions were prepared at 2 or 5% (w/v) for citric acid (pH 2.41 ± 0.29 and 2.08 ± 0.20, respectively) and malic acid (pH 2.10 ± 0.35 and 1.79 ± 0.56, respectively), or v/v or lactic acid (pH 2.03 ± 0.32 and 1.76 ± 0.29, respectively). Produce samples were submerged in 225 mL of water (control) or 2 or 5% of treatment solution in 710 mL-capacity stomacher bags for 2 or 5 min. After treatment, the produce was immediately removed from the water or treatment solution using sterile tongs and placed into 225 mL BPB in a new stomacher bag. For each trial, triplicate samples were evaluated for each produce type, pathogen, and treatment combination. Three independent trials were conducted.
2.4.2. Treatment with Organic Acids and Power Ultrasound
Produce samples were treated as described in Section 2.4.1 with the addition of power ultrasound. Ten liter-capacity power ultrasound bath units were used in this study (30 W/L, TH-SPQXJ-40A, Vevor, Shanghai, China) at 40 kHz. Prior to experiments, the units were degassed for 10 min. Samples were submerged in 225 mL of water (control), or 2 or 5% of treatment solution in 710 mL-capacity stomacher bags for 2 or 5 min inside the bath power ultrasound unit at ambient temperature (20–22 °C). During ultrasound treatment, the water temperature in the ultrasound unit increased by approximately 0.5 °C/min, resulting in temperatures of 21–23, 22.5–24.5, and 25–27 °C after 2 and 5 min, respectively. After treatment, the produce was immediately removed from the water or treatment solution using sterile tongs and placed into 225 mL of BPB in a new stomacher bag. For each trial, triplicate samples were evaluated for each produce type, pathogen, and treatment combination. Three independent trials were conducted.
2.5. Enumeration of L. monocytogenes and S. enterica
Produce samples were stomached for 1 min (Paddle Lab Blender-Masticator, NEU-TEC Group, Inc., Farmingdale, NY, USA). Samples were then serially diluted and plated onto Brain Heart Infusion Agar supplemented with rifampicin (BHIArif) (100 μg/mL). Agar plates were incubated at 37 °C for 24–48 h prior to enumeration. The limit of detection of the plate count assay was 2.25 log CFU/unit. Pathogen population data were expressed as log CFU/unit (see Section 2.1).
2.6. Statistical Analysis
Three independent trials were performed with triplicate samples for each condition (n = 9). Significant differences in population reductions in L. monocytogenes and S. enterica on produce treated with water for 2 or 5 min, without or with power ultrasound, were statistically determined using two-way analysis of variance (ANOVA) with Tukey’s post hoc test. Significant differences in population reductions in L. monocytogenes or S. enterica on produce treated with citric, malic, or lactic acid at 2 or 5% for 2 or 5 min, without or with power ultrasound, were also determined using ANOVA with Tukey’s post hoc test. A p-value ≤ 0.05 was considered significant.
3. Results
3.1. Efficacy of Water Alone or in Combination with Power Ultrasound to Reduce Pathogen Populations on Produce
Figure 1 displays the population reductions in both L. monocytogenes and S. enterica on cucumber, romaine lettuce, tomato, and strawberry treated with water for 2 or 5 min, without or with power ultrasound. For L. monocytogenes, the initial population levels were 8.54 ± 0.22, 8.98 ± 0.06, 8.49 ± 0.36, and 8.43 ± 0.09 log CFU/unit for cucumber, romaine lettuce, tomato, and strawberry, respectively. For S. enterica, the initial population levels were 8.34 ± 0.21, 8.41 ± 0.15, 7.41 ± 0.20, and 7.73 ± 0.31 log CFU/unit, respectively. With water alone, L. monocytogenes populations were reduced by 0.73 ± 0.10 (tomato) to 1.31 ± 0.27 log CFU/unit (cucumber) after 2 min and by 1.17 ± 0.24 (strawberry) to 1.82 ± 0.23 log CFU/unit (tomato) after 5 min. With the addition of the power ultrasound, no significant further reductions occurred for L. monocytogenes when treated for the same length of time. However, some differences were observed when comparing different treatment lengths, with or without the incorporation of ultrasound. For example, in the case of tomatoes, treatment for 5 min with water and power ultrasound resulted in a greater population reduction (2.33 ± 0.19 log CFU/unit) compared to treatment with water alone for 2 min (0.73 ± 0.10 log CFU/unit).

Figure 1.
Population reductions in (A) Listeria monocytogenes and (B) Salmonella enterica on cucumber, lettuce, strawberry, and tomato treated with water for 2 or 5 min, with or without power ultrasound. Data are mean values ± standard deviation (n = 9). Different lowercase letters indicate that means are significantly different in the same treatment groups (the same treatment length, with or without ultrasound). Data within a treatment group without lowercase letters are not significantly different.
For S. enterica, with water alone, populations were reduced by 0.81 ± 0.44 (tomato) to 1.23 ± 0.33 log CFU/unit (romaine lettuce) after 2 min and by 0.88 ± 0.74 (strawberry) to 3.04 ± 0.76 log CFU/unit (tomato) after 5 min. Similar to L. monocytogenes, With the addition of the power ultrasound, no significant further reductions occurred for S. enterica when treated for the same length of time. However, like L. monocytogenes, some differences were observed in S. enterica reductions when comparing different treatment lengths. For tomatoes, treatment for 5 min with water alone or in combination with power ultrasound resulted in greater population reductions (3.04 ± 0.76 and 3.12 ± 0.85 log CFU/unit) compared to the 2 min treatment (0.81 ± 0.44 and 0.85 ± 0.42 log CFU/unit), respectively.
3.2. Reduction in Pathogen Populations on Produce Treated with Organic Acids
Figure 2 displays the population reductions in L. monocytogenes on cucumber, romaine lettuce, tomato, and strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min. The higher concentration of organic acid and/or the longer treatment time did not always result in an increased reduction in L. monocytogenes. For example, no significant difference in the reduction in L. monocytogenes was observed on romaine lettuce when the concentration of organic acid increased from 2 to 5% or when the treatment length increased from 2 to 5 min; population reductions ranged from 1.27 ± 0.07 (2% citric, 2 min) to 2.70 ± 0.51 log CFU/unit (5% lactic, 2 min). For cucumber, the greatest population reduction was observed with 5% lactic acid when treatment occurred for 5 min (4.04 ± 0.49 log CFU/unit compared to 2.27 ± 0.26 log CFU/unit with the 2 min treatment). For tomato, the greatest L. monocytogenes reductions were observed with the use of 5% malic or lactic acid for 5 min (3.59 ± 0.68 and 4.53 ± 0.71 log CFU/unit, respectively). Similarly, for strawberry, 5% malic and lactic acid for 5 min were also the most effective; population reductions were 3.65 ± 0.79 and 3.29 ± 0.71 log CFU/unit, respectively.
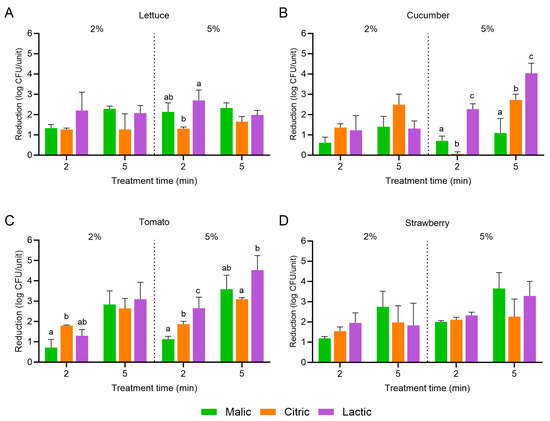
Figure 2.
Population reductions in Listeria monocytogenes on (A) lettuce, (B) cucumber, (C) tomato, and (D) strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min. Data are mean values ± standard deviation (n = 9). Different lowercase letters indicate that means are significantly different in the same treatment groups (the same treatment concentration and length). Data within a treatment group without lowercase letters are not significantly different.
Figure 3 depicts the population reductions in S. enterica on cucumber, romaine lettuce, tomato, and strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min. In general, greater population reductions on the produce matrices were observed for S. enterica than for L. monocytogenes for the same treatments. For romaine lettuce, both 2% and 5% malic and lactic acid were similarly effective at reducing S. enterica by 3.71 ± 0.25 and 3.00 ± 0.70 log CFU/unit after 5 min treatment, respectively. For cucumber, 5 min treatment with 2% malic or citric acid or 5% treatment with any of the three acids similarly reduced S. enterica; the greatest population reduction was achieved by 5% lactic acid after 5 min (5.00 ± 0.18 log CFU/unit compared to 0.97 ± 0.25 log CFU/unit for 2% lactic acid at the same treatment length). Lactic acid, even at 2% concentration, reduced S. enterica on tomatoes by >5.16 log CFU/unit after only 2 min. The same reduction on tomatoes was also observed with 5% malic acid after 5 min. For strawberry, all three acids at 5% similarly reduced the population of S. enterica after 5 min: the population was reduced by 2.89 ± 0.56 (citric) to 4.59 ± 1.03 log CFU/unit (lactic).

Figure 3.
Population reductions in Salmonella enterica on (A) lettuce, (B) cucumber, (C) tomato, and (D) strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min. Data are mean values ± standard deviation (n = 9). Different lowercase letters indicate that means are significantly different in the same treatment groups (the same treatment concentration and length). Data within a treatment group without lowercase letters are not significantly different. The asterisk (*) indicates that population reductions were >5.16 log CFU/unit.
3.3. Reduction in Pathogen Populations on Produce Treated with Organic Acids in Combination with Power Ultrasound
Figure 4 and Figure 5 display the population reductions in L. monocytogenes and S. enterica, respectively, on cucumber, romaine lettuce, tomato, and strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min with the combination of power ultrasound. On romaine lettuce and cucumber, no significant further population reduction was achieved with the combined organic acid and power ultrasound treatments compared to when the organic acid treatment was conducted alone for the same lengths of time. For tomato and strawberry, populations of L. monocytogenes were significantly further reduced for some of the combination organic acid and power ultrasound treatments. For tomato, the combination of 5% malic acid and power ultrasound for 2 min resulted in a L. monocytogenes population reduction of 2.45 ± 0.22 log CFU/unit, compared to a reduction of only 1.13 ± 0.14 log CFU/unit when the organic acid was used alone: a further 1.32 log CFU/unit reduction with the combination treatment. In addition, the combination of 5% malic or lactic acid with power ultrasound for 2 min reduced the population of L. monocytogenes on strawberry by 3.84 ± 0.47 and 3.25 ± 0.22 log CFU/unit, respectively, compared to the organic acids alone (2.00 ± 0.06 and 2.32 ± 0.16 log CFU/unit); the combination treatments reduced L. monocytogenes by an additional 1.84 and 0.93 log CFU/unit.
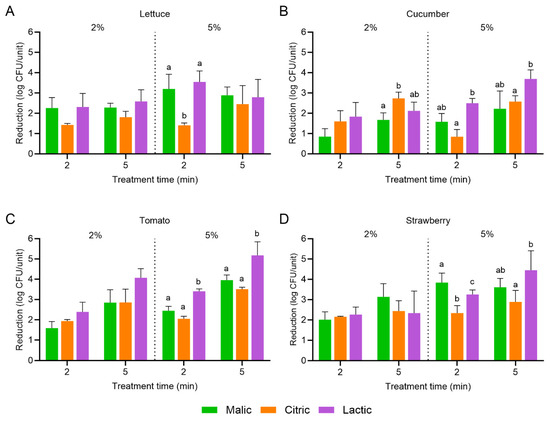
Figure 4.
Population reductions in Listeria monocytogenes on (A) lettuce, (B) cucumber, (C) tomato, and (D) strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min with power ultrasound. Data are mean values ± standard deviation (n = 9). Different lowercase letters indicate that means are significantly different in the same treatment groups (the same treatment concentration and length). Data within a treatment group without lowercase letters are not significantly different.
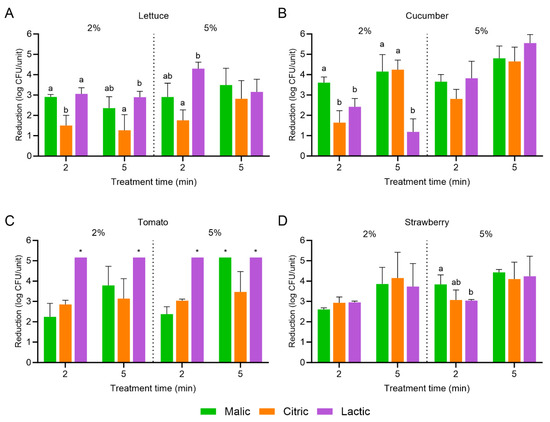
Figure 5.
Population reductions in Salmonella enterica on (A) lettuce, (B) cucumber, (C) tomato, and (D) strawberry treated with malic, citric, or lactic acid at 2 or 5% for 2 or 5 min with power ultrasound. Data are mean values ± standard deviation (n = 9). Different lowercase letters indicate that means are significantly different in the same treatment groups (the same treatment concentration and length). Data within a treatment group without lowercase letters are not significantly different. The asterisk (*) indicates that population reductions were >5.16 log CFU/unit.
For S. enterica, populations were significantly further reduced on romaine lettuce, cucumber, and strawberry for some of the combination organic acid and power ultrasound treatments; no significant further reductions were observed on tomato. On romaine lettuce, the combination of 5% lactic acid and power ultrasound for 2 min resulted in a population reduction of 4.30 ± 0.32 log CFU/unit, compared to a reduction of 2.69 ± 0.39 log CFU/unit when the organic acid was used alone for the same treatment length: a further 1.61 log CFU/unit reduction with the combination treatment. On cucumber, the combination of 2 or 5% malic acid and power ultrasound for 2 min resulted in population reductions of 3.61 ± 0.28 and 3.65 ± 0.35 log CFU/unit, respectively, compared to reductions of 1.74 ± 0.63 and 2.66 ± 0.07 log CFU/unit when the organic acids were used alone: the S. enterica population was further reduced by 1.87 and 0.99 log CFU/unit with the combination treatments. For strawberry, the combination of 2 or 5% malic acid and power ultrasound for 2 min resulted in population reductions of 2.61 ± 0.08 and 3.84 ± 0.47 log CFU/unit, respectively, compared to reductions of 1.19 ± 0.09 and 2.00 ± 0.06 log CFU/unit when the organic acids were used alone: the populations were further reduced by 1.42 and 1.84 log CFU/unit with the combination treatments.
4. Discussion
Traditional methods of washing fresh produce with chlorine or chlorine-based compounds have limitations, including the creation of harmful by-products, limited efficacy on certain produce items or pathogens, and a growing consumer concern regarding chemical treatments [4,5,6,7,8]. Due to the increasing interest in exploring alternative, non-thermal techniques that are both effective and safe for consumers, this study evaluated the use of organic acid and power ultrasound hurdle technology to reduce foodborne pathogens on four different types of fresh produce which are commonly eaten raw: romaine lettuce, cucumber, tomato, and strawberry. The different matrices were selected due to their vastly different surface characteristics to determine the efficacy of the individual or combined treatments to reduce the pathogen populations based on surface topology. Differences were observed in the reduction in L. monocytogenes and S. enterica depending on the produce matrix, the type and concentration of organic acid used, and if the treatment consisted of the organic acids alone or in combination with power ultrasound.
Attachment and the strength of attachment of bacterial cells to fresh produce surfaces is dependent upon many factors, including surface roughness, hydrophobicity, and other topology characteristics [4,26]. In our study, the individual and combination treatments were more effective at reducing both L. monocytogenes and S. enterica on the smoother surface of tomato, than on the rougher surfaces of romaine lettuce, cucumber, and strawberry. These results are similar to other studies which have determined that washing with antimicrobials, power ultrasound, or a combination of the two is more effective at reducing pathogens on produce surfaces, which are smoother and hydrophobic, including apples and tomatoes, compared to the rougher hydrophilic surfaces of different types of lettuce and spinach [11,14,20,27,28,29]. For example, the combination of sodium hypochlorite or peroxyacetic acid and power ultrasound hurdle technology was more effective at reducing both L. monocytogenes and S. enterica on the smoother surface of grape tomatoes than on the rougher surfaces of spinach and iceberg lettuce [14]. In another study, the use of organic acid (malic, citric, or lactic) and power ultrasound resulted in greater reductions in L. monocytogenes and S. enterica on the smoother surface of whole apples compared to the rougher surface of whole peaches [29]. Roughness of the surfaces of the leafy greens, including depressions, crevices, and elevations, can also create a larger surface area for bacterial attachment and colonization and offer protection from antimicrobial and power ultrasound treatments [4].
Malic and lactic acids were more effective at reducing L. monocytogenes and S. enterica on the four produce matrices, especially at the higher 5% concentration with the combination of power ultrasound. Lactic acid, even at the lower concentration of 2%, was also most effective at reducing S. enterica populations on tomato, even without the incorporation of power ultrasound (resulting in a reduction of >5.16 after only 2 min). While L. monocytogenes was not as affected by organic acids as S. enterica, lactic and malic acid treatment at 5% for 5 min with the combination of power ultrasound still resulted in reductions of 3.96 and 5.18 log CFU/unit on tomato, respectively. Malic and lactic acids have been shown to be effective at reducing pathogen populations on other fresh produce matrices, including apples, radish, and lettuce, both with and without the combination of power ultrasound [17,26,28,30]. In one study, the combinations of malic or lactic acid and power ultrasound were more effective at reducing biofilms of L. monocytogenes and E. coli on lettuce than when acetic or citric acids were used [17].
Synergistic effects were observed with some of the organic acid and power ultrasound hurdle treatments in this study. L. monocytogenes and S. enterica populations were significantly further reduced by 0.93–1.84 and 1.42–1.87 log CFU/unit, respectively, on some of the produce matrices (i.e., on tomato and strawberry for L. monocytogenes and on romaine lettuce, cucumber, and strawberry for S. enterica) when power ultrasound was used in combination with either malic or lactic acid. Synergistic effects of organic acid and power ultrasound for the reduction in foodborne pathogens have also been observed in other studies [20,26,30,31]. In one study, the use of citric, lactic, or malic acid with the combination of power ultrasound resulted in an additional 0.8 to 1.0 log reduction in E. coli O157:H7, S. enterica, and L. monocytogenes on romaine lettuce compared to when the treatment consisted of only the organic acid [20]. In another study, the use of lactic or acetic acid with power ultrasound resulted in an additional 0.8–1.1 log/cm2 reduction in S. enterica and E. coli on green pepper and an additional 0.4–1.3 log/cm2 reduction in populations on muskmelon [26].
This study evaluated the efficacy of organic acids, power ultrasound, and combined hurdle treatments to reduce L. monocytogenes and S. enterica on four different fresh produce matrices. Results of this study indicate that produce matrix topology, including surface roughness, may play a role in the effectiveness of these treatments, as greater pathogen reductions were observed on tomatoes, compared to the rougher surfaces of romaine lettuce, cucumber, and strawberry. In addition, synergistic effects of the hurdle technology were also observed, especially when malic and lactic acids were used. Results of this study suggest that the combination of organic acids and power ultrasound may be an effective hurdle technology to reduce foodborne pathogens on select produce matrices. However, further research is still needed to evaluate the feasibility and effectiveness of this hurdle approach in industrial food processing environments. Future studies can incorporate industrial processing equipment, the use of agitation during treatment, different treatment temperatures, and the use of an inoculum at lower pathogen populations to assist with the validation of this hurdle technology for practical industry use.
Author Contributions
Conceptualization, J.K.S., P.B. and W.Z.; methodology, B.A.K., P.B., X.Z., W.Z. and J.K.S.; formal analysis, M.L.F., P.B., B.A.K., D.S.S., X.Z. and J.K.S.; investigation, P.B., M.L.F., D.S.S., X.Z., W.Z. and J.K.S.; data curation, P.B., M.L.F., C.W.Y.W., D.S.S., X.Z., W.Z. and J.K.S.; writing—original draft preparation, M.L.F., P.B. and J.K.S.; writing—review and editing, M.L.F., P.B., B.A.K., C.W.Y.W., D.S.S., X.Z., W.Z. and J.K.S.; visualization, P.B., M.L.F. and J.K.S.; supervision, X.Z., W.Z. and J.K.S.; All authors have read and agreed to the published version of the manuscript.
Funding
M. Fay and B. Khouja were supported by the Oak Ridge Institute for Science and Education Research Participation Program to the U.S. Food and Drug Administration. The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
Data used in this study is presented in the article. Inquiries may be made to the corresponding author.
Acknowledgments
The authors thank Karl Reineke for obtaining the produce used in this study and Jayaram Thatavarthi for laboratory assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aiyedun, S.O.; Onarinde, B.A.; Swainson, M.; Dixon, R.A. Foodborne outbreaks of microbial infection from fresh produce in Europe and North America: A systematic review of data from this millennium. Int. J. Food Sci. Technol. 2021, 56, 2215–2223. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, B.; Feng, H. Chapter 2: Surface Characteristics of Fresh Produce and their Impact on Attachment and Removal of Human Pathogens on Produce Surfaces. In Decontamination of Fresh and Minimally Processed Produce; Gomez-Lopez, M., Ed.; Wiley-Blackwell: Ames, IA, USA, 2012. [Google Scholar]
- Beuchat, L.R.; Adler, B.B.; Lang, M.M. Efficacy of chlorine and a peroxyacetic acid sanitizer in killing Listeria monocytogenes on iceberg and Romaine lettuce using simulated commercial processing conditions. J. Food Prot. 2004, 67, 1238–1242. [Google Scholar] [CrossRef]
- Chinchkar, A.V.; Singh, A.; Singh, S.V.; Acharya, A.M.; Kamble, M.G. Potential sanitizers and disinfectants for fresh fruits and vegetables: A comprehensive review. J. Food Process. Preserv. 2022, 46, e16495. [Google Scholar] [CrossRef]
- Sethi, S.; Nayak, S.L.; Joshi, A.; Sharma, R.R. Chapter 5: Sanitizers for Fresh-cut Fruits and Vegetables. In Fresh-Cut Fruits and Vegetables: Technologies and Mechanisms for Safety Control; Siddiqi, M.W., Ed.; Elsevier-Academic Press: San Diego, CA, USA, 2020. [Google Scholar]
- Chaidez, C.; Castro-del Campo, J.; Heredia, B.; Contreras-Angulo, L.; Gonzalez-Aguilar, G.; Ayala-Zavala, J.F. Chapter 7: Chlorine. In Decontamination of Fresh and Minimally Processed Produce; Gomez-Lopez, M., Ed.; Wiley-Blackwell: Ames, IA, USA, 2012. [Google Scholar]
- Gonzalez-Aguilar, G.; Ayala-Zavala, J.F.; Chaidez-Quiroz, C.; Heredia, J.B.; Castro-del Campo, N. Chapter 12: Peroxyacetic acid. In Decontamination of Fresh and Minimally Processed Produce; Gomez-Lopez, M., Ed.; Wiley-Blackwell: Ames, IA, USA, 2012. [Google Scholar]
- José, J.S.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Bilek, S.E.; Turantaş, F. Decontamination efficiency of high power ultrasound in the fruit and vegetable industry, a review. Int. J. Food Microbiol. 2013, 166, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Piyasena, P.; Mohareb, E.; McKellar, R. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Kovach, S.M. Research: Ensuring Cavitation in a Medical Device Ultrasonic Cleaner. Biomed. Instrum. Technol. 2019, 53, 280–285. [Google Scholar] [CrossRef]
- Zhou, X.; Salazar, J.K.; Fay, M.L.; Zhang, W. Efficacy of Power Ultrasound-Based Hurdle Technology on the Reduction of Bacterial Pathogens on Fresh Produce. Foods 2023, 12, 2653. [Google Scholar] [CrossRef]
- Rafeeq, S.; Ovissipour, R. The Effect Ultrasound and Surfactants on Nanobubbles Efficacy against Listeria innocua and Escherichia coli O157:H7, in Cell Suspension and on Fresh Produce Surfaces. Foods 2021, 10, 2154. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wu, Z.X.; Wang, H.B. Combination of ultrasound-peracetic acid washing and ultrasound-assisted aerosolized ascorbic acid: A novel rinsing-free disinfection method that improves the antibacterial and antioxidant activities in cherry tomato. Ultrason. Sonochem. 2022, 86, 106001. [Google Scholar] [CrossRef] [PubMed]
- Turhan, E.U.; Polat, S.; Erginkaya, Z.; Konuray, G. Investigation of synergistic antibacterial effect of organic acids and ultrasound against pathogen biofilms on lettuce. Food Biosci. 2022, 47, 101643. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Zhang, M.; Xu, B. Application of ultrasonic technology in postharvested fruits and vegetables storage: A review. Ultrason. Sonochem. 2020, 69, 105261. [Google Scholar] [CrossRef]
- Huang, T.S.; Xu, C.; Walker, K.; West, P.; Zhang, S.; Weese, J. Decontamination efficacy of combined chlorine dioxide with ultrasonication on apples and lettuce. J. Food Sci. 2006, 71, M134–M139. [Google Scholar] [CrossRef]
- Sagong, H.G.; Lee, S.-Y.; Chang, P.-S.; Heu, S.; Ryu, S.; Choi, Y.-J.; Kang, D.-H. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011, 145, 287–292. [Google Scholar] [CrossRef]
- FDA. CFR-Code of Federal Regulations § 184 Direct Food Substances Affirmed as Generally Recognized as Safe (GRAS). 2024. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184&showFR=1&subpartNode=21:3.0.1.1.14.1 (accessed on 29 October 2024).
- Taylor, T.M.; Doores, S.X. Organic acids. In Antimicrobials in Foods, 4th ed.; Davidson, P.M., Taylor, T.M., David, J.R.D., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- CDC. Multistate Outbreak of Salmonella Poona Infections Linked to Imported Cucumbers. 2016. Available online: https://archive.cdc.gov/www_cdc_gov/salmonella/poona-09-15/index.html (accessed on 4 April 2019).
- CDC. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August–September 2011. MMWR 2011, 60, 1357–1358. [Google Scholar]
- CDC. 2014 Outbreak of Listeria Infections Linked to Commercially Produced, Prepackaged Caramel Apples Made from Bidart Bros. Apples. 2015. Available online: https://archive.cdc.gov/www_cdc_gov/listeria/outbreaks/caramel-apples-12-14/index.html (accessed on 19 March 2015).
- José, J.F.B.D.; de Medeiros, H.S.; Bernardes, P.C.; de Andrade, N.J. Removal of Salmonella enterica Enteritidis and Escherichia coli from green peppers and melons by ultrasound and organic acids. Int. J. Food Microbiol. 2014, 190, 9–13. [Google Scholar] [CrossRef]
- Liao, C.H.; Sapers, G.M. Attachment and growth of Salmonella Chester on apple fruits and in vivo response of attached bacteria to sanitizer treatments. J. Food Prot. 2000, 63, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, M.R.; Park, J.W.; Park, K.H.; Chung, M.S.; Ryu, S.; Kang, D.H. Use of organic acids to inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef]
- Khouja, B.A.; Mathias, H.; Joshi, M.; Fay, M.L.; Korade, S.; Wong, C.W.Y.; Stewart, D.S.; Zhou, X.; Zhang, W.; Salazar, J.K. Power Ultrasound- and Organic Acid-Based Hurdle Technology to Reduce Listeria monocytogenes and Salmonella enterica on Whole Apples and Peaches. Foods 2025, 14, 1744. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Abhari, K.; Khaneghah, A.M. The combined effects of ultrasound and lactic acid in inactivating microorganisms on fresh radish (Raphans raphanistrum subsp. sativus): Microbiological and quality changes. Food Sci. Nutr. 2020, 8, 162–169. [Google Scholar] [CrossRef] [PubMed]
- José, J.F.B.D.; Ramos, A.M.; Vanetti, M.C.D.; de Andrade, N.J. Inactivation of Salmonella Enteritidis on cherry tomatoes by ultrasound, lactic acid, detergent, and silver nanoparticles. Can. J. Microbiol. 2021, 67, 259–270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).