Abstract
Sourdough fermentation has emerged as a promising biotechnological approach to reducing gluten content and modifying gluten proteins in wheat-based products. This review assesses the current scientific literature on the enzymatic degradation and hydrolysis of gluten during lactic acid bacteria (LAB) sourdough fermentation. It explores implications for individuals with gluten-related disorders, including celiac disease, non-celiac gluten sensitivity and intolerance, as well as irritable bowel syndrome (IBS). In addition, LAB sourdough effect on fermentable oligo-, di-, monosaccharides and polyols (FODMAPs), amylase-trypsin inhibitors (ATIs), and phytate are revised. Selected homo- and heterofermentative LAB are capable of degrading gluten proteins, especially the polypeptides derived from the action of native cereal proteases. Mixed cultures of LAB degrade gluten peptides more effectively than monocultures. However, LAB sourdough is not sufficient to remove the toxic peptides to the minimal level (<20 ppm). This goal is achieved only if sourdough is combined with fungal proteases during sourdough fermentation. LAB sourdough directly contributes to lower FODMAPs but not ATIs and phytate. Phytate is reduced by the endogenous cereal phytases activated at acidic pHs (pH < 5.0), conditions generated during sourdough fermentation. ATIs are also lowered by endogenous cereal proteases instead of LAB proteases/peptidases. Despite LAB sourdough not fully degrading the gluten or directly reducing the ATIs and phytate, it participates through peptidases activity and acidic pH that trigger the action of endogenous cereal proteases and phytases.
1. Introduction
Sourdough fermentation is among the oldest traditional methods in food technology [1] to make bread and other baked goods. Sourdough is a mixture of flour (commonly wheat or rye) and water with or without sourdough starters, allowed to ferment for various hours between 28 and 37 °C. Sourdough technology for breadmaking implies a high number of proteolytic enzymes secreted by microorganisms involved in fermentation, especially lactic acid bacteria (LAB). At the beginning of sourdough fermentation, LAB reduces the dough’s pH, favoring the endogenous cereal enzymes activity, which includes proteases and phytases. Cereal proteases disassemble albumin, globulins, gliadins, and glutenins (the main cereal proteins) into polypeptides susceptible to peptidases secreted or produced by LAB.
Gluten-related disorders describe a group of diseases that include celiac disease (CD), wheat allergy, and non-celiac wheat sensitivity, where gluten is the main external trigger [2]. Gluten is a group of proteins found in different cereals (wheat, rye, and barley). It is composed of gliadins and glutenins, both of which are resistant to complete degradation in the gastrointestinal tract due to their high content of prolines and glutamines [3,4]. In CD patients, the intake of gluten and immunogenic peptides (toxic peptides) provokes inflammation of the small intestine and villous atrophy, leading to malabsorption of essential nutrients; thus, a gluten-free diet is a known effective treatment [5].
During sourdough fermentation, gluten proteins are broken down into harmless fragments. However, the degradation of toxic peptides during sourdough fermentation is often incomplete, and residual peptides are sufficient to trigger deleterious effects on people with CD [6]. Mixed cultures of lactic acid bacteria in sourdough are more effective in reducing gluten and their toxic peptides than monocultures. The addition of fungal proteases during sourdough improves gluten degradation, reaching levels <20 ppm [7,8,9]. LAB of sourdough utilizes fructans, oligosaccharides, raffinose, and polyols during fermentation, which are effectively reduced to lower levels (<0.5%), resulting in baked products suitable for individuals with IBS. Undoubtedly, LAB sourdough contributes to reducing gluten toxicity, FODMAPs, ATIs, and phytates, which are detrimental to the quality of baked goods. Various reviews have been published summarizing the main findings about the strategies to diminish the effects of gluten on CD patients and gluten-related disorders patients, including probiotics [10], the description of gluten-related disorders and sourdough effects on wheat components [11], the proteolytic activity of LAB sourdough [12], sourdough to low-FODMAP bread [13], enzyme technology to degrade gluten [14], therapies for CD and non-celiac wheat sensitivity [2], and pros and cons of gluten-free diet (GFD), improving the GFD, and dietary alternatives to GFD [15]. However, the impact of lactic acid bacteria on gluten, FODMAPs, ATIs, and phytate during sourdough fermentation has been less reported. In addition, the implications of complete gluten degradation are also discussed in this review. Therefore, this review assesses the current understanding of gluten’s enzymatic degradation and hydrolysis during LAB sourdough fermentation. It explores implications for individuals with gluten-related disorders, including celiac disease, non-celiac gluten sensitivity and intolerance, as well as irritable bowel syndrome (IBS). In addition, LAB sourdough effects on fermentable oligo-, di-, monosaccharides and polyols (FODMAPs), amylase-trypsin inhibitors (ATIs), and phytate are revised.
2. Disorders Related to Components of Cereals Containing Gluten
Gluten-related disorders cover a spectrum of immune-mediated, allergic, and non-immune conditions triggered by ingesting gluten-containing cereals, including wheat, barley, and rye [16]. While gluten proteins are the primary causes, other wheat components, including amylase/trypsin inhibitors (ATIs) and fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs), have also been implicated in the onset of symptoms among sensitive individuals. Increased awareness and prevalence of these disorders have driven research into food processing strategies designed to reduce the immunogenic potential of cereal-based food products.
One strategy is sourdough fermentation, an ancient biotechnological process utilizing LAB and yeasts [17]. This technique has seen a resurgence in interest due to its ability to break down gluten and other reactive components in wheat. It is proposed that the degradation of gluten in sourdough bread may enhance its suitability for individuals with gluten-related disorders [12,18,19]. Sourdough fermentation, a key process, significantly reduces FODMAP content by breaking down fructans, thereby enhancing digestibility for individuals with irritable bowel syndrome (IBS) [18,19,20]. This reduction in FODMAP content is a crucial aspect of sourdough fermentation, providing consumers with important knowledge about its potential benefits.
Although FODMAPs may benefit gut microbiota in healthy individuals, their ingestion can provoke intestinal symptoms in people with IBS or non-celiac wheat sensitivity (NCWS), alongside the inflammatory effects of ATIs [21]. Sourdough fermentation has shown promise in reducing both FODMAP levels and ATI activity [22], offering symptom relief for patients with IBS and non-celiac gluten sensitivity (NCGS) [22,23]. While ATIs may be inactivated or degraded during fermentation, more research is needed to elucidate the mechanisms involved [22,24]. The acidic environment of sourdough alters the gluten matrix. It enhances digestibility, providing reassurance and confidence in its potential to reduce postprandial glycemic responses and produce better-tolerated wheat-based products.
2.1. Celiac Disease
Celiac disease is a chronic autoimmune enteropathy triggered by gluten ingestion in genetically predisposed individuals, specifically those carrying HLA-DQ2 or HLA-DQ8 alleles (Figure 1) [25,26]. Gluten’s resistance to gastrointestinal proteolysis results in the formation of immunogenic peptides, particularly the 33-mer from α-gliadin [27]. These peptides undergo deamidation by tissue transglutaminase and are presented by antigen-presenting cells, which activate T cells and induce mucosal damage. Immune and inflammatory responses ultimately lead to a characteristic increase in intraepithelial lymphocytes and villous atrophy (Figure 1) [28]. In patients with CD, prolonged exposure to gluten causes varying degrees of intestinal damage, and in most cases, the intestinal mucosa regenerates when following a gluten-free diet. Clinical presentations vary from gastrointestinal disturbances to extraintestinal manifestations, including symptoms such as (fatty) diarrhea, weight loss, micronutrient deficiencies, anemia, osteoporosis, and neurological symptoms [29].
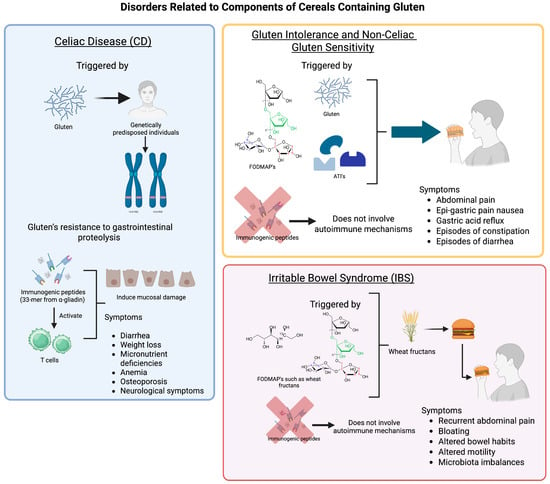
Figure 1.
Differences between disorders related to components of cereals containing gluten.
CD diagnosis is supported by serological markers including anti-tissue transglutaminase IgA (tTG-IgA), anti-endomysium IgA (EMA), and anti-deamidated gliadin peptide IgG (DPG) [30,31]. Affecting approximately 1% of the global population, however, in some meta-analyses, the global seroprevalence of CD is 1.4%, with a range of 1.1% to 1.7%. In contrast, based on biopsy sample analysis, it is 0.7%, ranging from 0.5% to 0.9% [28]. Currently, CD is managed solely through strict, lifelong adherence to a gluten-free diet. However, a substantial proportion of commercial food products, particularly convenience foods and ready-to-eat meals, are formulated with wheat flour or contain one or more wheat-derived components, such as wheat proteins, starch, glucose syrup, or maltodextrins, which serve various functional purposes, including as fillers, processing aids, binding agents, or stabilizers [28]. These challenges in entirely avoiding unintentional gluten exposure have led to a growing interest in complementary strategies, such as enzymatic gluten degradation and microbial fermentation.
2.2. Gluten Intolerance
Gluten intolerance, a broader term frequently confused with non-celiac gluten sensitivity (NCGS), refers to gastrointestinal and systemic symptoms that arise after consuming gluten, without evidence of enteropathy, such as mucosal damage or brush border flattening of CD or wheat allergy (WA). While the immune system is not implicated in conditions such as CD or WA (Figure 1), affected individuals may still experience bloating, fatigue, and cognitive complaints, albeit without diagnostic biomarkers [32].
Previously thought to be limited to CD and WA [33], gluten intolerance is now recognized as a heterogeneous condition encompassing three entities: CD, WA, and NCGS. Among these, NCGS appears to be the most prevalent [33]. Unlike CD and WA, which involve autoimmune and IgE-mediated mechanisms, respectively, some individuals with gluten sensitivity display no identifiable immunological abnormalities [30,31]. Wheat allergy, characterized by type I and IV hypersensitivity reactions, is typically mediated by IgE antibodies [31].
2.3. Non-Celiac Gluten Sensitivity
NCGS refers to gluten-triggered symptoms in individuals who lack serological and histological evidence of CD or immunoglobulin E-mediated wheat allergy. These symptoms resolve upon gluten withdrawal and recur with reintroduction. The term “non-celiac wheat sensitivity” (NCWS) is often preferred, as it more accurately reflects the potential role of other wheat components, such as FODMAPs and ATIs, in symptom development (Figure 1) [31].
Unlike CD, NCGS does not involve autoimmune mechanisms but may activate the innate immune system [16,34], but enteropathy (mucosal damage and flattening of the brush border) is absent. Given its symptomatic overlap with IBS, diagnosis remains clinical, and double-blind placebo-controlled gluten challenges are recommended in research settings [35]. For example, a double-blind, placebo-controlled crossover study conducted by Biesiekierski et al. [36] demonstrated a significant worsening of overall gastrointestinal symptoms regardless of the intervention (i.e., placebo, low-gluten diet, or high-gluten diet). Interestingly, symptom scores were highest during the initial phase of the dietary challenge. This finding indicates an order effect, reflecting a strong anticipatory symptomatic response. Moreover, in a randomized, controlled crossover trial, Herfndal et al. [37] investigated the effects of FOS-fructans and gluten on the composition and diversity of the fecal microbiota in 59 self-reported NCWS or wheat sensitivity participants. Overall, the study found that FOS-fructans induced more pronounced gastrointestinal symptoms than gluten intake. Similarly, no significant changes in fecal microbiota composition were observed that could account for the participants’ gastrointestinal symptoms.
2.4. Irritable Bowel Syndrome (IBS)
Irritable bowel syndrome (IBS) (Figure 1) affects about 10% of the global population and is characterized by recurrent abdominal pain, bloating, and altered bowel habits [38,39]. Though not immunologically mediated, IBS often shares symptomatology with gluten-related disorders such as CD and NCGS [40]. Its pathogenesis involves dysregulation of the gut–brain axis, altered motility, and microbiota imbalances.
Fructans (Figure 1), a subtype of FODMAPs found in wheat, are poorly absorbed and highly fermentable, making them significant dietary triggers in IBS [41,42]. It remains uncertain whether the intake of as little as 0.5 g to slightly over 1 g of FODMAPs, amounts typically found in most breads, is sufficient to cause significant gas production [28]. A low-FODMAP diet, which often includes limiting wheat-based foods, has demonstrated clinical efficacy in managing IBS symptoms [18,43]. Roncoroni et al. [44] reported improved outcomes in celiac patients with residual functional symptoms when combining a gluten-free diet with a low-FODMAP diet, highlighting the potential for dual dietary strategies.
2.5. Grain Components That Trigger Adverse Reactions
Wheat has nourished human populations for over 10,000 years, offering energy, fiber, and essential micronutrients. Nevertheless, some components in wheat can provoke adverse reactions in susceptible individuals [28,45]. Despite popular belief, most people tolerate wheat without any issues, and there is no scientific justification for the widespread avoidance of wheat among the general population [46]. However, the growing influence of media and pseudoscientific narratives has led many consumers to adopt wheat-free diets, often without a medical requirement [28,46].
Aside from gluten, ATIs and FODMAPs are primary non-gluten components linked to adverse responses. ATIs, naturally occurring pest-resistant proteins, can resist digestion and activate toll-like receptor 4 (TLR4), triggering innate immune responses and inflammation [47]. FODMAPs, by contrast, cause symptoms via fermentation and water retention in the colon, aggravating IBS and NCGS. Wheat germ agglutinin has also been shown to increase intestinal permeability, which may contribute to the development of symptoms [48].
To mitigate these effects, LABs are a promising tool due to their proteolytic capacity [49,50,51]. Sourdough LAB can reduce wheat allergens during fermentation [52,53]. Di Cagno et al. [54] demonstrated that specific LAB strains degrade immunogenic prolamin peptides, enabling bread production with significantly reduced gliadin toxicity when optimal fermentation conditions are applied.
3. Sourdough as an Alternative for Improving Gut Health
3.1. Microorganisms and Fermentation in Sourdough
Sourdough is a fermented mixture of flour and water that undergoes spontaneous fermentation by lactic acid bacteria (LAB) and yeast, exhibiting both acidifying and leavening properties [55]. Sourdough starters have a long tradition of use in artisanal bakeries. Renewed interest in sourdough fermentation is owing to positive effects on texture, nutritional, sensory, and shelf life of derived products [56]. Lactic acid bacteria dominate sourdough fermentation compared with yeast at a ratio of approximately 100:1 [57]. During sourdough fermentation, LAB and yeast metabolize carbohydrates, starch, and proteins while acidifying the medium and producing a wide variety of metabolites, including peptides, free amino acids, and short-chain fatty acids, among others. Acid production in sourdough reduces the pH to values below 4. Viable LAB increases up to reach populations of 9–10 logs CFU/g sourdough [58,59,60,61]. Approximately 62 species of homofermentative LAB and 49 species of heterofermentative LAB have been isolated from sourdoughs [62]. The most common LAB isolated from spontaneous sourdough are Fructilactobacillus sanfranciscensis, Lactiplantibacillus plantarum, Levilactobacillus brevis, Limosilactobacillus pontis, Companilactobacillus paralimentarius, Furfurilactobacillus rossiae, Leuconostoc mesenteroides, Pediococcus pentosaceus, Pediococcus parvulus, Weissella cibaria, Lactilactobacillus sakei, Leuconostoc citreum, Laticaseibacillus paracasei, Leuconostoc lactis, Latilactobacillus curvatus, Limosilactobacillus fermentum, Lacticaseibacillus casei, Weissella confusa, and Lactiplantibacillus pentosus. Saccharomyces cerevisiae, Torulaspora delbrueckii, Kazachstania humilis, and Saccharomyces pastorianus are common yeasts in sourdoughs.
Sourdoughs can be classified into three types based on their preparation process and the metabolic activity of the primary LAB and yeasts present. Type I sourdough is prepared with flour and water, inoculated with previously fermented sourdough, and incubated at temperatures <30 °C. Type I sourdoughs are typically firm doughs and use a single leavening agent. Type II sourdough is a mixture of flour and water incubated at temperatures >30 °C for prolonged times (up to 5 days). S. cerevisiae is added to leaven the dough. Type II sourdoughs can be liquid and have been widely adopted by the industry. Type III sourdough is a dried dough “ready to use” where the microorganisms are in a dormant state by the drying process and require reactivation and resuspension in water before use as a bread ingredient [17].
3.2. Enzymatic Proteolysis of Grain Proteins
Wheat is one of the world’s most important crops due to its essential role in food security, as baked goods and pasta form a significant part of diverse diets [46]. Wheat flour is the ideal staple food for breadmaking, highlighting its excellent properties for developing a wide range of doughs and breads, partly due to its high gluten content. The main proteins in wheat are albumins (water soluble), globulins (10% NaCl solution soluble), gliadins (70–90% ethanol soluble), and glutenins (soluble in acid and basic solutions) [63]. Gluten, a complex molecule found in wheat but also in rye, barley, triticale, and oats, is directly associated with gluten-related disorders [2,14].
Wheat gluten, a group of water-insoluble proteins, accounts for ~72% of wheat protein and consists mainly of wheat storage proteins, comprising gliadins (28–50 kDa) and glutenins (100 kDa–10 MDa), with roughly equal proportions of each. Glutamine and proline represent ≥50% of the peptide-bound amino acids. The gliadins are primary monomeric polypeptides classified as α- (α/β 28–33%), γ- (23–31%), and ω1,2- and ω5 (7–13%) based on their amino acid sequences and mobility at low pH in gel electrophoresis. Glutenins consist of low (40–55 kDa)- and high (80–120 kDa)-molecular-weight glutenins (LMW-GS and HMW-GS, respectively) that aggregate as a result of interchain disulfide bonds [64]. In other cereals, gluten proteins are γ-secalins and HMW secalins for rye, D-, B-, γ-, and C-hordeins for barley, and avenins (prolamins) for oat [12,14]. Gluten peptides (derived from the partial digestion of proteins) contain repeated amino acid (Pro and Gln) sequences (commonly referred to as CD-active epitopes) that are recognized by immune cells and induce the inflammatory responses in the gut epithelium [11]. More than 1000 CD-active peptides (CD-toxic and CD-immunogenic) from gluten proteins of cereals have been identified [65]. The length of these CD-active peptides varies from 7/9 to 91 amino acids; the most important is the 33-mer peptide [7]. The γ3-gliadin provides four peptides and ω-gliadin one more associated with CD peptides [6]. The 33-mer peptide from α2-gliadin contains three overlapping T-cell epitopes, and it is the most resistant to proteolysis [11,14].
Flour protein hydrolysis during sourdough fermentation is primarily attributed to the action of grain proteases (mainly aspartic proteinases and serine carboxypeptidases) activated at acidic pH levels (3.0–4.5) within a moderate temperature range. Native gluten, glutenin, and gliadin suffer structural changes during fermentation, beginning with secondary structure changes (decreases in α-helixes and enhancing in β-sheets) and disaggregation of proteins due to disulfide-bond rupture, leading to protein depolymerization promoted by LAB [66]. Yeast isolated from sourdough (S. cerevisiae and T. delbrueckii) and later used for sourdough fermentation in monoculture had minimal impact on peptide hydrolysis, gluten depolymerization, or immunogenicity [66]. Despite the controversial topic about LAB contribution to cereal proteins degradation, peptide accumulation in sourdough is mediated by induced wheat proteases, while the production of smaller oligopeptides is via LAB proteinases [61]. Hence, medium-sized polypeptides resulting from primary hydrolysis are efficiently transported into Lactobacillus’ cytoplasm and subjected to peptidase activity as occurs with the 33-mer peptide [59]. Moreover, chemically acidified dough with similar pH values to sourdoughs did not degrade gluten up to the same end level when it was compared with the sourdough (reducing between 3.6- and 6-fold depending on the LAB mixture) [59].
3.3. Protein Hydrolysis Through Lactic Acid Fermentation
LAB degraded specific proteins, β-amylase, serpins, α-amylase trypsin inhibitors, and oleosin, thus enhancing the digestibility of wheat-derived foods [61]. Proteolytic activity of LAB comprises an extracellular serine protease, di- and oligopeptide-specific transport systems, and a wide range of intracellular peptidases that modify and affect gluten integrity [10]. Low pH (<4.0) induces changes in the molecular conformation and the solubility of monomeric glutenins; thus, high-molecular-weight glutenin subunits were hydrolyzed during fermentation, resulting in depolymerization of the glutenin macropolymer (GMP) [67]. These changes (proteolytic degradation of glutenins subunits) were observed after 6 h of sourdough fermentation in a dough formulated with flour, salt, water, and Lactobacillus sanfranciscensis LTH2581 or Lactobacillus pontis TMW1.397 [67]. Various LAB have been evaluated for their proteolytic activity and their effect on gluten. Table 1 presents selected LAB with a proteolytic effect on gluten.

Table 1.
Lactic acid bacteria (LAB) capable of degrading gluten during sourdough fermentation.
A screening of LAB’s gluten hydrolysis capability is recommended because proteolytic activity is strain-dependent. The capability of individual strains or cellular extracts to degrade toxic peptides or fragments in liquid or solid media is evaluated to identify potential LAB with gluten degradation capacity. Exploratory studies on fermented gluten could be conducted to assess the degradation of gluten levels, the peptide profile, and the antigenicity of the resulting peptide fractions, as El Mecherfi et al. [49] and Zadeike et al. [68] have performed. Afterwards, LAB’s proteolytic and peptidase activity should be tested in real sourdough preparation. For example, cell extracts (both cell wall and intracellular) from Lactobacillus plantarum CRL 759 and CRL 778 hydrolyzed 73% and 36% of the 31–43 α-gliadin fragments after 4 h in vitro [69]. Alvarez-Sieiro et al. [70] isolated various LAB (20 strains) with gliadin-metabolizing activity from sourdoughs. Most LAB were identified as Lactobacillus casei (15 strains). Half of the L. casei strains metabolized 50% of the 33-mer peptide in vitro after 24 h; one strain degraded 82% of the peptide in 8 h and fully hydrolyzed it in 12 h.
Five LAB (Lev. brevis A6, Co. alimentarius G4, F. sanfranciscensis SB52, and Ln. pseudomesenteroides D4 and W2) were evaluated individually for their proteolytic activity during sourdough fermentation [61]. Each bacterium extensively hydrolyzed γ-, α-gliadin, and high-molecular-weight (HMW) glutenins after 48 h of fermentation. The hydrolysis level during fermentation showed insignificant changes in γ-gliadin after six hours and slight reductions after 24 h. ω-gliadins were resistant to LAB proteolysis due to their high proline content; glutenins also showed great resistance to LAB starters. Moreover, the central region of α–gliadin–encoding immunotoxic sequences for celiac patients was not affected by LAB’s peptidases. Thus, the action of individual LAB strains does not contribute to abolishing the toxic effects of CD-active peptides [61]. Individual LAB is not capable of reducing or eliminating the wheat flour toxicity during sourdough fermentation. An individual strain does not produce the entire mixture of peptidases required for extensive gluten degradation; thus, a combination of effective strains can act synergistically [14]. It is evident that LAB have different proteolytic activity; consequently, gluten degradation also varies between species and/or strains. The specific LAB capabilities to degrade gluten are attributed to its proline-specific peptidases (PSP) and post-glutamine cleaving peptidases content. PSPs can hydrolyze proline-rich peptides, while post-glutamine cleaving peptidases act against glutamine-rich peptides, including both CD-active peptides [27]. The most common PSPs are prolyloligopeptidase, prolylendopeptidase, fibroblast activation protein, dipeptidylpeptidases, prolylcarboxypeptidase, aminopeptidases, and prolidase [27]. Thus, LAB’s peptidase activity is crucial to determine their gluten degradation capability. Gerez et al. [71] evaluated the gliadin degradation during sourdough fermentation of a mixture of two LAB (L. plantarum CRL 775 and P. pentosaceus CRL 792) or the cell-free extract (CFE) containing proline-rich proteolytic enzymes. The peptidase activity was previously investigated in both bacteria. The pool of bacteria has peptidase activities of prolyl iminopeptidase (PepI, 2.0 units), X-prolyl-dipeptidyl aminopeptidase (PepX 36.1 units), gluteryl aminopeptidase (PepA 2.7 units), and prolidase (PepQ 2.7 units). After 24 h, gliadin hydrolysis was 70.3% and 82.7% for LAB fermentation and CFE, respectively. It appears that CFE degraded gliadin more rapidly because the free enzyme cocktail encountered gliadin immediately. In contrast, in LAB, peptides must be transported across the cell membrane into the cytoplasm. It is worth mentioning that the pH in dough added with CFE remained >5.0; thus, gliadin degradation was exclusively from LAB peptidases since flour endogenous enzymes were not activated [71]. Brzozowski [72] obtained the enzymatic content, including endopeptidase and PSP activity, from L. acidophilus 5e2 and L. sanfranciscensis DSM20663, and evaluated their proteolytic activity in wheat flour. Wheat flour (1.5 g) suspended in water (3 mL) was treated with enzyme preparations at 5 U/mL and incubated at 30 °C for three hours. Gliadin content and immunoreactivity were reduced by 43% and 45% for L. acidophilus and L. sanfranciscensis enzymes, respectively. Later, a mixture of peptidases from L. acidophilus 5e2 and A. niger improved the gliadin hydrolysis at pH 4.0 reaching 66 and 74% when incubated at 30 or 37 °C, respectively. The enzymatic activity was tested on gliadin solution [73]. A current approach to identify LAB capability to degrade gluten and their CD-toxic peptides is searching in the LAB’s genome for genes encoding peptidases with the potential to hydrolyze bonds in proline-rich peptides, as Leszczyńska et al. [74] performed. They analyzed three genomes of LAB (L. casei LC310, L. paracasei LPC100, and L. plantarum LP140) and identified a high number of genes encoding typical proline-specific enzymes in L. casei LC310 and L. paracasei LCP100, which exhibited higher gliadin degradation. Moreover, the prolyl oligopeptidase (POP) and pyrrolidone-carboxylate peptidase (Pcp) are present in the efficient gliadin degradation bacteria (L. casei LC310 and L. paracasei LCP100) compared with L. plantarum LP140 (the inefficient one). The Pcp is involved in the removal of L-pyroglutamic acid from the amino-terminus of pyroglutamyl proteins and peptides. At the same time, POP is a key enzyme in the hydrolysis of immunogenic peptides. Other peptidases that cleave prolyl bonds, such as metalloendopeptidases (PepO and PepF) and proline iminopeptidase PepI, were also encoded in higher copy numbers in L. casei LC310 and L. paracasei LCP100 [74]. Despite this, genome analysis of LAB provides a useful tool for identifying their gluten degradation capability; it is necessary to confirm their functionality in sourdough and test the immunogenicity of food products in CD patients.
LAB mixtures are more effectively degrading toxic peptides derived from gluten due to the presence of proteases/peptidases with different specificities that completely break the toxic peptides derived from gliadin and glutenin. For instance, a mixture of L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B fully hydrolyzed the fragment 62–75 of A-gliadin and 33-mer peptide in vitro due to their enzymatic activity, including iminopeptidase, dipeptidyl-peptidase, prolyl endopeptidase, prolidase, prilinase, and aminopeptidase P. In sourdough (mixture of wheat, oat, millet, and buckwheat at 3:1:4:2), wheat prolamins almost disappeared after 24 h of fermentation. In contrast, those from oats, millet, and buckwheat were affected less or not at all [8]. The authors also evaluated the proteolytic activity of sourdough using viable cells and viable cells + cytoplasmic extract from the 4 Lactobacillus and compared gluten degradation. There were no differences in gluten hydrolysis because long-chain polypeptides hydrolysis started with cell wall-associated proteinase [8]. In another study, the hydrolysis of 33-mer peptide was evaluated in the extracellular environment and the cell cytoplasm of two mixtures of LAB for 48 h; 70% of the 33-mer was reduced after 6 h and completely hydrolyzed in 18 h; thus, no traces were detectable in the cell cytoplasm [59]; the tested LAB were L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B or six strains of L. sanfranciscensis (LS3, LS10, LS19, LS23, LS38, and LS47). Table 2 presents selected studies about sourdoughs using LAB and their effect on gluten. Most studies have been reported by the Gobbetti’s group using 4 or 10 Lactobacillus strains (in two effective mixtures) with great gluten hydrolysis. After that, other researchers have investigated the gluten-degrading capability of other LAB strains with similar results. According to Table 2, most studies focus on wheat and rye flour, with sourdough conditions ranging from 8 to 48 h of fermentation, commonly at 28, 30, or 37 °C. Interestingly, no one mixture of LAB was capable of completely degrading the gluten and its toxic peptides. Thus, the addition of fungal proteases was necessary to achieve this goal [75].

Table 2.
Selected studies of lactic acid bacteria (LAB) used in sourdoughs and their effect on gluten proteins.
LAB sourdough starters comprise a mixture of various LAB; they are more effective than individual bacteria in gluten degradation. A key factor is the fermentation time of sourdough; longer fermentation times result in a greater degradation of gluten. However, 48 h appears to be the maximum, as low pH reduces microbial growth and slows down enzymatic (peptidolytic) activity [61]. In this regard, 53% of gluten was reduced by a sourdough starter after 45 h and 42% within 21 h [76]. Therefore, short-time fermentations (60–90 min) did not result in gluten degradation since acid conditions did not activate the native proteases of flour [78]. The sourdough fermentation does not degrade all gluten, but it reduces it importantly, thus adding enzymatic treatment with prolyl endopeptidases could result in levels below the limit of quantification (10 mg gluten/kg) to perform a sourdough free of CD peptides [76]. Rizzello et al. [59] used a cocktail of six strains of L. sanfranciscensis (LS3, LS10, LS19, LS23, LS38, and LS47) with peptidase system (active towards Pro-rich peptides) and two proteases (one from Aspergillus oryzae and one from Aspergillus niger) commonly used for bakery applications to prepare a wheat flour sourdough, and incubated at 37 °C for 48 h (stirring 200 rpm), reaching a gluten level of 12 ppm. The 2D electrophoresis analysis indicated no albumin-globulin polypeptides after fermentation and enzymatic treatment. In contrast, gliadin polypeptides and some glutenin polypeptides remained in the sourdough (160/193 spots showed 95–100% hydrolysis, 24 exhibited 50–70%, and 9 demonstrated 30–50% hydrolysis). Gliadin was reduced to a few peptides of ~8 kDa with no immunogenic activity, while gliadin peptides residues 62–75 had concentrations < 1 ppm. Additionally, LAB sourdough + proteases produced high amounts of free amino acids (14,622 mg/kg) compared with chemically acidified dough (1050 mg/kg) [59]; similarly, De Angelis et al. [7] obtained gluten content <200 ppm for 9 cultivars of durum wheat semolina fermented with the same LAB pools, 400 ppm of proteases, and 72 h of fermentation under the same conditions. The prolyl endo peptidases (PEP) from A. niger cleaved efficiently whole gliadins and their peptic-tryptic hydrolysates at a wide range of pH values (pH 2–8, with optimal activity pH 4–5) [27]. PEP from A. niger degrades CD-active peptides along with intact α-gliadins, γ-gliadins, HMW-GS, and LMW-GS [79]. A. oryzae produces peptidases (non-specific aminopeptidase and X-prolyldipeptidylpeptidase) with effective hydrolyzing activity against proline-containing peptides at acidic conditions (pH values 2–6, optimal pH 4.0) [27]. The optimal pH for PEP and peptidases is closely related to the dough pH produced during sourdough fermentation, resulting in a synergistic combination to degrade gluten, as studies have demonstrated. Complete gluten degradation by LAB requires fungal PEP and peptidases produced during sourdough fermentation; only the combination ensures the low required levels of CD-active peptides in dough. Fungal protease alone does not destroy the whole gluten proteins or gliadin content [72,79].
The identification of enzymes from LAB capable of degrading gluten proteins to peptides <9 amino acid residues could effectively eliminate CD-immunoreactivity peptides [80]. De Angelis et al. [7] investigated the capability of 9 partial purified peptidases from Lactobacillus to degrade the 33-mer peptide. Peptidases were from the pooled cytoplasmic extracts of 10 lactobacilli (L. sanfranciscensis 7A, LS3, LS10, LS19, LS23, LS38, and LS47, L. alimentarius 15M, L. brevis 14G, and L. hilgardii 51B). Enzymatic combinations of 6 to 8 peptidases were required to completely hydrolyze a 33-mer peptide after 14 h of incubation; the same peptidases hydrolyzed other synthetic immunogenic epitopes (fragments 57–68 of α9-gliadin, 62–75 of A-gliadin, and 134–153 of γ-gliadin) after 12–14 h. In the same study, the polypeptide soluble extracts from sourdough (fermented with 10 lactobacilli and added with two fungal proteases) of durum wheat semolina have been tested in cellular cultures after pepsin and trypsin digestion to assess their inflammatory response; the extracts do not induce synthesis of IFN-γ and IL-2, revealing its non-toxic effect [7].
Sourdoughs can be intentionally inoculated with health-beneficial bacteria, such as probiotics, to study their impact on the sourdough beyond their health benefits or in vivo gluten hydrolysis. For instance, the preparation VSL#3, a mixture of LAB + Bifidobacterium (Streptococcus thermophilus, L. plantarum, L. acidophilus, L. casei, L. delbrueckii spp. bulgaricus, Bifidobacterium breve, B. longum, and B. infantis), presented high hydrolytic activity on gluten during dough fermentation, degrading 79 of the 84 gliadin polypeptides, of which 65 of the 79 polypeptides presented hydrolysis factors > 80% [81]. Recently, the use of re-assembly LAB (Lpb. plantarum DSM33366, DSM33364, Lcb. paracasei DSM33373, Lim. reuteri DSM33374) and Bacillus (B. megaterium DSM33300, B. pumilus DSM33297, DSM33355) consortia (probiotic and gluten hydrolysis activity) supplemented with commercial protease preparation (Promod™ D24MDP, Biocatalysts, Cardiff, Wales, UK) or a prolyl-oligopeptidase (Tolerase G®, DSM-Firmenich) was evaluated on the gluten metabolism during the simulated gastrointestinal conditions with a total time of 48 h. For white wheat bread, gluten reduction was achieved <20 ppm in less than 24 h [82].
The renewed interest in sourdough uses and benefits has driven studies to isolate and identify bacteria from specific and regional sourdoughs, investigating their benefits and properties. For example, Khan et al. [64] isolated from Pakistani fermented wheat sourdough Bacillus subtilis LZU-GM, which demonstrated gluten protein degradation of 73.7% gluten peptides (γ- α/β gliadins) in 24 h in vitro and 3-fold higher in the small intestine of mice model colonized compared with the untreated group. Similar results have been reported by Rashmi et al. [83] for Bacillus spp. (GS 188). Therefore, new genera and strains will be isolated and identified in the sourdoughs in further studies.
3.4. Changes in Non-Protein Components (FODMAPs), Phytate, and Antinutritional Compounds
The fermentable oligo-, di-, monosaccharides and polyols (FODMAPs) include fructans and raffinose-family oligosaccharides (oligosaccharides), disaccharides (lactose), monosaccharides (fructose), and polyols (sorbitol and mannitol among others). Fructo-oligosaccharides (polymerization degree 3–10) are the main FODMAPs in wheat and rye flours, with levels between 1–3% and 3–5%, respectively [11], other carbohydrates present in cereals are excess fructose (1.1–3.8%), sorbitol (traces), mannitol (traces to 1.6%), raffinose (1.4–3.8%), mannans, galactans, and galacturonans (1–1.5% in wheat), and stachyose (traces) [13,41]. The main antinutrients include phytate, lectins, and ATIs.
FODMAPs are susceptible to baker’s yeast and LAB from sourdough fermentation. Thus, the dough development method, dough fermentation time, proofing time, and temperature, as well as sourdough fermentation (time, single culture, and mixed culture), have been investigated regarding FODMAP content in bread. The effect of the breadmaking process and the efficacy of the sourdough strategy in reducing FODMAPs depend on the type of flour used, due to its initial content. In this context, rye flour and whole meal wheat require a multi-strain sourdough to achieve adequate reductions of FODMAPs, effectively transforming oligosaccharides into monosaccharides and polyols, followed by their metabolization to low levels in the dough [84]. FODMAP cut-off levels have been established to identify a low-FODMAP food as follows: 0.5 g of total FODMAPs, 0.15 g fructose, 0.3 g total oligosaccharides, 0.4 g total polyols (or 0.2 mannitol or sorbitol), and 1 g lactose per serving (50 g of bread) [85].
During breadmaking, partial degradation of wheat or rye fructans (5–6) is achieved by yeast invertase. In most lactobacilli, fructans are internalized and then hydrolyzed intracellularly, which leads to partial degradation of fructans [86]. Straight dough reduced <50% of fructans in bread, conventional sourdough between 65 and 70%, and dough supplemented with L. crispatus reached >90% [86]. Sourdough fermentation (12 h, using a commercial type I sourdough starter) reduced up to 69% of fructans, 69% of raffinose, and 41% of ATIs in wheat doughs; however, an increase in mannitol was observed, reaching 550% at the same time [21]. Some LAB have extracellular fructosidases induced by certain sugars (fructose, sucrose) or oligosaccharides (inulin) and repressed by others. Fos E (an extracellular β-fructosidase) has been identified in L. paracasei and Liquorilactobacillus salivarius, while Fru A (an extracellular β-fructosidase) was found in L. crispatus DSM29598 [86]. Rye sourdough supplemented with a fructosidase-producing strain decreased the fructan content by up to 0.5% after 12 h of fermentation. Fru A is necessary for to rapid hydrolysis of fructans [86]. The initial content of fructan in rye flour was ~2.3%. LAB transform raffinose to melibiose and fructose by the action of extracellular levansucrase, followed by cell internalization to hydrolyze by the action of the α-galactosidase [13]. Heterofermentative LAB, such as Limosilactobacillus, convert fructose to mannitol (also a FODMAP); thus, fructose and mannitol should be monitored during sourdough [86]. Other LAB, such as L. delbrueckii, L. casei, L. plantarum, and L. salivarius, metabolize mannitol [13].
Depending on the dough and its FODMAP content, prolonged dough proofing (>4 h) may be sufficient to reduce FODMAP levels in the bread product by up to 90% [87]. To improve FODMAP reductions in flours with higher sourdough content, proofing time can be combined. Schmidt and Sciurba [84] evaluated the effect of proofing time and the commercial use of a sourdough starter (F. sanfranciscensis (1 × 109 CFU/g) and Candida milleri (5 × 106 CFU/g, fermented at 26 °C for 16 h) on the FODMAP content of standard wheat bread, Ciabatta-style breads, whole meal wheat bread, and rye breads. The extended proofing time significantly reduced the excess of fructose due to the increased consumption of the sugars by the yeast. Proofing temperature also plays a key role in the FODMAP reduction. At suboptimal temperature, yeast metabolic activity is slowed down, resulting in less consumption of the monosaccharides, including fructose. The incorporation of sourdough in bread only alters the FODMAP composition, reducing fructans and releasing polyols. All the wheat bread reached low-FODMAP classification while bread from whole meal wheat and rye bread were sorted as high-FODMAPs [84]. In this regard, the inclusion of fructophilic LAB (Apilactobacillus kunkeii and Fructobacillus fructosus) could improve the reduction of fructose during sourdough [20]. The production and accumulation of polyols from LAB fermentation during sourdough could be driven if adequate strains are chosen. Borowska et al. [88] studied the content of FODMAPs in whole-wheat bread using homofermentative LAB (Lpb. plantarum FST1.7, Lcb. paracasei R3, and P. pentosaceus RYE106) to avoid the polyols production during sourdough, while reducing fructans. The resulting breads fulfill the low-FODMAP criteria; however, the contribution of LAB is unclear since the bread without sourdough also met the criteria. As previously mentioned, the use of strains with fructosidase activity favors FODMAP degradation in the product. L. paracasei FJSSZ3L1 with β-fructosidase (Fos E) was used in sourdough (24 h fermentation) to formulate steamed bread. The bread satisfied the criteria of low-FODMAPs [89].
ATIs are proteins part of the albumin and globulin fraction of wheat. They degrade when pH is <4.0 and can have inflammatory effects on human epithelium cells. As gluten proteins, ATIs could also be hydrolyzed by LAB during sourdough fermentation. P. pentasaceus Pp3, L. coryniformis Lco4, and L. paracasei Lpa4 exhibited ATIs degradation capacity with reducing levels between 85% and 70.2% in a model system [90]. As it occurs with gluten degradation, ATI disassembly could improve with a mixed culture rather than with individual strains. Only 30% of the ATIs remained as tetramers in wheat dough when rye sourdough starter with a multi-species mixture was used to ferment the dough (24 h, 30 °C, 9 log CFU/g of F. sanfranciscensis, L. crispatus, W. cibaria, and yeast Kazachstania humilis in 7 log CFU/g) [24]. In contrast, Stefańska et al. [91] obtained similar levels of degradation in albumin/globulin fraction after sourdough fermentation (24 h at 37 °C) using LAB (L. curvatus 750, P. acidilactici EKO26, P. pentosaceus 1850, L. coryniformis pA, W. cibaria EKO31, L. plantarum KKP593, L. helveticus Lh10) in mono- or mixed cultures. The tested LAB completely hydrolyzed IgE-binding polypeptides with a molecular weight of ~24 kDa and partially the polypeptide of ~19 kDa, reducing the allergenicity of wheat sourdoughs. Huang et al. [24] recorded lower pro-inflammatory bioactivity in human monocytes of bread prepared with sourdough fermentation using F. sanfranciscensis DSM20451T, Lat. sakei LS8, and Lim. reuteri LTH5448. According to studies, the proteolytic/peptidolytic activity of LAB contributes to ATIs degradation during sourdough; however, the mechanisms and specific enzymes involved are unknown, as they have been less investigated.
The pH conditions generated during sourdough fermentation favor enzyme degradation of phytate, a complex of phytic acid with proteins and polyvalent cations (Fe, Zn, Ca, Mg) present in cereals. Phytate reductions increase the solubility and bioavailability of cations. Phytic acid breakdown was associated with dough’s acidic pH (5.5–4.5) produced by LAB, which provides the optimum pH for the flour’s endogenous phytases activity [92]. Slight decreases in pH during sourdough, with an acidic pH of ~5.0, activate endogenous phytases present in the flour, resulting in phytate reductions of ~70% in whole-wheat flour, comparable to chemical acidification [93]. Magnesium solubility increased during conventional bread fermentation, while phytic acid breakdown occurred during 5 h when 35% of sourdough from L. plantarum S18 and Ln. mesenteroides S50 (18 h at 30 °C) was used in the formulation of whole-wheat bread [92]. After 5 h of fermentation, phytic acid was reduced by 62% or 38% when dough was added with sourdough or yeast, respectively. Moreover, bran inoculated with sourdough and incubated for 4 h before breadmaking improved phytate reduction by up to 90%. Reale et al. [94] demonstrated that phytate conversion is owing to endogenous cereal phytases at lower pH (LAB fermentation) and not to LAB’s enzyme activity. They showed that heat-treated and LAB inoculated dough retained a steady content of phytate after fermentation. Therefore, phytate decreases in the studied flours were not strain-dependent, and the reduction levels were 100% in rye flour, 95–100% in wheat, and 39–47% in oat flour after 24 h of fermentation. Other studies investigated whether LAB possesses phytases and tested their activity. De Angelis et al. [95] studied the effect of L. sanfranciscensis CB1 (phytase active strain) or its cytoplasmic extract on the Na-phytate content during sourdough fermentation (8 h at 37 °C). Authors obtained marked reductions in Na-phytate content (64–74%) compared with the control dough. Most studies have revealed that phytate is removed by cereal endogenous phytases activated at low pH (through fermentation or chemical treatment). Probably, in selected LAB strains, the intracellular bacterial phytases may contribute to phytate degradation.
The degradation of wheat germ agglutinin (WGA) during sourdough fermentation was also investigated. WGA is a lectin located in the germ of the wheat grain with a dimer size of 35 kDa (pH 3.5 to 7.4); each monomer is stabilized by 16 intramolecular disulfide bonds [96]. WGA could be associated with NCWS; however, their contribution is unclear [97,98]. F. sanfranciscensis DSM20451 and Lat. sakei TMW 1.22 reduced the content of WGA in the sourdough (24 h at 30 °C) due to the biological reduction in oxidized glutathione by the glutathione reductase [99]. WGA reduction was mediated by the thiol metabolism via oxidation of thiol groups (as Lat. sakei) or by increasing the thiol content, as F. sanfranciscensis did. In both cases, the redox potential of Lactobacillus is associated with the mechanisms of WGA conversion. Reductions between 36 and 59% of WGA were observed after 0.5 h of fermentation with Lat. sakei and F. sanfranciscensis, respectively; the WGA content increased slightly after 24 h.
3.5. Impact of Sourdough Products on Gluten Intolerance, Non-Celiac Gluten Sensitivity, and IBS
The impact of food products formulated from sourdough and proteases, where gluten was fully hydrolyzed, has been evaluated in CD patients. Outstanding results demonstrate that wheat bread containing non-toxic peptides can be produced and consumed safely. The intestinal permeability of CD patients with a gluten-free diet did not change when intake bread from wheat sourdough using 4 LAB (L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, and L. hilgardii 51B) and their cytoplasmic extract, 24 h at 37 °C) and mixed with adequate amounts of oat, millet, and buckwheat flours; in addition, the rhamnose and lactulose absorption as well as the lactulose/rhamnose ratio did not differ significantly from the baseline values after the intake of 80 g of bread [8]. The BAL mixture completely hydrolyzed the α2-gliadin-derived epitopes 62–75 and the 33-mer peptide in sourdough [8]. Baked goods prepared with sourdoughs and fungal proteases have been tested on CD patients to evaluate their safety. The ingestion for 60 days of 200 g/day sweet baked goods (biscuits and cakes) made with fully hydrolyzed wheat flour (8 ppm gluten) by CD patients did not modify the serological and immunohistochemistry analyses (TG2 antibodies, CD3 cells, TCRγδ cells, CD25 cells), the histologic Marsh classification, or clinical symptoms at the end of the study [9]. Likewise, Di Cagno et al. [100] recorded normal values of hematology, serology (total serum IgA, IgG and IgA antigluten, endomysial and tissue transglutaminase IgA antibodies), and intestinal permeability in young CD patients, after 60 days of daily intake of 200 g of sweet baked goods (equivalent to 10 g of native gluten) prepared with spray dried wheat sourdough (using LAB and fungal proteases) with degraded gluten to <10 ppm. In other studies, the inflammatory markers (interleukin-2 and interleukin-10) and interferon gamma (IFN-γ) reduced up to basal levels in cultures from duodenal biopsy specimens (CD patients) after the intake of bread prepared from sourdough mixture 1 (L. casei, L. delbrueckii subsp. bulgaricus, L. paracasei LPC01 and BGP2, L. plantarum BGP12, LP27, LP35, LP40, LP47, and SP1; Francavilla et al., 2017) or mixture 2 (Lpb. plantarum DSM33363 and DSM33364, Lcb. paracasei DSM33373, Lim. reuteri DSM33374, Bacillus megaterium DSM33300, Bacillus pumilus DSM33297 and DSM33355, and fungal proteases) [101]. The microbial enzymes from mixture 1 fully hydrolyzed immunogenic epitopes (the gliadin 33-mer peptide, the peptide spanning residues 57 to 68 of the α9-gliadin, A-gliadin peptide 62–75, and γ-gliadin peptide 134–153) in vitro [102]. Mandile et al. [103] investigated the effects of the intake of baked food (200 g/day equivalent to 10 g gluten) prepared with hydrolyzed wheat flour (sourdough using lactobacilli and fungal proteases) in CD patients (12–24 years old) for 6 days. The intake of baked food maintained the immunogenicity of T cells and peripheral blood immune response (IFN-γ) at a similar level to the baseline. Additionally, no clinical symptoms (abdominal pain, vomiting, diarrhea, or headache) were reported by patients during the study.
In IBS patients, the intake of sourdough (>12 h fermentation) bread does not induce inflammatory or gastrointestinal symptoms compared with yeast bread. In a randomized and double-blind crossover trail, the intake of pasta and wheat bread reduced in gluten (50%, using sourdough fermentation (8 h) and fungal proteases) after two weeks, significantly decreased Visual Analogue Scale (VAS) score while no differences were found in the Hospital Anxiety and Depression Scale and Irritable Bowel Syndrome Quality of Life in IBS patients; 50% of patients improved at least 30% of VAS (pain descriptors) and/or Irritable Bowel Syndrome Severity Score [60]. However, in another pilot study, the intake of 150 g of bread (either sourdough or yeast bread) for 7 days by patients with IBS did not result in significant differences in gastrointestinal symptoms or inflammatory markers [104]. In another study, the effects of consuming low-FODMAP rye bread prepared using a specific sourdough system and standard rye bread were compared in IBS patients. The intestinal microbiota and IBS symptoms were analyzed in patients who consumed 7–8 slices of rye bread per day for 4 weeks. The microbial taxa were affected by bread type due to a decline in Klebsiella when low-FODMAP bread was consumed. Flatulence, dyspepsia, and heartburn symptoms decreased in patients who consumed low-FODMAP rye bread, indicating an improvement in the long-term management of IBS-associated symptoms and overall gut health in IBS patients [105].
4. Technological and Processing Considerations
The microbiological complexity of sourdough presents additional challenges. Spontaneous fermentation can lead to variability in microbial populations, causing inconsistent bread quality and making large-scale industrial production less predictable and more demanding [106]. The use of selected and characterized microbial consortia has been proposed to address these issues. Gélinas and Théolier [107] highlighted that patented inventions employing specific proteolytic bacteria offer promising solutions for producing gluten-reduced foods from gluten-containing cereals. However, these products are more accurately described as “gluten-reduced” rather than “gluten-free,” according to current regulatory standards.
Among the patented technologies, Giuliani et al. [108] developed a method for partial degradation of gluten, where a liquid dough containing 70% water and 30% gluten-rich flour (wheat, barley, rye, or oat) is fermented at 30–37 °C for approximately 12 h using L. sanfranciscensis DSM22063 and L. plantarum DSM22064, along with fungal proteases. This method yields a dough with residual gluten concentrations between 20,000 and 80,000 ppm, suitable for producing foods with reduced gluten content. Bread formulated with the detoxified sourdough and supplemented with 20% gluten-free flours (maize, rice, quinoa, teff, amaranth, buckwheat) reportedly resulted in no alteration of intestinal permeability in celiac patients. However, gluten quantification techniques were not specified. Extending this work, Giuliani et al. [109] patented a biotechnological process aimed at complete gluten degradation to levels below 20 ppm, suitable for producing gluten-free foods. This approach also uses L. sanfranciscensis DSM22063 and L. plantarum DSM22064 with fungal proteases to ferment liquid doughs derived from gluten-containing flours. Although detoxified flours followed a standardized protocol for gluten reduction, results showed a residual gluten range of 20,000–80,000 ppm when assessed with non-specific ELISA, indicating partial degradation better suited for gluten-sensitive individuals rather than celiac patients.
Another approach was proposed by Pedroza-Islas [110], who patented a microencapsulated bacterial consortium for gluten degradation. This consortium includes three lactic acid bacteria strains (L. plantarum ATCC 8014, L. sanfranciscensis ATCC 27652, L. brevis ATCC 14869), combined with milk serum protein isolate, maltodextrin, Arabic gum, maguey honey, trehalose, and both bacterial and fungal proteases. Although the patent claims achieving gluten concentrations below 10 ppm, this figure is based on non-specific ELISA assays and lacks full validation.
These technologies demonstrate the potential of targeted microbial fermentations to control gluten degradation more consistently than spontaneous sourdough processes. However, standardization, reproducibility, and validated gluten quantification remain critical hurdles for the broader industrial application of these methods. Beyond its structural aspects, sourdough fermentation has a significant impact on sensory properties and shelf life. It enhances flavor by producing organic acids and volatile compounds, offsetting the blandness often associated with gluten-free or gluten-reduced products [111]. Furthermore, antimicrobial metabolites produced by LAB contribute to extended shelf life by inhibiting spoilage organisms [112,113,114,115]. Nonetheless, balancing these sensory and preservation benefits with process scalability remains a major hurdle for industrial applications [116].
Sourdough application to reduce gluten content in wheat-based products offers notable opportunities but also introduces technological challenges. During fermentation, partial hydrolysis of gluten alters dough rheology, potentially compromising gas retention and dough structure [117]. This often results in products with reduced loaf volume and modified texture [106], especially where gluten’s viscoelastic properties are critical.
Several factors influence the quality of sourdough bread, including fermentation conditions, flour particle size, protein quality, starch characteristics, and dietary fiber composition [106]. To mitigate negative effects, strategies such as the addition of hydrocolloids, enzymes, or adjustments to fermentation parameters (e.g., extended times or optimized hydration levels) have been explored [106,118,119]. Sourdough fermentation critically affects dough systems when gluten is reduced or hydrolyzed [120]. As shown in Table 3, enzymatic and microbial activity weakens the gluten network, reducing dough extensibility and gas retention [117,118], typically resulting in lower loaf volume. To address these issues, incorporating hydrocolloids and protein fortification has been explored with promising results.

Table 3.
Effects of lactic acid bacteria (LAB) sourdough fermentation on technological properties of wheat-based products.
Hydrocolloids such as xanthan gum, guar gum, and hydroxypropyl methylcellulose (HPMC) are widely utilized to replicate gluten’s viscoelastic properties, improving dough handling and final bread quality [122]. Torres-Pérez et al. [123] demonstrate that HPMC enhances dough elasticity and loaf volume, psyllium husk improves moisture retention, and xanthan gum refines crumb structure. Similarly, Irondi et al. [124] highlighted the functional advantages of guar gum, locust bean gum, and pectin in improving texture, moisture, and shelf life in gluten-free baked products. Capriles and Arêas [125] also reported that HPMC enhances gas retention and crumb structure in gluten-reduced formulations.
Protein fortification strategies, employing sources such as egg white, whey protein isolate, and soy protein, have improved reduced-gluten breads’ nutritional profile and mechanical strength [126]. Combining hydrocolloids with protein ingredients often produces synergistic effects, yielding better textural properties and improved sensory acceptance. Castellanos-Fuentes et al. [127] demonstrated that soybean extruded-expelled meal fortified with probiotics enhances bread volume, texture, and protein content. Peñalver et al. [128] successfully developed gluten-free functional bread enriched with olive, acerola, citrus, spinach, calcium, iron, and linseed, improving antioxidant capacity, mineral content, and fiber.
Despite structural challenges, sourdough fermentation offers substantial advantages in flavor development and shelf-life extension. LABs generate a complex profile of organic acids and volatile compounds [111] while inhibiting spoilage organisms [112,113,114,121]. According to Alkay et al. [1], metabolites produced by sourdough microbiota, including yeasts, enhance the bread’s technological and nutritional features and extend shelf life. However, over-degradation of the gluten network can lead to undesirable gumminess [106], highlighting the importance of controlled fermentation and careful ingredient selection [129]. De Bondt et al. [116] emphasized the substantial variations in fermentation conditions, leavening agents, and resulting bread quality, particularly regarding loaf volume and acidification. Integrating sourdough fermentation into breadmaking improves shelf life and flavor beyond what is achievable with baker’s yeast alone [1], offering potential nutritional benefits [116]. Optimizing sourdough fermentation for gluten-reduced products requires balancing the achievement of desirable technological properties and maintaining consistent product quality. Microbial selection, ingredient functionality, and process control continue to enhance the feasibility of producing high-quality, gluten-reduced baked goods at an industrial scale.
5. Challenges and Limitations
Although interest in sourdough fermentation has increased over the past two decades, its widespread adoption as a strategy for gluten reduction faces significant scientific and regulatory obstacles. A major challenge lies in the variability of microbial communities during fermentation, which can lead to inconsistent gluten degradation [10,130]. Standardized starter cultures with proven gluten-hydrolyzing activity are crucial for ensuring reproducibility and safety, particularly for individuals with gluten-related disorders. Although certain lactic acid bacteria strains exhibit promising gluten-degrading activity, most sourdough fermentation protocols—without the addition of exogenous fungal proteases—do not reliably reduce gluten to levels below the <20 ppm threshold considered safe for individuals with celiac disease [131,132,133] therefore, such products should not be marketed as gluten-free unless their gluten content is verified using certified analytical methods [134].
Research into the physiology and biochemistry of sourdough LAB has improved the understanding of sourdough microbial ecology and its effects on flavor, texture, shelf life, and nutritional value [135]. However, achieving the degree of gluten degradation necessary for gluten-free labeling, typically less than 20 ppm, according to Codex Alimentarius, is not consistently possible through fermentation alone [15,119]. This presents concerns for CD patients, where even trace amounts of gluten can trigger immune responses. Thus, while sourdough fermentation holds promise for individuals with non-celiac gluten sensitivity or gluten intolerance, its application for producing safe foods for CD patients is possible by combining the use of selected sourdoughs with proteases from fungi, as explained in Section 3.3 and Section 3.5. Moreover, in selected markets such as Italy, bread and other baked goods made with hydrolyzed wheat flour are currently distributed and available [15].
From a consumer perspective, distinguishing between “gluten-reduced” and “gluten-free” products poses ethical and marketing dilemmas [136,137]. Discrepancies exist between in vitro and in vivo findings on sourdough fermentation, and human clinical evidence remains insufficient for all populations. Consequently, regulatory bodies such as EFSA (in the EU) and the FDA (in the United States) have not authorized health claims about sourdough’s potential gluten-reducing benefits anywhere [11]. Transparent regulatory frameworks and clear definitions are critical to maintaining consumer trust [15].
As summarized in Table 4, microbial variability, particularly with undefined or spontaneous fermentations, continues to hinder standardization and safe gluten reduction [12]. This inconsistency can lead to unpredictable degrees of gluten hydrolysis, posing a risk for sensitive populations. Defining microbial consortia with documented proteolytic capabilities is increasingly recommended [11]. Even under optimized conditions, complete gluten degradation below the 20 ppm safety threshold is difficult to guarantee [138], and the use of exogenous glutenases has been proposed as a complementary strategy [23,119].

Table 4.
Main technological challenges in developing gluten-reduced sourdough products.
Regulatory challenges further complicate product labeling. For example, many jurisdictions prohibit gluten-free claims on wheat-derived products, regardless of residual gluten levels [7,134]. Accurate gluten detection is crucial for safeguarding individuals with CD. Analytical methods must be sensitive, specific, and validated [138]. Table 5 presents the techniques commonly used, including ELISA (especially the R5 sandwich ELISA), Lateral Flow Devices (LFDs), PCR, LC-MS/MS, and Western blots [138,147,148,149]. ELISA remains the most widely used method, though it can be less accurate for hydrolyzed gluten in fermented foods. LFDs provide rapid on-site testing [150], while PCR can detect gluten-containing cereal DNA in processed foods [138,151]. LC-MS/MS offers high specificity and sensitivity, particularly useful for validating gluten-free claims in complex matrices [152]. Western blotting is also employed for confirmatory analyses [153]. Further research in this topic should include the standardization of ELISA for detecting residual gluten [154] and HPLC-MS/MS for peptide profiling [138].

Table 5.
Analytical methods for gluten detection in foods.
Beyond scientific and regulatory challenges, scaling up gluten-reduced sourdough production is difficult due to the artisanal nature of many fermentation protocols. Industrial reproducibility requires rigorous process control and possibly genetic optimization of starter strains [117,118]. Lima et al. [139] emphasized the importance of controlling fermentation parameters, such as temperature, pH, dough yield, and flour type, for industrial scalability.
Finally, there is an urgent need for clearer labeling standards distinguishing “gluten-free” from “gluten-reduced” products [144,145,146]. Transparent communication with consumers will be key to building trust and ensuring the success of sourdough-based gluten-reduced products.
6. Final Comments and Remarks
Selected sourdough starters could eliminate the risk of contamination with gluten and enhance the nutritional properties of gluten-free bread. Physical and sensory properties of bread from almost completely degraded gluten are unpleasant. However, a suitable gluten-free protein or hydrocolloid could replace the lost functionality of gluten. In that case, it is possible to obtain bread with better sensory and nutritional quality than that obtained with natural gluten-free raw materials, because the typical flavor is preserved. LAB sourdough contributes to reducing FODMAPs, ATIs, and phytate directly or indirectly, leading to suitable baked goods for gluten-related disorders and IBS patients. Fungal proteases, as well as those from other bacteria, such as Bacillus, are required in sourdough fermentation to achieve a gluten level safe for CD patients. The intake of bread and baked goods (prepared using hydrolyzed wheat flour from sourdough) in the short and long term is an alternative strategy for CD patients, as the immune-stimulatory properties of gluten are eliminated. Sourdough products can be beneficial for patients with non-celiac gluten sensitivity and IBS, as they undergo partial gluten breakdown and have lower FODMAP content. To address current limitations, such as the lack of standard fermentation methods, a limited number of LAB with gluten hydrolysis capacity, inadequate reporting of LAB strain identification, and gluten residual analysis, which affect reproducibility, regulatory approval, and consumer safety, it is recommended to use validated analytical tools. Further research could focus on identifying new strains with gluten degradation capability, as a limited pool of LAB has been studied over the last two decades. The analysis of LAB genomes to identify genes encoding proline-rich peptides could support the potential discovery of new bacterial strains with gluten degradation capabilities. Additionally, the combination and use of proteases from diverse origins, such as bacteria, fungi, plants, or animals, could enhance gluten hydrolysis during sourdough fermentation. The new LAB sourdough strains and/or mixtures with alternative proteases must also be evaluated in food manufacturing and their immunogenicity in CD patients. LAB genera and species must be mentioned in the studies to allow the reproducibility and application of sourdough technology.
Author Contributions
Conceptualization, R.H.H.-F., A.L.-M., and E.M.-L.; formal analysis, R.H.H.-F., A.L.-M., and E.M.-L.; investigation, R.H.H.-F., A.L.-M., and E.M.-L.; resources, A.L.-M.; data curation, R.H.H.-F. and E.M.-L.; writing—original draft preparation, R.H.H.-F., A.L.-M., and E.M.-L.; writing—review and editing, R.H.H.-F., A.L.-M., and E.M.-L.; visualization, R.H.H.-F. and E.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
This review article did not generate new data.
Acknowledgments
The authors thank Universidad de las Américas Puebla for its support in carrying out this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alkay, Z.; Falah, F.; Cankurt, H.; Dertli, E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods 2024, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Serena, G.; D’Avino, P.; Fasano, A. Celiac Disease and Non-Celiac Wheat Sensitivity: State of Art of Non-Dietary Therapies. Front. Nutr. 2020, 7, 152. [Google Scholar] [CrossRef]
- Skovbjerg, H.; Koch, C.; Anthonsen, D.; Sjöström, H. Deamidation and Cross-Linking of Gliadin Peptides by Transglutaminases and the Relation to Celiac Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2004, 1690, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of Gluten-Related Disorders: Consensus on New Nomenclature and Classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Muskovics, G.; Farkas, A.; Bugyi, Z.; Tömösközi, S. Changes of Gluten Protein Composition during Sourdough Fermentation in Rye Flour. Cereal Chem. 2024, 101, 1354–1363. [Google Scholar] [CrossRef]
- Barre, A.; Benoist, H.; Rougé, P. Impacts of Sourdough Technology on the Availability of Celiac Peptides from Wheat α- and γ-Gliadins: In Silico Approach. Allergies 2023, 3, 39–57. [Google Scholar] [CrossRef]
- De Angelis, M.; Cassone, A.; Rizzello, C.G.; Gagliardi, F.; Minervini, F.; Calasso, M.; Di Cagno, R.; Francavilla, R.; Gobbetti, M. Mechanism of Degradation of Immunogenic Gluten Epitopes from Triticum Turgidum L. Var. Durum by Sourdough Lactobacilli and Fungal Proteases. Appl. Environ. Microbiol. 2010, 76, 508–518. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough Bread Made from Wheat and Nontoxic Flours and Started with Selected Lactobacilli Is Tolerated in Celiac Sprue Patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef]
- Greco, L.; Gobbetti, M.; Auricchio, R.; Di Mase, R.; Landolfo, F.; Paparo, F.; Di Cagno, R.; De Angelis, M.; Rizzello, C.G.; Cassone, A.; et al. Safety for Patients With Celiac Disease of Baked Goods Made of Wheat Flour Hydrolyzed During Food Processing. Clin. Gastroenterol. Hepatol. 2011, 9, 24–29. [Google Scholar] [CrossRef]
- Cristofori, F.; Francavilla, R.; Capobianco, D.; Dargenio, V.N.; Filardo, S.; Mastromarino, P. Bacterial-Based Strategies to Hydrolyze Gluten Peptides and Protect Intestinal Mucosa. Front. Immunol. 2020, 11, 567801. [Google Scholar] [CrossRef]
- D’Amico, V.; Gänzle, M.; Call, L.; Zwirzitz, B.; Grausgruber, H.; D’Amico, S.; Brouns, F. Does Sourdough Bread Provide Clinically Relevant Health Benefits? Front. Nutr. 2023, 10, 1230043. [Google Scholar] [CrossRef]
- Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation 2021, 7, 246. [Google Scholar] [CrossRef]
- Loponen, J.; Gänzle, M.G. Use of Sourdough in Low FODMAP Baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Wieser, H.; Koehler, P. Novel Approaches for Enzymatic Gluten Degradation to Create High-Quality Gluten-Free Products. Food Res. Int. 2018, 110, 62–72. [Google Scholar] [CrossRef]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; De Angelis, M.; Di Cagno, R. How to Improve the Gluten-Free Diet: The State of the Art from a Food Science Perspective. Food Res. Int. 2018, 110, 22–32. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Figueroa, R.H.; Mani-López, E.; Palou, E.; López-Malo, A. Sourdoughs as Natural Enhancers of Bread Quality and Shelf Life: A Review. Fermentation 2023, 10, 7. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The Low FODMAP Diet: Recent Advances in Understanding Its Mechanisms and Efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Minervini, F.; Filannino, P.; Sardaro, M.L.S.; Gatti, M.; Lindner, J.D.D. Effects of Sourdough on FODMAPs in Bread and Potential Outcomes on Irritable Bowel Syndrome Patients and Healthy Subjects. Front. Microbiol. 2018, 9, 1972. [Google Scholar] [CrossRef]
- Albiac, M.A.; Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. How Fructophilic Lactic Acid Bacteria May Reduce the FODMAPs Content in Wheat-Derived Baked Goods: A Proof of Concept. Microb. Cell Fact. 2020, 19, 182. [Google Scholar] [CrossRef]
- Boakye, P.G.; Kougblenou, I.; Murai, T.; Okyere, A.Y.; Anderson, J.; Bajgain, P.; Philipp, B.; LaPlante, B.; Schlecht, S.; Vogel, C.; et al. Impact of Sourdough Fermentation on FODMAPs and Amylase-Trypsin Inhibitor Levels in Wheat Dough. J. Cereal Sci. 2022, 108, 103574. [Google Scholar] [CrossRef]
- Geisslitz, S.; Scherf, K.A. Impact of Sourdough Microbiota on FODMAPs and ATI Content in Bakery Products. In Sourdough Microbiota and Starter Cultures for Industry; Ceresino, E.B., Juodeikiene, G., Schwenninger, S.M., da Rocha, J.M.F., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 425–459. ISBN 978-3-031-48603-6. [Google Scholar]
- Lamacchia, C.; Camarca, A.; Picascia, S.; Di Luccia, A.; Gianfrani, C. Cereal-Based Gluten-Free Food: How to Reconcile Nutritional and Technological Properties of Wheat Proteins with Safety for Celiac Disease Patients. Nutrients 2014, 6, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schuppan, D.; Tovar, L.E.R.; Zevallos, V.F.; Loponen, J.; Gänzle, M. Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity. Foods 2020, 9, 943. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac Disease. Nat. Rev. Dis. Primers 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Makharia, G.K.; Singh, P.; Catassi, C.; Sanders, D.S.; Leffler, D.; Ali, R.A.R.; Bai, J.C. The Global Burden of Coeliac Disease: Opportunities and Challenges. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 313–327. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Belozersky, M.A.; Filippova, I.Y.; Oppert, B.; Elpidina, E.N. Effective Degradation of Gluten and Its Fragments by Gluten-Specific Peptidases: A Review on Application for the Treatment of Patients with Gluten Sensitivity. Pharmaceutics 2021, 13, 1603. [Google Scholar] [CrossRef]
- Brouns, F.; Van Rooy, G.; Shewry, P.; Rustgi, S.; Jonkers, D. Adverse Reactions to Wheat or Wheat Components. Comp. Rev. Food Sci. Food Saf. 2019, 18, 1437–1452. [Google Scholar] [CrossRef]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac Disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Dale, H.F.; Biesiekierski, J.R.; Lied, G.A. Non-Coeliac Gluten Sensitivity and the Spectrum of Gluten-Related Disorders: An Updated Overview. Nutr. Res. Rev. 2019, 32, 28–37. [Google Scholar] [CrossRef]
- Roszkowska, A.; Pawlicka, M.; Mroczek, A.; Bałabuszek, K.; Nieradko-Iwanicka, B. Non-Celiac Gluten Sensitivity: A Review. Medicina 2019, 55, 222. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Boschetti, E.; Stanghellini, V.; De Giorgio, R. Features and Progression of Potential Celiac Disease in Adults. Clin. Gastroenterol. Hepatol. 2016, 14, 686–693.e1. [Google Scholar] [CrossRef]
- Czaja-Bulsa, G. Non Coeliac Gluten Sensitivity—A New Disease with Gluten Intolerance. Clin. Nutr. 2015, 34, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal Cell Damage and Systemic Immune Activation in Individuals Reporting Sensitivity to Wheat in the Absence of Coeliac Disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef]
- De Graaf, M.C.; Timmers, E.; Bonekamp, B.; Van Rooy, G.; Witteman, B.J.; Shewry, P.R.; Lovegrove, A.; America, A.H.; Gilissen, L.J.; Keszthelyi, D.; et al. Two Randomized Crossover Multicenter Studies Investigating Gastrointestinal Symptoms after Bread Consumption in Individuals with Noncoeliac Wheat Sensitivity: Do Wheat Species and Fermentation Type Matter? Am. J. Clin. Nutr. 2024, 119, 896–907. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients With Self-Reported Non-Celiac Gluten Sensitivity After Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Herfindal, A.M.; Nilsen, M.; Aspholm, T.E.; Schultz, G.I.G.; Valeur, J.; Rudi, K.; Thoresen, M.; Lundin, K.E.A.; Henriksen, C.; Bøhn, S.K. Effects of Fructan and Gluten on Gut Microbiota in Individuals with Self-Reported Non-Celiac Gluten/Wheat Sensitivity—A Randomised Controlled Crossover Trial. BMC Med. 2024, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Ford, A.C. Global Burden of Irritable Bowel Syndrome: Trends, Predictions and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional Gastrointestinal Disorders: Advances in Understanding and Management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Usai-Satta, P.; Bassotti, G.; Bellini, M.; Oppia, F.; Lai, M.; Cabras, F. Irritable Bowel Syndrome and Gluten-Related Disorders. Nutrients 2020, 12, 1117. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten Causes Gastrointestinal Symptoms in Subjects Without Celiac Disease: A Double-Blind Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef]
- Spiller, R. Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Bascuñán, K.A.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Branchi, F.; Ferretti, F.; Dell’Osso, B.; Montanari, V.; Bardella, M.T.; et al. A Low FODMAP Gluten-Free Diet Improves Functional Gastrointestinal Disorders and Overall Mental Health of Celiac Disease Patients: A Randomized Controlled Trial. Nutrients 2018, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Sabença, C.; Ribeiro, M.; de Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. The Two Faces of Wheat. Front. Nutr. 2020, 7, 517313. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat Amylase Trypsin Inhibitors Drive Intestinal Inflammation via Activation of Toll-like Receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Carcea, M.; Melloni, S.; Narducci, V.; Turfani, V. Wheat Germ Agglutinin (WGA): Its Nature, Biological Role, Significance in Human Nutrition, and Possibility to Be Used as Marker of Whole-Grain Status in Wheat-Based Foods. Foods 2024, 13, 2990. [Google Scholar] [CrossRef]
- El Mecherfi, K.-E.; Lupi, R.; Cherkaoui, M.; Albuquerque, M.A.C.; Todorov, S.D.; Tranquet, O.; Klingebiel, C.; Rogniaux, H.; Denery-Papini, S.; Onno, B.; et al. Fermentation of Gluten by Lactococcus Lactis LLGKC18 Reduces Its Antigenicity and Allergenicity. Probiot. Antimicro. Prot. 2022, 14, 779–791. [Google Scholar] [CrossRef]
- Biscola, V.; Albuquerque Cavalcanti de Albuquerque, M.; Pacheco, T.; Silva, D.; Gombossy, B. Lactic Acid Bacteria Application to Decrease Food Allergies. In Lactic Acid Bacteria. A Functional Approach; CRC Press: Boca Raton, FL, USA, 2020; p. 21. [Google Scholar]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the Proteolytic Enzymes Produced by Lactic Acid Bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Fu, W.; Xue, W.; Liu, C.; Tian, Y.; Zhang, K.; Zhu, Z. Screening of Lactic Acid Bacteria and Yeasts from Sourdough as Starter Cultures for Reduced Allergenicity Wheat Products. Foods 2020, 9, 751. [Google Scholar] [CrossRef]
- Fu, W.; Liu, C.; Meng, X.; Tao, S.; Xue, W. Co-Culture Fermentation of Pediococcus Acidilactici XZ31 and Yeast for Enhanced Degradation of Wheat Allergens. Int. J. Food Microbiol. 2021, 347, 109190. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by Sourdough Lactic Acid Bacteria: Effects on Wheat Flour Protein Fractions and Gliadin Peptides Involved in Human Cereal Intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; Di Cagno, R.; Gobbetti, M. Use of Sourdough Fermented Wheat Germ for Enhancing the Nutritional, Texture and Sensory Characteristics of the White Bread. Eur. Food Res. Technol. 2010, 230, 645–654. [Google Scholar] [CrossRef]
- Fraberger, V.; Ammer, C.; Domig, K.J. Functional Properties and Sustainability Improvement of Sourdough Bread by Lactic Acid Bacteria. Microorganisms 2020, 8, 1895. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Coda, R.; Gobbetti, M. Use of Selected Sourdough Lactic Acid Bacteria to Hydrolyze Wheat and Rye Proteins Responsible for Cereal Allergy. Eur. Food Res. Technol. 2006, 223, 405–411. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, M.D.; Palmisano, F.; Maurano, F.; et al. Highly Efficient Gluten Degradation by Lactobacilli and Fungal Proteases during Food Processing: New Perspectives for Celiac Disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef]
- Calasso, M.; Francavilla, R.; Cristofori, F.; De Angelis, M.; Gobbetti, M. New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2018, 10, 1873. [Google Scholar] [CrossRef]
- Reale, A.; Di Stasio, L.; Di Renzo, T.; De Caro, S.; Ferranti, P.; Picariello, G.; Addeo, F.; Mamone, G. Bacteria Do It Better! Proteomics Suggests the Molecular Basis for Improved Digestibility of Sourdough Products. Food Chem. 2021, 359, 129955. [Google Scholar] [CrossRef]
- De Vuyst, L.; González-Alonso, V.; Wardhana, Y.R.; Pradal, I. Taxonomy and Species Diversity of Sourdough Lactic Acid Bacteria. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 97–160. ISBN 978-3-031-23083-7. [Google Scholar]
- Radomir, L. The Chemistry of Cereal Proteins, 2nd ed.; Routledge: Oxford, UK, 2017; ISBN 978-0-203-73705-7. [Google Scholar]
- Khan, A.; Li, S.; Han, H.; Jin, W.-L.; Ling, Z.; Ji, J.; Iram, S.; Liu, P.; Xiao, S.; Salama, E.-S.; et al. A Gluten Degrading Probiotic Bacillus Subtilis LZU-GM Relieve Adverse Effect of Gluten Additive Food and Balances Gut Microbiota in Mice. Food Res. Int. 2023, 170, 112960. [Google Scholar] [CrossRef]
- Sollid, L.M.; Qiao, S.-W.; Anderson, R.P.; Gianfrani, C.; Koning, F. Nomenclature and Listing of Celiac Disease Relevant Gluten T-Cell Epitopes Restricted by HLA-DQ Molecules. Immunogenetics 2012, 64, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, S.; Xue, W. Mechanism of Carbohydrate and Protein Conversion during Sourdough Fermentation: An Analysis Based on Representative Chinese Sourdough Microbiota. Int. J. Food Microbiol. 2023, 410, 110487. [Google Scholar] [CrossRef]
- Thiele, C.; Grassl, S.; Gänzle, M. Gluten Hydrolysis and Depolymerization during Sourdough Fermentation. J. Agric. Food Chem. 2004, 52, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Zadeike, D.; Cipkute, K.; Cizeikiene, D. Effects of Co-Fermentation with Lactic Acid Bacteria and Yeast on Gliadin Degradation in Whole-Wheat Sourdough. Fermentation 2025, 11, 238. [Google Scholar] [CrossRef]
- Rollan, G.; De Angelis, M.; Gobbetti, M.; De Valdez, G.F. Proteolytic Activity and Reduction of Gliadin-like Fractions by Sourdough Lactobacilli. J. Appl. Microbiol. 2005, 99, 1495–1502. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Redruello, B.; Ladero, V.; Martín, M.C.; Fernández, M.; Alvarez, M.A. Screening sourdough samples for gliadin-degrading activity revealed Lactobacillus casei strains able to individually metabolize the coeliac-disease-related 33-mer peptide. Can. J. Microbiol. 2016, 62, 422–430. [Google Scholar] [CrossRef]
- Gerez, C.L.; Dallagnol, A.; Rollán, G.; Font De Valdez, G. A Combination of Two Lactic Acid Bacteria Improves the Hydrolysis of Gliadin during Wheat Dough Fermentation. Food Microbiol. 2012, 32, 427–430. [Google Scholar] [CrossRef]
- Brzozowski, B. Immunoreactivity of Wheat Proteins Modified by Hydrolysis and Polymerisation. Eur. Food Res. Technol. 2016, 242, 1025–1040. [Google Scholar] [CrossRef]
- Brzozowski, B.; Stasiewicz, K.; Ostolski, M.; Adamczak, M. Reducing Immunoreactivity of Gliadins and Coeliac-Toxic Peptides Using Peptidases from L. Acidophilus 5e2 and A. Niger. Catalysts 2020, 10, 923. [Google Scholar] [CrossRef]
- Leszczyńska, J.; Szczepankowska, A.K.; Majak, I.; Mańkowska, D.; Smolińska, B.; Ścieszka, S.; Diowksz, A.; Cukrowska, B.; Aleksandrzak-Piekarczyk, T. Reducing Immunoreactivity of Gluten Peptides by Probiotic Lactic Acid Bacteria for Dietary Management of Gluten-Related Diseases. Nutrients 2024, 16, 976. [Google Scholar] [CrossRef]
- Bradauskiene, V.; Vaiciulyte-Funk, L.; Shah, B.; Cernauskas, D.; Tita, M. Recent Advances in Biotechnological Methods for Wheat Gluten Immunotoxicity Abolishment—A Review. Pol. J. Food Nutr. Sci. 2021, 71, 5–20. [Google Scholar] [CrossRef]
- Walter, T.; Wieser, H.; Koehler, P. Degradation of Gluten in Rye Sourdough Products by Means of a Proline-Specific Peptidase. Eur. Food Res. Technol. 2015, 240, 517–524. [Google Scholar] [CrossRef]
- Cano, R.H.; Guergoletto, K.B.; Garcia, S. Isolamento e Identificação de Bactérias Láticas Com Potencial Proteolítico a Partir de Sourdough Produzidas Em Londrina, Paraná, Brasil. Semin. Ciênc. Agrár. 2025, 46, 827–842. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Sogi, D.S.; Sehajpal, P.K. Effect of Short-Term Sourdough Fermentation on Wheat Protein. Cogent Food Agric. 2016, 2, 1132983. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. Hydrolytic Enzymes and Their Directly and Indirectly Effects on Gluten and Dough Properties: An Extensive Review. Food Sci. Nutr. 2021, 9, 3988–4006. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Montserrat, V.; Mujico, J.R.; Loh, K.L.; Beringer, D.X.; Van Lummel, M.; Thompson, A.; Mearin, M.L.; Schweizer, J.; Kooy-Winkelaar, Y.; et al. T-Cell Receptor Recognition of HLA-DQ2–Gliadin Complexes Associated with Celiac Disease. Nat. Struct. Mol. Biol. 2014, 21, 480–488. [Google Scholar] [CrossRef]
- De Angelis, M.; Rizzello, C.G.; Fasano, A.; Clemente, M.G.; De Simone, C.; Silano, M.; De Vincenzi, M.; Losito, I.; Gobbetti, M. VSL#3 Probiotic Preparation Has the Capacity to Hydrolyze Gliadin Polypeptides Responsible for Celiac Sprue Probiotics and Gluten Intolerance. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2006, 1762, 80–93. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Nikoloudaki, O.; Speckmann, B.; Calabrese, F.M.; Gobbetti, M.; De Angelis, M. Metabolic Characterization of Selected Probiotic Consortia during Gluten and Wheat Bread Simulated Digestion. Food Sci. Human. Wellness 2025, 14, 9250033. [Google Scholar] [CrossRef]
- Rashmi, B.S.; Gayathri, D.; Vasudha, M.; Prashantkumar, C.S.; Swamy, C.T.; Sunil, K.S.; Somaraja, P.K.; Prakash, P. Gluten Hydrolyzing Activity of Bacillus Spp Isolated from Sourdough. Microb. Cell Fact. 2020, 19, 130. [Google Scholar] [CrossRef]
- Schmidt, M.; Sciurba, E. Determination of FODMAP Contents of Common Wheat and Rye Breads and the Effects of Processing on the Final Contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastro Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Loponen, J.; Gänzle, M.G. Characterization of the Extracellular Fructanase FruA in Lactobacillus Crispatus and Its Contribution to Fructan Hydrolysis in Breadmaking. J. Agric. Food Chem. 2020, 68, 8637–8647. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steiner, D.; Longin, C.F.H.; Würschum, T.; Schweiggert, R.M.; Carle, R. Wheat and the Irritable Bowel Syndrome—FODMAP Levels of Modern and Ancient Species and Their Retention during Bread Making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Borowska, M.; Ispiryan, L.; Neylon, E.; Sahin, A.W.; Murphy, C.P.; Zannini, E.; Arendt, E.K.; Coffey, A. Screening and Application of Novel Homofermentative Lactic Acid Bacteria Results in Low-FODMAP Whole-Wheat Bread. Fermentation 2023, 9, 336. [Google Scholar] [CrossRef]
- Fang, S.; Yan, B.; Tian, F.; Lian, H.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. β-Fructosidase FosE Activity in Lactobacillus Paracasei Regulates Fructan Degradation during Sourdough Fermentation and Total FODMAP Levels in Steamed Bread. LWT 2021, 145, 111294. [Google Scholar] [CrossRef]
- Fraberger, V.; Ladurner, M.; Nemec, A.; Grunwald-Gruber, C.; Call, L.M.; Hochegger, R.; Domig, K.J.; D’Amico, S. Insights into the Potential of Sourdough-Related Lactic Acid Bacteria to Degrade Proteins in Wheat. Microorganisms 2020, 8, 1689. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, I.; Piasecka-Jóźwiak, K.; Kotyrba, D.; Kolenda, M.; Stecka, K.M. Selection of Lactic Acid Bacteria Strains for the Hydrolysis of Allergenic Proteins of Wheat Flour. J. Sci. Food Agric. 2016, 96, 3897–3905. [Google Scholar] [CrossRef]
- Lopez, H.W.; Krespine, V.; Guy, C.; Messager, A.; Demigne, C.; Remesy, C. Prolonged Fermentation of Whole Wheat Sourdough Reduces Phytate Level and Increases Soluble Magnesium. J. Agric. Food Chem. 2001, 49, 2657–2662. [Google Scholar] [CrossRef]
- Leenhardt, F.; Levrat-Verny, M.-A.; Chanliaud, E.; Rémésy, C. Moderate Decrease of pH by Sourdough Fermentation Is Sufficient To Reduce Phytate Content of Whole Wheat Flour through Endogenous Phytase Activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef]
- Reale, A.; Konietzny, U.; Coppola, R.; Sorrentino, E.; Greiner, R. The Importance of Lactic Acid Bacteria for Phytate Degradation during Cereal Dough Fermentation. J. Agric. Food Chem. 2007, 55, 2993–2997. [Google Scholar] [CrossRef]
- De Angelis, M. Phytase Activity in Sourdough Lactic Acid Bacteria: Purification and Characterization of a Phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 2003, 87, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Raikhel, N. Sequence Variability in Three Wheat Germ Agglutinin Isolectins: Products of Multiple Genes in Polyploid Wheat. J. Mol. Evol. 1989, 28, 327–336. [Google Scholar] [CrossRef]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of Wheat Germ Agglutinin on Human Gastrointestinal Epithelium: Insights from an Experimental Model of Immune/Epithelial Cell Interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, V.J.; Brouns, F.J.P.H. Health Effects of Wheat Lectins: A Review. J. Cereal Sci. 2014, 59, 112–117. [Google Scholar] [CrossRef]
- Tovar, L.E.R.; Gänzle, M.G. Degradation of Wheat Germ Agglutinin during Sourdough Fermentation. Foods 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Barbato, M.; Di Camillo, C.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; De Vincenzi, M.; Gobbetti, M.; Cucchiara, S. Gluten-free Sourdough Wheat Baked Goods Appear Safe for Young Celiac Patients: A Pilot Study. J. Pediatr. Gastroenterol. nutr. 2010, 51, 777–783. [Google Scholar] [CrossRef]
- De Angelis, M.; Siragusa, S.; Vacca, M.; Di Cagno, R.; Cristofori, F.; Schwarm, M.; Pelzer, S.; Flügel, M.; Speckmann, B.; Francavilla, R.; et al. Selection of Gut-Resistant Bacteria and Construction of Microbial Consortia for Improving Gluten Digestion under Simulated Gastrointestinal Conditions. Nutrients 2021, 13, 992. [Google Scholar] [CrossRef]
- Francavilla, R.; De Angelis, M.; Rizzello, C.G.; Cavallo, N.; Bello, F.D.; Gobbetti, M. Selected Probiotic Lactobacilli Have the Capacity To Hydrolyze Gluten Peptides during Simulated Gastrointestinal Digestion. Appl. Environ. Microbiol. 2017, 83, e00376-17. [Google Scholar] [CrossRef]
- Mandile, R.; Picascia, S.; Parrella, C.; Camarca, A.; Gobbetti, M.; Greco, L.; Troncone, R.; Gianfrani, C.; Auricchio, R. Lack of Immunogenicity of Hydrolysed Wheat Flour in Patients with Coeliac Disease after a Short-term Oral Challenge. Aliment. Pharmacol. Ther. 2017, 46, 440–446. [Google Scholar] [CrossRef]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.-M.; Loponen, J.; Poussa, T.; Huang, X.; Sontag-Strohm, T.; Salmenkari, H.; Korpela, R. Pilot Study: Comparison of Sourdough Wheat Bread and Yeast-Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome. Nutrients 2017, 9, 1215. [Google Scholar] [CrossRef]
- Laatikainen, R.; Jalanka, J.; Loponen, J.; Hongisto, S.-M.; Hillilä, M.; Koskenpato, J.; Korpela, R.; Salonen, A. Randomised Clinical Trial: Effect of Low-FODMAP Rye Bread versus Regular Rye Bread on the Intestinal Microbiota of Irritable Bowel Syndrome Patients: Association with Individual Symptom Variation. BMC Nutr. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Islam, S. Sourdough Bread Quality: Facts and Factors. Foods 2024, 13, 2132. [Google Scholar] [CrossRef]
- Gélinas, P.; Théolier, J. How to Reduce Gluten in Foods: A Critical Review of Patents. Int. J. Food Sci. Technol. 2024, 59, 8069–8081. [Google Scholar] [CrossRef]
- Giuliani, G.; Benedusi, A.; Di Cagno, R.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M.; Cassone, A. Method for Partial Degradation of Gluten. U.S. Patent 20140065262A1, 6 March 2014. [Google Scholar]
- Giuliani, G.; Benedusi, A.; Di Cagno, R.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M.; Cassone, A. Process of Microbic Biotechnology for Completely Degrading Gluten in Flours. U.S. Patent 9386777B2, 12 July 2016. [Google Scholar]
- Pedroza-Islas, R. Microencapsulated Bacterial Consortium for the Degradation of Gluten into Sourdough and Method for Producing Said Sourdough. EP Patent 2818048A1, 9 January 2019. [Google Scholar]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and Cereal Fermentation in a Nutritional Perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in Sourdough Fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal Activity of Wheat-Flour Sourdough (Type II) from Two Different Lactobacillus in Vitro and Bread. Appl. Food Res. 2023, 3, 100319. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal Capacity of Poolish-Type Sourdough Supplemented with Lactiplantibacillus Plantarum and Its Aqueous Extracts In Vitro and Bread. Antibiotics 2022, 11, 1813. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Morales-Camacho, J.I.; Mani-López, E.; López-Malo, A. Assessment of Antifungal Activity of Aqueous Extracts and Protein Fractions from Sourdough Fermented by Lactiplantibacillus Plantarum. Future Foods 2024, 9, 100314. [Google Scholar] [CrossRef]
- De Bondt, Y.; Verdonck, C.; Brandt, M.J.; De Vuyst, L.; Gänzle, M.G.; Gobbetti, M.; Zannini, E.; Courtin, C.M. Wheat Sourdough Breadmaking: A Scoping Review. Annu. Rev. Food Sci. Technol. 2024, 15, 265–282. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Bello, F.D. Impact of Sourdough on the Texture of Bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Moroni, A.V.; Bello, F.D.; Arendt, E.K. Sourdough in Gluten-Free Bread-Making: An Ancient Technology to Solve a Novel Issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of Fungal Proteases and Selected Sourdough Lactic Acid Bacteria for Making Wheat Bread with an Intermediate Content of Gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in Sourdough Fermentations: Mechanisms and Potential for Improved Bread Quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Optimizing Lactic Acid Bacteria Proportions in Sourdough to Enhance Antifungal Activity and Quality of Partially and Fully Baked Bread. Foods 2024, 13, 2318. [Google Scholar] [CrossRef]
- Salehi, F. Improvement of Gluten-free Bread and Cake Properties Using Natural Hydrocolloids: A Review. Food Sci. Nutr. 2019, 7, 3391–3402. [Google Scholar] [CrossRef]
- Torres-Pérez, R.; Martínez-García, E.; Siguero-Tudela, M.M.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Enhancing Gluten-Free Bread Production: Impact of Hydroxypropyl Methylcellulose, Psyllium Husk Fiber, and Xanthan Gum on Dough Characteristics and Bread Quality. Foods 2024, 13, 1691. [Google Scholar] [CrossRef]
- Irondi, E.A.; Imam, Y.T.; Ajani, E.O.; Alamu, E.O. Natural and Modified Food Hydrocolloids as Gluten Replacement in Baked Foods: Functional Benefits. Grain Oil Sci. Technol. 2023, 6, 163–171. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A.G. Novel Approaches in Gluten-Free Breadmaking: Interface between Food Science, Nutrition, and Health. Comp. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Fuentes, A.P.; Genevois, C.E.; Flores, S.K.; Pla, M.F.d.E. Novel Application of a Food Ingredient Based on Soybean Extruded-expelled Meal Containing Probiotics for Improving Gluten-free Bread Quality. Int. J. Food Sci. Technol. 2023, 58, 6855–6861. [Google Scholar] [CrossRef]
- Peñalver, R.; Ros, G.; Nieto, G. Development of Gluten-Free Functional Bread Adapted to the Nutritional Requirements of Celiac Patients. Fermentation 2023, 9, 631. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough Improves the Quality of Whole-Wheat Flour Products: Mechanisms and Challenges—A Review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; Cerrone, R.; Capobianco, D.; Filardo, S.; Mancini, P.; Zanni, F.; Fanelli, S.; Mastromarino, P.; Mosca, L. A Probiotic Preparation Hydrolyzes Gliadin and Protects Intestinal Cells from the Toxicity of Pro-Inflammatory Peptides. Nutrients 2020, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the Sourdough May Affect the Functional Features of Leavened Baked Goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel Insights on the Functional/Nutritional Features of the Sourdough Fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough Technology as a Tool for the Development of Healthier Grain-Based Products: An Update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L. Impact of Sourdough Fermentation on Nutrient Transformations in Cereal-Based Foods: Mechanisms, Practical Applications, and Health Implications. Grain Oil Sci. Technol. 2024, 7, 124–132. [Google Scholar] [CrossRef]
- Gänzle, M.; Gobbetti, M. Physiology and Biochemistry of Sourdough Lactic Acid Bacteria and Their Impact on Bread Quality. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 213–256. ISBN 978-3-031-23083-7. [Google Scholar]
- McCabe, M.S. Balancing Consumer Protection and Scientific Integrity in the Face of Uncertainty: The Example of Gluten-Free Foods. Food Drug LJ 2010, 65, 367–390. [Google Scholar]
- Grabowicz, A.; Czaja-Bulsa, G. Misleading Labelling of Gluten-Free Products in the Light of EU Regulations: Time for a Change? J. Consum. Prot. Food Saf. 2019, 14, 93–95. [Google Scholar] [CrossRef]
- Xhaferaj, M.; Alves, T.O.; Ferreira, M.S.L.; Scherf, K.A. Recent Progress in Analytical Method Development to Ensure the Safety of Gluten-Free Foods for Celiac Disease Patients. J. Cereal Sci. 2020, 96, 103114. [Google Scholar] [CrossRef]
- Lima, T.T.M.; Hosken, B.D.O.; De Dea Lindner, J.; Menezes, L.A.A.; Pirozi, M.R.; Martin, J.G.P. How to Deliver Sourdough with Appropriate Characteristics for the Bakery Industry? The Answer May Be Provided by Microbiota. Food Biosci. 2023, 56, 103072. [Google Scholar] [CrossRef]
- Bruins Slot, I.D.; Bremer, M.G.E.G.; Hamer, R.J.; Van Der Fels-Klerx, H.J. Part of Celiac Population Still at Risk despite Current Gluten Thresholds. Trends Food Sci. Technol. 2015, 43, 219–226. [Google Scholar] [CrossRef]
- Di Sario, F.; Monachesi, C.; Verma, A.K.; Catassi, C. Everything That Must Be Known About the Relationship of Gluten to Human Health. In Designing Gluten Free Bakery and Pasta Products; De Escalada Pla, M.F., Genevois, C.E., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–17. ISBN 978-3-031-28343-7. [Google Scholar]
- Raju, N.; Joshi, A.K.R.; Vahini, R.; Deepika, T.; Bhaskarachari, K.; Devindra, S. Gluten Contamination in Labelled and Naturally Gluten-Free Grain Products in Southern India. Food Addit. Contam. Part. A 2020, 37, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kaur, A.; Chopra, C.S. Gluten-Free Products for Celiac Susceptible People. Front. Nutr. 2018, 5, 116. [Google Scholar] [CrossRef]
- Arslain, K.; Gustafson, C.R.; Baishya, P.; Rose, D.J. Determinants of Gluten-Free Diet Adoption among Individuals without Celiac Disease or Non-Celiac Gluten Sensitivity. Appetite 2021, 156, 104958. [Google Scholar] [CrossRef]
- Verrill, L.; Zhang, Y.; Kane, R. Food Label Usage and Reported Difficulty with Following a Gluten-free Diet among Individuals in the USA with Coeliac Disease and Those with Noncoeliac Gluten Sensitivity. J. Human. Nutr. Diet. 2013, 26, 479–487. [Google Scholar] [CrossRef]
- Gutowski, E.D.; Weiten, D.; Green, K.H.; Rigaux, L.N.; Bernstein, C.N.; Graff, L.A.; Walker, J.R.; Duerksen, D.R.; Silvester, J.A. Can Individuals with Celiac Disease Identify Gluten-Free Foods Correctly? Clin. Nutr. ESPEN 2020, 36, 82–90. [Google Scholar] [CrossRef]
- Ribeiro, M.; De Sousa, T.; Sabença, C.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Advances in Quantification and Analysis of the Celiac-related Immunogenic Potential of Gluten. Comp. Rev. Food Sci. Food Saf. 2021, 20, 4278–4298. [Google Scholar] [CrossRef]
- Scherf, K.A.; Poms, R.E. Recent Developments in Analytical Methods for Tracing Gluten. J. Cereal Sci. 2016, 67, 112–122. [Google Scholar] [CrossRef]
- Bustamante, M.Á.; Simón, E.; Churruca, I.; Del Pilar Fernández-Gil, M. Techniques for Analyzing Gluten. In Nutritional and Analytical Approaches of Gluten-Free Diet in Celiac Disease; SpringerBriefs in Food, Health, and Nutrition; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–46. ISBN 978-3-319-53341-4. [Google Scholar]
- Morón, B.; Bethune, M.T.; Comino, I.; Manyani, H.; Ferragud, M.; López, M.C.; Cebolla, Á.; Khosla, C.; Sousa, C. Toward the Assessment of Food Toxicity for Celiac Patients: Characterization of Monoclonal Antibodies to a Main Immunogenic Gluten Peptide. PLoS ONE 2008, 3, e2294. [Google Scholar] [CrossRef]
- Osorio, C.E.; Mejías, J.H.; Rustgi, S. Gluten Detection Methods and Their Critical Role in Assuring Safe Diets for Celiac Patients. Nutrients 2019, 11, 2920. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Smits, N.G.E.; Blázquez, D.; Albero-Pérez, C.; Pallás-Tamarit, Y.; Maquieira, Á.; Morais, S. Simultaneous Quantification of Six Major Allergens in Commercial Foods for Children Using a Multiplex Array on a Digital Versatile Disc. Food Chem. 2023, 404, 134570. [Google Scholar] [CrossRef] [PubMed]
- Panda, R.; Garber, E.A.E. Western Blot Analysis of Fermented-Hydrolyzed Foods Utilizing Gluten-Specific Antibodies Employed in a Novel Multiplex Competitive ELISA. Anal. Bioanal. Chem. 2019, 411, 5159–5174. [Google Scholar] [CrossRef] [PubMed]
- Amnuaycheewa, P.; Niemann, L.; Goodman, R.E.; Baumert, J.L.; Taylor, S.L. Challenges in Gluten Analysis: A Comparison of Four Commercial Sandwich ELISA Kits. Foods 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.C.; Sousa, C. Analytical Tools for Gluten Detection. Policies and Regulation. In Advances in the Understanding of Gluten related Pathology and the Evolution of Gluten-Free Foods; Arranz, E., Fernández-Bañares, F., M.Rosell, C., Rodrigo, L., Peña, A.S., Eds.; OmniaScience: Barcelona, Spain, 2015; pp. 527–564. ISBN 978-84-943418-2-3. [Google Scholar]
- Alves, T.O.; D’Almeida, C.T.S.; Scherf, K.A.; Ferreira, M.S.L. Modern Approaches in the Identification and Quantification of Immunogenic Peptides in Cereals by LC-MS/MS. Front. Plant Sci. 2019, 10, 1470. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, H.; Chen, Y.; Li, Z.; Timira, V. A Systematic Review on the Recent Advances of Wheat Allergen Detection by Mass Spectrometry: Future Prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 12324–12340. [Google Scholar] [CrossRef]
- Lock, S. Gluten Detection and Speciation by Liquid Chromatography Mass Spectrometry (LC-MS/MS). Foods 2013, 3, 13–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).