Effects of Glomus iranicum Inoculation on Growth and Nutrient Uptake in Potatoes Associated with Broad Beans Under Greenhouse Conditions
Abstract
1. Introduction
Importance and Applications of Biofertilizers in Agricultural Crops
2. Materials and Methods
2.1. Description of Site, Plants, and Fungi
2.2. Experimental Design
2.3. Measurement of Plant Growth Parameters
2.4. Statistical Analysis
3. Results
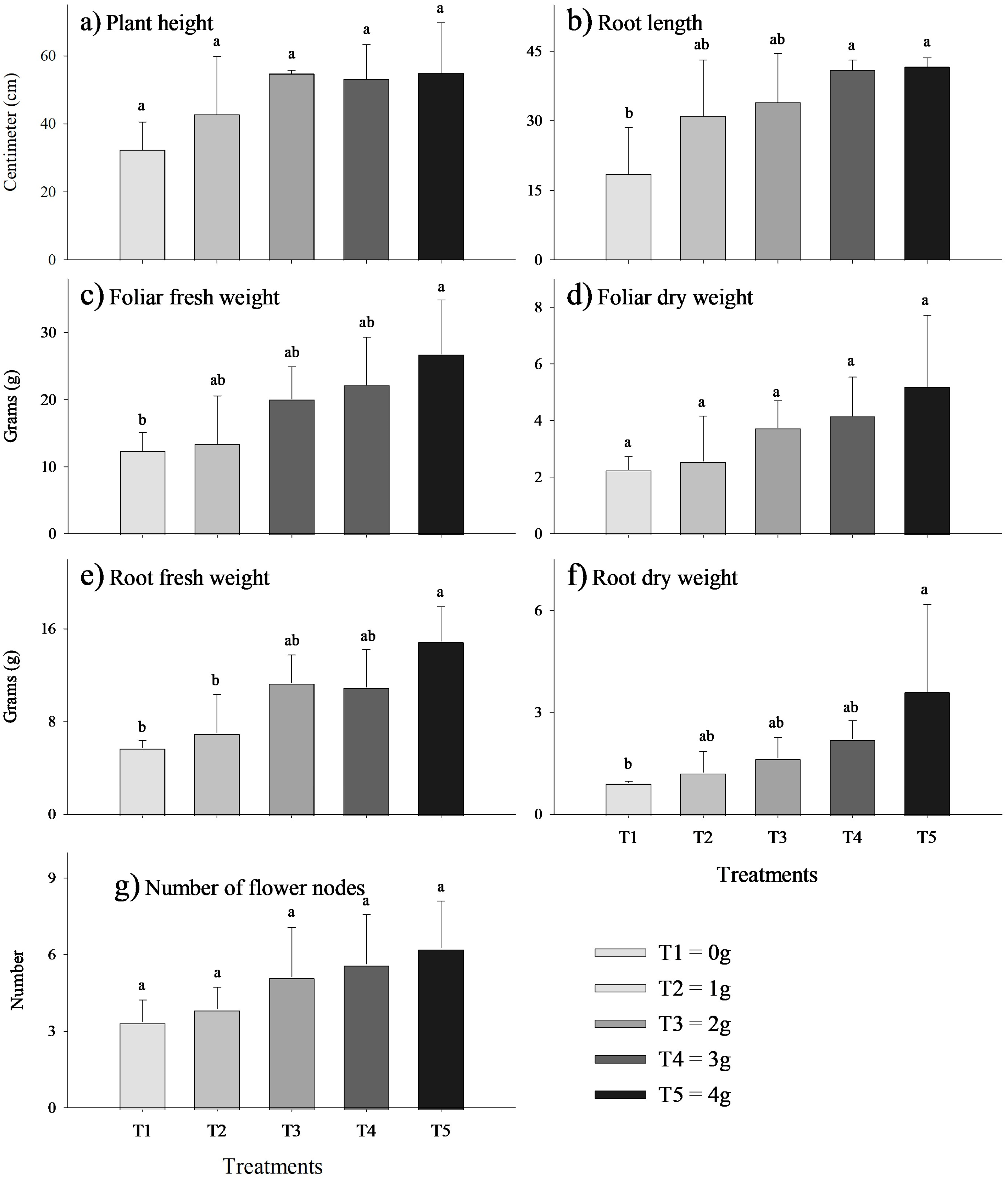
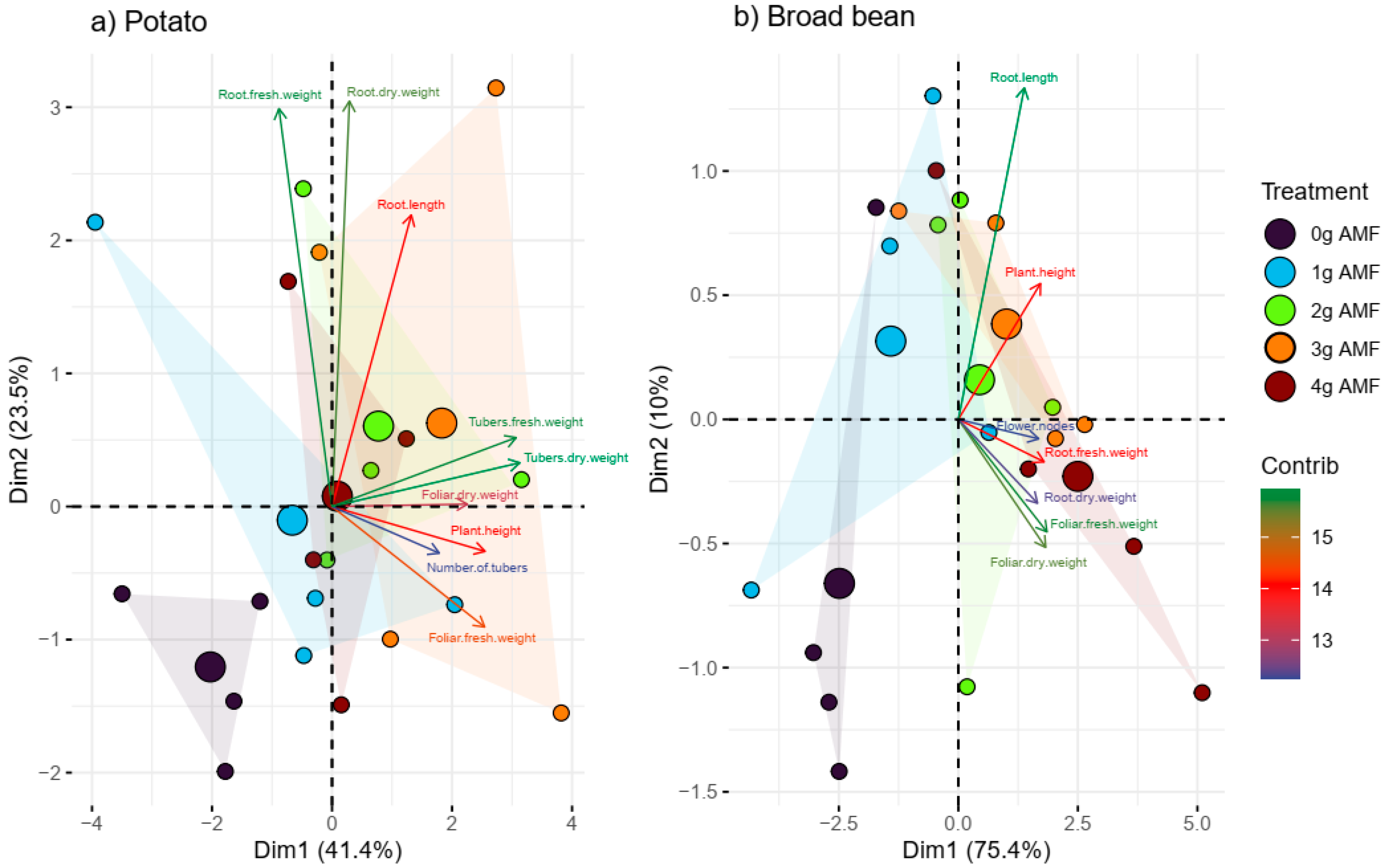
3.1. Plant Growth Parameters
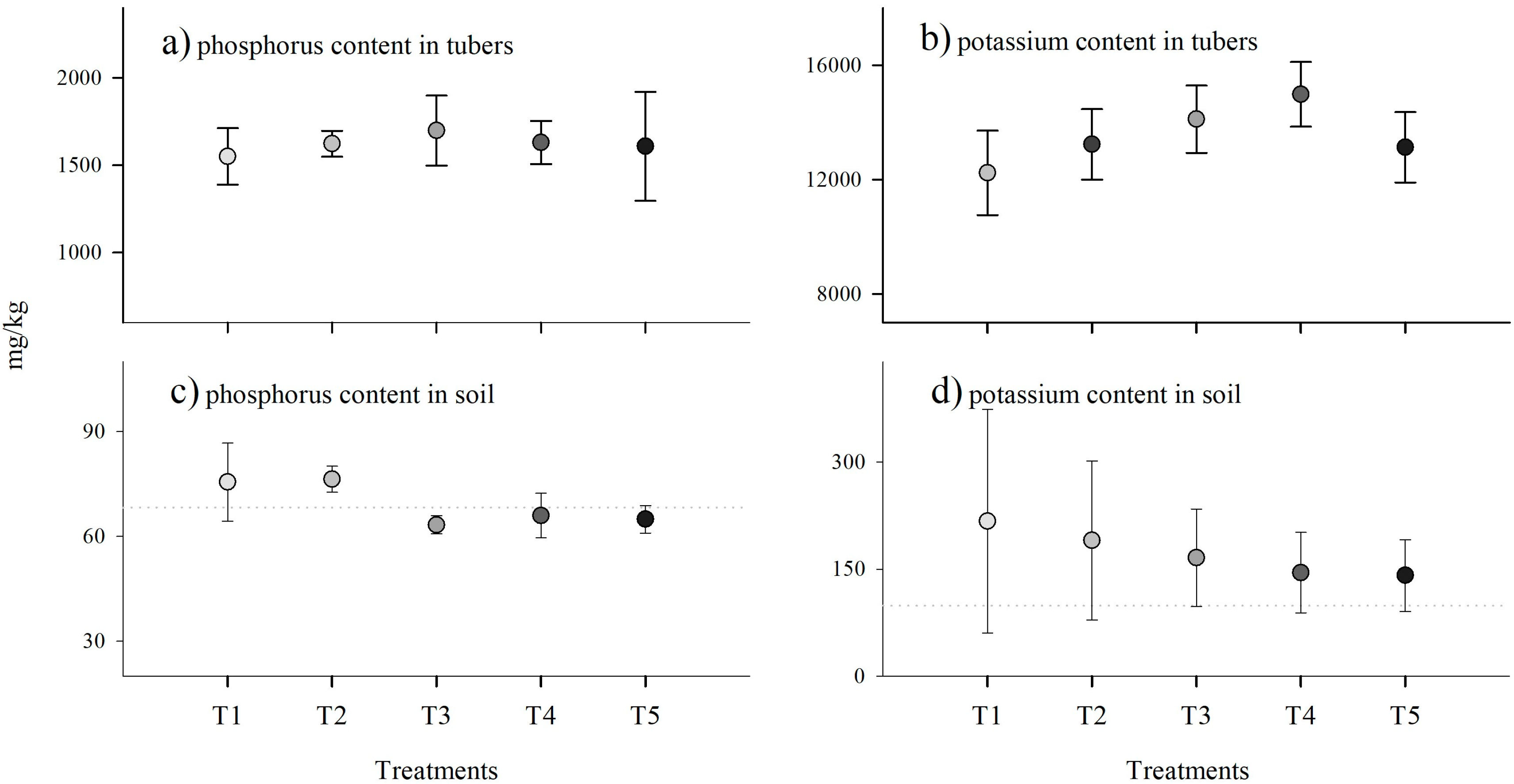
3.2. Tuber and Soil Nutrient Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Bhardwaj, A.; Singh, L.P.; Singh, G. Quantifying Ecological Impacts: A Comparative Life Cycle Assessment of Conventional and Organic Potato Cultivation. Ecol. Modell. 2023, 486, 110510. [Google Scholar] [CrossRef]
- Mrabet, R. Sustainable Agriculture for Food and Nutritional Security. In Sustainable Agriculture and the Environment; Press: Cambridge, MA, USA, 2023; pp. 25–90. ISBN 9780323905008. [Google Scholar]
- Odewale, S.A.; Odekanle, E.L.; Fakinle, B.S. Global Environmental Sustainability and Agrochemical Use. In One Health Implications of Agrochemicals and Their Sustainable Alternatives; Springer Nature: Singapore, 2023; pp. 735–764. ISBN 978-981-99-3439-3. [Google Scholar]
- Haverkort, A.J.; de Ruijter, F.J.; van Evert, F.K.; Conijn, J.G.; Rutgers, B. Worldwide Sustainability Hotspots in Potato Cultivation. 1. Identification and Mapping. Potato Res. 2013, 56, 343–353. [Google Scholar] [CrossRef]
- Meena, K.K.; Kumar, P.; Sorty, A.M.; Bitla, U.; Pathak, H. Ecology of Arbuscular Mycorrhizae and Influence on Drought Tolerance in Crop Plants. In Microbial BioTechnology for Sustainable Agriculture Volume 1; Springer: Singapore, 2022; pp. 261–285. ISBN 978-981-16-4843-4. [Google Scholar]
- Arévalo-Gardini, E.; Canto, M.; Alegre, J.; Loli, O.; Julca, A.; Baligar, V. Changes in Soil Physical and Chemical Properties in Long Term Improved Natural and Traditional Agroforestry Management Systems of Cacao Genotypes in Peruvian Amazon. PLoS ONE 2015, 10, e0132147. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, O.; Poly, F.; Bonnemoy, F.; Bru, D.; Batisson, I.; Bohatier, J.; Philippot, L.; Mallet, C. Functional and Structural Responses of Soil N-Cycling Microbial Communities to the Herbicide Mesotrione: A Dose-Effect Microcosm Approach. Environ. Sci. Pollut. Res. Int. 2016, 23, 4207–4217. [Google Scholar] [CrossRef]
- Pan, Z.; Fan, D.; Jiang, R.; Abbasi, N.; Song, D.; Zou, G.; Wei, D.; He, P.; He, W. Improving Potato Productivity and Mitigating Nitrogen Losses Using Enhanced-Efficiency Fertilizers: A Global Meta-Analysis. Agric. Ecosyst. Environ. 2023, 348, 108416. [Google Scholar] [CrossRef]
- Ma, H.; Xie, C.; Zheng, S.; Li, P.; Cheema, H.N.; Gong, J.; Xiang, Z.; Liu, J.; Qin, J. Potato Tillage Method Is Associated with Soil Microbial Communities, Soil Chemical Properties, and Potato Yield. J. Microbiol. 2022, 60, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hijri, M. Analysis of a Large Dataset of Mycorrhiza Inoculation Field Trials on Potato Shows Highly Significant Increases in Yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Betencourt, E.; Duputel, M.; Colomb, B.; Desclaux, D.; Hinsinger, P. Intercropping Promotes the Ability of Durum Wheat and Chickpea to Increase Rhizosphere Phosphorus Availability in a Low P Soil. Soil Biol. Biochem. 2012, 46, 181–190. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Six, J.; Sun, J.H.; Bao, X.G.; Zhang, F.S.; Van Der Werf, W. Intercropping Enhances Soil Carbon and Nitrogen. Glob. Change Biol. 2015, 21, 1715–1726. [Google Scholar] [CrossRef]
- Chai, Q.; Nemecek, T.; Liang, C.; Zhao, C.; Yu, A.; Coulter, J.A.; Wang, Y.; Hu, F.; Wang, L.; Siddique, K.H.M.; et al. Integrated Farming with Intercropping Increases Food Production While Reducing Environmental Footprint. Proc. Natl. Acad. Sci. USA 2021, 118, e2106382118. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad Agraria Guía para la Implementación de Buenas Prácticas Agrícolas (BPA). Para El Cultivo de Papa; SENASA: Lima, Peru, 2020. [Google Scholar]
- Dubova, L.; Šenberga, A.; Alsiņa, I. The Effect of Double Inoculation on the Broad Beans (Vicia faba L.) Yield Quality. Res. Rural Dev. 2015, 1, 34–39. [Google Scholar]
- Youseif, S.H.; Fayrouz, H.A.E.M.; Saleh, S.A. Improvement of Faba Bean Yield Using Rhizobium/Agrobacterium Inoculant in Low-Fertility Sandy Soil. Agronomy 2017, 7, 2. [Google Scholar] [CrossRef]
- Yin, J.; Sui, Z.; Li, Y.; Yang, H.; Yuan, L.; Huang, J. A New Function of White-Rot Fungi Ceriporia Lacerata HG2011: Improvement of Biological Nitrogen Fixation of Broad Bean (Vicia faba). Microbiol. Res. 2022, 256, 126939. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Cao, J. Intercropping Increases Soil N-Targeting Enzyme Activities: A Meta-Analysis. Rhizosphere 2023, 26, 100686. [Google Scholar] [CrossRef]
- Virk, A.L.; Lin, B.J.; Kan, Z.R.; Qi, J.Y.; Dang, Y.P.; Lal, R.; Zhao, X.; Zhang, H.L. Simultaneous Effects of Legume Cultivation on Carbon and Nitrogen Accumulation in Soil. Adv. Agron. 2022, 171, 75–110. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Enany, A.W.E.; Nafady, N.A.; Khalaf, D.M.; Morsy, F.M. Synergistic Interaction of Rhizobium Leguminosarum Bv. Viciae and Arbuscular Mycorrhizal Fungi as a Plant Growth Promoting Biofertilizers for Faba Bean (Vicia faba L.) in Alkaline Soil. Microbiol. Res. 2014, 169, 49–58. [Google Scholar] [CrossRef]
- Nicolás, E.; Maestre-Valero, J.F.; Alarcón, J.J.; Pedrero, F.; Vicente-Sánchez, J.; Bernabé, A.; Gómez-Montiel, J.; Hernández, J.A.; Fernández, F. Effectiveness and Persistence of Arbuscular Mycorrhizal Fungi on the Physiology, Nutrient Uptake and Yield of Crimson Seedless Grapevine. J. Agric. Sci. 2015, 153, 1084–1096. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh Perspectives on the Roles of Arbuscular Mycorrhizal Fungi in Plant Nutrition and Growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Ortuño, M.F.; Álvarez, S.; Sánchez-Blanco, M.J. Influence of Mycorrhizal or Microbial Complex Inoculation on Laurustinus Plants Irrigated with Reclaimed Water. J. Hortic. Sci. Biotechnol. 2020, 95, 661–672. [Google Scholar] [CrossRef]
- Pereira, S.; Mucha, Â.; Gonçalves, B.; Bacelar, E.; Látr, A.; Ferreira, H.; Oliveira, I.; Rosa, E.; Marques, G. Improvement of Some Growth and Yield Parameters of Faba Bean (Vicia faba) by Inoculation with Rhizobium Laguerreae and Arbuscular Mycorrhizal Fungi. Crop Pasture Sci. 2019, 70, 595–605. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the Productivity and Product Quality of Vegetable Crops Using Arbuscular Mycorrhizal Fungi: A Review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular Mycorrhizal Fungi Modify Nutrient Allocation and Composition in Wheat (Triticum aestivum L.) Subjected to Heat-Stress. Plant Soil 2016, 408, 385–399. [Google Scholar] [CrossRef]
- Jiménez-Martínez, A.; del, C. Gutiérrez-Castorena, M.; Montaño, N.M.; Gutiérrez-Castorena, E.V.; Alarcón, A.; Gavito, M.E. Micromorphology and Thematic Micro-Mapping Reveal Differences in the Soil Structuring Traits of Three Arbuscular Mycorrhizal Fungi. Pedobiologia 2024, 104, 150953. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Rillig, M.C. Soil Biota Contributions to Soil Aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified Crop Rotation: An Approach for Sustainable Agriculture Production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Viguier, L.; Cavan, N.; Bockstaller, C.; Cadoux, S.; Corre-Hellou, G.; Dubois, S.; Duval, R.; Keichinger, O.; Toqué, C.; Toupet de Cordoue, A.L.; et al. Combining Diversification Practices to Enhance the Sustainability of Conventional Cropping Systems. Eur. J. Agron. 2021, 127, 126279. [Google Scholar] [CrossRef]
- Castillo, C.; Solano, J.; Collinao, M.; Catalán, R.; Campos, P.; Aguilera, P.; Sieverding, E.; Borie, F. Intercropping Wheat with Ancestral Non-Mycorrhizal Crops in a Volcanic Soil at Early Growth Stage. Chil. J. Agric. Res. 2022, 82, 663–672. [Google Scholar] [CrossRef]
- Meza, K.; Vanek, S.J.; Sueldo, Y.; Olivera, E.; Ccanto, R.; Scurrah, M.; Fonte, S.J. Grass–Legume Mixtures Show Potential to Increase Above-and Belowground Biomass Production for Andean Forage-Based Fallows. Agronomy 2022, 12, 142. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.; Wen, Y.; Zhu, J.; Di, X.; Zeng, Y.; Han, X.; Que, Z.; Mediatrice, H.; Rensing, C.; et al. Short-Term Effects of Cenchrus Fungigraminus/Potato or Broad Bean Interplanting on Rhizosphere Soil Fertility, Microbial Diversity, and Greenhouse Gas Sequestration in Southeast China. Microorganisms 2024, 12, 1665. [Google Scholar] [CrossRef]
- Taurinanda, A.P.; Banjarnahor, D.R.V. Mycorrhiza Diversity in Some Intercropping Systems of Potato (Solanum tuberosum L.) and Faba Bean (Vicia faba L.). J. Tek. Pertan. Lampung 2023, 12, 495. [Google Scholar] [CrossRef]
- Al-Zubaidi, A.H.A. Biofertilizer Impact on the Productivity of Broad Bean (Vicia faba L.). SABRAO J. Breed. Genet. 2024, 56, 1705–1711. [Google Scholar] [CrossRef]
- Carrara, J.E.; Reddivari, L.; Lehotay, S.J.; Zinati, G.; Heller, W.P. Arbuscular Mycorrhizal Fungi Increase the Yield and Nutritional Quality of Yellow and Purple Fleshed Potatoes (Solanum tuberosum). Am. J. Potato Res. 2023, 100, 210–220. [Google Scholar] [CrossRef]
- Kuila, D.; Ghosh, S. Arbuscular Mycorrhizae and Mycorrhizae Helper Organism---A Synergistic Movement Towards Soil and Crop Sustainability. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Parihar, M., Rakshit, A., Adholeya, A., Chen, Y., Eds.; Springer Nature: Singapore, 2024; pp. 429–451. ISBN 978-981-97-0300-5. [Google Scholar]
- Sharma, U.C.; Datta, M.; Sharma, V. Soil Microbes and Biofertilizers. In Soils in the Hindu Kush Himalayas: Management for Agricultural Land Use; Springer International Publishing: Cham, Switzerland, 2022; pp. 117–144. ISBN 978-3-031-11458-8. [Google Scholar]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking Crop Nutrition in Times of Modern Microbiology: Innovative Biofertilizer Technologies. Front. Sustain. Food Syst. 2021, 5, 1–23. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Hassan, G.I.; Samoon, S.A.; Rather, H.A.; Dar, S.A.; Zehra, B. Bio-Fertilizers In Organic Agriculture. J. Phytol. 2010, 2, 42–54. [Google Scholar]
- Bhat, T.A.; Ahmad, L.; Ganai, M.A.; Shams-Ul-Haq; Khan, O.A. Nitrogen Fixing Biofertilizers; Mechanism and Growth Promotion: A Review. J. Pure Appl. Microbiol. 2015, 9, 1675–1690. [Google Scholar]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving Crop Yield and Nutrient Use Efficiency via Biofertilization—A Global Meta-Analysis. Front. Plant Sci. 2018, 8. [Google Scholar] [CrossRef]
- Choudhury, A.T.M.A.; Kennedy, I.R. Prospects and Potentials for Systems of Biological Nitrogen Fixation in Sustainable Rice Production. Biol. Fertil. Soils 2004, 39, 219–227. [Google Scholar] [CrossRef]
- Susniak, K.; Krysa, M.; Kidaj, D.; Szymanska-Chargot, M.; Komaniecka, I.; Zamlynska, K.; Choma, A.; Wielbo, J.; Ilag, L.L.; Sroka-Bartnicka, A. Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors. Int. J. Mol. Sci. 2021, 22, 12991. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, S.; Malgioglio, A. Azolla-Anabaena as a Biofertilizer for Rice Paddy Fields in the Po Valley, a Temperate Rice Area in Northern Italy. Int. J. Agron. 2010, 2010, 152158. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Damayani, M.; Herdiyantoro, D.; Suryatmana, P.; Anggraini, D.; Khumairah, F.H. The Application Dosage of Azolla Pinnata in Fresh and Powder Form as Organic Fertilizer on Soil Chemical Properties, Growth and Yield of Rice Plant. In Proceedings of the AIP Conference Proceedings, Jatinangor, Indonesia, 8–9 August 2017; Volume 1927. [Google Scholar]
- Baset Mia, M.A.; Shamsuddin, Z.H. Rhizobium as a Crop Enhancer and Biofertilizer for Increased Cereal Production. Afr. J. Biotechnol. 2010, 9, 6001–6009. [Google Scholar]
- Revillas, J.J.; Rodelas, B.; Pozo, C.; Martínez-Toledo, M.V.; González López, J. Production of Amino Acids by Azotobacter Vinelandii and Azotobacter Chroococcum with Phenolic Compounds as Sole Carbon Source under Diazotrophic and Adiazotrophic Conditions. Amino Acids 2005, 28, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Kizilkaya, R. Nitrogen Fixation Capacity of Azotobacter spp. Strains Isolated from Soils in Different Ecosystems and Relationship between Them and the Microbiological Properties of Soils. J. Environ. Biol. 2009, 30, 73–82. [Google Scholar]
- Chungopast, S.; Thongjoo, C.; Islam, A.K.M.M.; Yeasmin, S. Efficiency of Phosphate-Solubilizing Bacteria to Address Phosphorus Fixation in Takhli Soil Series: A Case of Sugarcane Cultivation, Thailand. Plant Soil 2021, 460, 347–357. [Google Scholar] [CrossRef]
- Nguyen Quoc, K.; Le Vinh, T.; Le Thanh, Q.; Tran Ngoc, H.; Do Thi, X.; Huynh Huu, D.; Ly Ngoc Thanh, X.; Le Thi My, T. Effects of Biofertilizer Supplementation, Rhodopseudomonas Spp., on Nitrogen and Phosphorus Uptakes, Growth, and Yield of Sesame (Sesamum indicum L.) on Salt-Affected Soil. J. Plant Nutr. 2024, 47, 1–17. [Google Scholar] [CrossRef]
- Singh, Z.; Singh, G.; Aggarwal, N.; Virk, H.K.; Gupta, R.K. Applications of Biofertilizers and Phosphorus Influence Nutrient Uptake and Nutrient Use Efficiency in Chickpea (Cicer arietinum L.). Commun. Soil Sci. Plant Anal. 2024, 55, 1093–1104. [Google Scholar] [CrossRef]
- Seenivasagan, R.; Babalola, O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef]
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate Solubilizing Microbes: An Effective and Alternative Approach as Biofertilizers. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Din, M.; Nelofer, R.; Salman, M.; Abdullah; Khan, F.H.; Khan, A.; Ahmad, M.; Jalil, F.; Din, J.U.; Khan, M. Production of Nitrogen Fixing Azotobacter (SR-4) and Phosphorus Solubilizing Aspergillus Niger and Their Evaluation on Lagenaria Siceraria and Abelmoschus Esculentus. Biotechnol. Rep. 2019, 22, e00323. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.F.; He, L.Y. Solubilization of Potassium-Bearing Minerals by a Wild-Type Strain of Bacillus Edaphicus and Its Mutants and Increased Potassium Uptake by Wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef]
- Basak, B.B.; Biswas, D.R. Co-Inoculation of Potassium Solubilizing and Nitrogen Fixing Bacteria on Solubilization of Waste Mica and Their Effect on Growth Promotion and Nutrient Acquisition by a Forage Crop. Biol. Fertil. Soils 2010, 46, 641–648. [Google Scholar] [CrossRef]
- Han, H.S.; Supanjani; Lee, K.D. Effect of Co-Inoculation with Phosphate and Potassium. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar] [CrossRef]
- ISO 11265:1994; Soil Quality-Determination of the Specific Electrical Conductivity. ISO: Geneva, Switzerland, 1996.
- USEPA. Method 9045D Soil and Waste PH; USEPA: Washington, DC, USA, 2004; Volume 8, pp. 104–110. [Google Scholar]
- Walkley, A.; Black, I. An Examination of the Degtjareff Method and a Proposed Modification of the Chromic Matter and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 34, 29–38. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of Total Organic and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Norma Oficial Mexicana NOM-021-RECNAT-2000. 2002. Available online: http://www.ordenjuridico.gob.mx/Documentos/Federal/wo69255.pdf (accessed on 10 December 2024).
- Bouyoucos, G.J. Directions for Making Mechanical Analysis of Soils by the Hydrometer Method. Soil Sci. 1936, 42, 225–230. [Google Scholar] [CrossRef]
- Tahir, M.; Ahmad, I.; Shahid, M.; Shah, G.M.; Farooq, A.B.U.; Akram, M.; Tabassum, S.A.; Naeem, M.A.; Khalid, U.; Ahmad, S.; et al. Regulation of Antioxidant Production, Ion Uptake and Productivity in Potato (Solanum tuberosum L.) Plant Inoculated with Growth Promoting Salt Tolerant Bacillus Strains. Ecotoxicol. Environ. Saf. 2019, 178, 33–42. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 466052. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-Inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef]
- Karagatzides, J.D.; Wilton, M.J.; Tsuji, L.J.S. Soil Nutrient Supply in Cultivated Bush Bean–Potato Intercropping Grown in Subarctic Soil Managed with Agroforestry. Sustainability 2021, 13, 8185. [Google Scholar] [CrossRef]
- Fernández Martín, F.; Molina, J.J.; Nicolás Nicolás, E.; Alarcón, J.J.; Kirchmair, M.; García, F.J.; Bernabe, A.J.; Bernal, C. Application of Arbuscular Mycorrhizae Glomus Iranicum Var. Tenuihypharum Var. Nova in Intensive Agriculture: A Study Case. J. Agric. Sci. Technol. B 2017, 7, 221–247. [Google Scholar] [CrossRef]
- Duffy, E.M.; Cassells, A.C. The Effect of Inoculation of Potato (Solanum tuberosum L.) Microplants with Arbuscular Mycorrhizal Fungi on Tuber Yield and Tuber Size Distribution. Appl. Soil Ecol. 2000, 15, 137–144. [Google Scholar] [CrossRef]
- Lone, R.; Shuab, R.; Sharma, V.; Kumar, V.; Mir, R.; Koul, K.K. Effect of Arbuscular Mycorrhizal Fungi on Growth and Development of Potato (Solanum tuberosum) Plant. Asian J. Crop Sci. 2015, 7, 233–243. [Google Scholar] [CrossRef]
- Davies, F.T.; Calderón, C.M.; Huaman, Z.; Gómez, R. Influence of a Flavonoid (Formononetin) on Mycorrhizal Activity and Potato Crop Productivity in the Highlands of Peru. Sci. Hortic. 2005, 106, 318–329. [Google Scholar] [CrossRef]
- Cozzolino, V.; Di Meo, V.; Piccolo, A. Impact of Arbuscular Mycorrhizal Fungi Applications on Maize Production and Soil Phosphorus Availability. J. Geochem. Explor. 2013, 129, 40–44. [Google Scholar] [CrossRef]
- Vicente-Sánchez, J.; Nicolás, E.; Pedrero, F.; Alarcón, J.J.; Maestre-Valero, J.F.; Fernández, F. Arbuscular Mycorrhizal Symbiosis Alleviates Detrimental Effects of Saline Reclaimed Water in Lettuce Plants. Mycorrhiza 2014, 24, 339–348. [Google Scholar] [CrossRef]
- Chafai, W.; Bouchentouf, H.; Khalid, A. Comparative Impact of Arbuscular Mycorrhizal Fungi from Moroccan Soils and Commercial Inoculum on Potato Yield and Nutrient Composition. Aust. J. Crop Sci. 2024, 18, 160–166. [Google Scholar] [CrossRef]
- Salim, H.A.; Ali, A.F.; Alsaady, M.H.M.; Saleh, U.N.; Jassim, N.H.; Hamad, A.R.; Attia, J.A.; Darwish, J.J.; Hassan, A.F. Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Growth of Cauliflower (Brassica oleracea L. Var. botrytis). Plant Arch. 2020, 20, 782–786. [Google Scholar]
- Schellenbaum, L.; Berta, G.; Ravolanirina, F.; Tisserant, B.; Gianinazzi, S.; Fitter, A.H. Influence of Endomycorrhizal Infection on Root Morphology in a Micropropagated Woody Plant Species (Vitis vinifera L.). Ann. Bot. 1991, 68, 135–141. [Google Scholar] [CrossRef]
- Hooker, J.E.; Munro, M.; Atkinson, D. Vesicular-Arbuscular Mycorrhizal Fungi Induced Alteration in Poplar Root System Morphology. Plant Soil 1992, 145, 207–214. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Influence of Arbuscular Mycorrhizal Fungi and Salinity on Growth, Biomass, and Mineral Nutrition of Acacia Auriculiformis. Biol. Fertil. Soils 2003, 38, 170–175. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine Root Architecture of Nine North American Trees. Ecol. Monogr. 2002, 72, 293. [Google Scholar] [CrossRef]
- Nurbaity, A.; Hamdani, J.S.; Rahayu, R.P. Effect of Arbuscular Mycorrhizal Fungi and Different Composition of Growing Medium on Growth and Production of Potato Seed Cultivars Medians in Inceptisols Jatinangor. IOP Conf. Ser. Earth Environ. Sci. 2019, 393, 012052. [Google Scholar] [CrossRef]
- Naji, N.S.; Dhiyab, N.S.; Abed, R.M. Efficiency of Some Local Isolates of Arbuscular Mycorrhizae in the Growth and Productivity of Potatoes (Solanum tuberosum L.) in Plastic Pots. Plant Sci. Today 2024, 11, 274–280. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The Influence of Arbuscular Mycorrhizal Colonisation on Key Growth Parameters and Fruit Yield of Pepper Plants Grown at High Salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhizae on the Root System of Maize Plants under Salt Stress. Can. J. Microbiol. 2009, 55, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Abiala, M.A.; Popoola, O.O.; Olawuyi, O.J.; Oyelude, J.O.; Akanmu, A.O.; Killani, A.S.; Osonubi, O.; Odebode, A.C. Harnessing the Potentials of Vesicular Arbuscular Mycorrhizal (VAM) Fungi to Plant Growth—A Review. Int. J. Pure Appl. Sci. Technol. 2013, 14, 61–79. [Google Scholar]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V.; Hunegnaw, D.K. Legume-Rhizobium Specificity Effect on Nodulation, Biomass Production and Partitioning of Faba Bean (Vicia faba L.). Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Elliott, A.J.; Daniell, T.J.; Cameron, D.D.; Field, K.J. A Commercial Arbuscular Mycorrhizal Inoculum Increases Root Colonization across Wheat Cultivars but Does Not Increase Assimilation of Mycorrhiza-Acquired Nutrients. Plants People Planet 2021, 3, 588–599. [Google Scholar] [CrossRef]
- Ghobadi, M.; Dehnavi, M.M.; Yadavi, A.R.; Parvizi, K.; Zafari, D. Reduced P Fertilization Improves Fe and Zn Uptake in Potato When Inoculated with AMF in P, Fe and Zn Deficient Soil. Rhizosphere 2020, 15, 100239. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Torta, L.; Esposito, A.; Moncada, A. Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes. Agronomy 2023, 13, 440. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Prasanna, B.M. Analysis of Genetic Diversity in Crop Plants—Salient Statistical Tools and Considerations. Crop Sci. 2003, 43, 1235–1248. [Google Scholar] [CrossRef]
- Hanoon, M.B.; Haran, M.S.; Sahi, M.K. Effect of Rhizobium Inoculation and Different Levels of Organic and Nitrogen Fertilizers on Growth and Production of Broad Bean (Vicia faba L.) and Nitrogen Readiness in Soil. Int. J. Agric. Stat. Sci. 2020, 16, 229–236. [Google Scholar]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal Symbiosis Modulates the Rhizosphere Microbiota to Promote Rhizobia–Legume Symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef]
- He, J.; Zhang, L.; Van Dingenen, J.; Desmet, S.; Goormachtig, S.; Calonne-Salmon, M.; Declerck, S. Arbuscular Mycorrhizal Hyphae Facilitate Rhizobia Dispersal and Nodulation in Legumes. ISME J. 2024, 18. [Google Scholar] [CrossRef]
- Mateo, N.; Tapia, M. High Mountain Environment and Farming Systems in the Andean Region of Latin America. In Proceedings of the International Workshop on Mountain Crops and Genetic Resources, Katmandu, Nepal, 16–19 February 1987; p. 51. [Google Scholar]

| pH | CE (mS/m) | OM (%) | P (mg/kg) | K (mg/kg) | Sand (%) | Silt (%) | Clay (%) | Texture |
|---|---|---|---|---|---|---|---|---|
| 6.1 | 7.3 | 1.8% | 68.3 | 98.8 | 33 | 47 | 20 | Loamy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Pino, D.L.; Pizarro, S.; Verastegui-Martinez, P.; Solórzano-Acosta, R.; Requena-Rojas, E.J. Effects of Glomus iranicum Inoculation on Growth and Nutrient Uptake in Potatoes Associated with Broad Beans Under Greenhouse Conditions. Microbiol. Res. 2025, 16, 164. https://doi.org/10.3390/microbiolres16070164
Contreras-Pino DL, Pizarro S, Verastegui-Martinez P, Solórzano-Acosta R, Requena-Rojas EJ. Effects of Glomus iranicum Inoculation on Growth and Nutrient Uptake in Potatoes Associated with Broad Beans Under Greenhouse Conditions. Microbiology Research. 2025; 16(7):164. https://doi.org/10.3390/microbiolres16070164
Chicago/Turabian StyleContreras-Pino, Duglas Lenin, Samuel Pizarro, Patricia Verastegui-Martinez, Richard Solórzano-Acosta, and Edilson J. Requena-Rojas. 2025. "Effects of Glomus iranicum Inoculation on Growth and Nutrient Uptake in Potatoes Associated with Broad Beans Under Greenhouse Conditions" Microbiology Research 16, no. 7: 164. https://doi.org/10.3390/microbiolres16070164
APA StyleContreras-Pino, D. L., Pizarro, S., Verastegui-Martinez, P., Solórzano-Acosta, R., & Requena-Rojas, E. J. (2025). Effects of Glomus iranicum Inoculation on Growth and Nutrient Uptake in Potatoes Associated with Broad Beans Under Greenhouse Conditions. Microbiology Research, 16(7), 164. https://doi.org/10.3390/microbiolres16070164








