Comparative Evaluation of Recombinant Chlamydia abortus and Chlamydia trachomatis Major Outer Membrane Proteins for Diagnosing Human Chlamydial Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Participants and Clinical Samples
2.3. Obtaining Recombinant MOMP (rMOMP)
2.4. Recombinant ELISA
2.5. Molecular Detection of Chlamydia trachomatis Infection
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Sensitivity and Specificity of ELISA with Recombinant MOMP
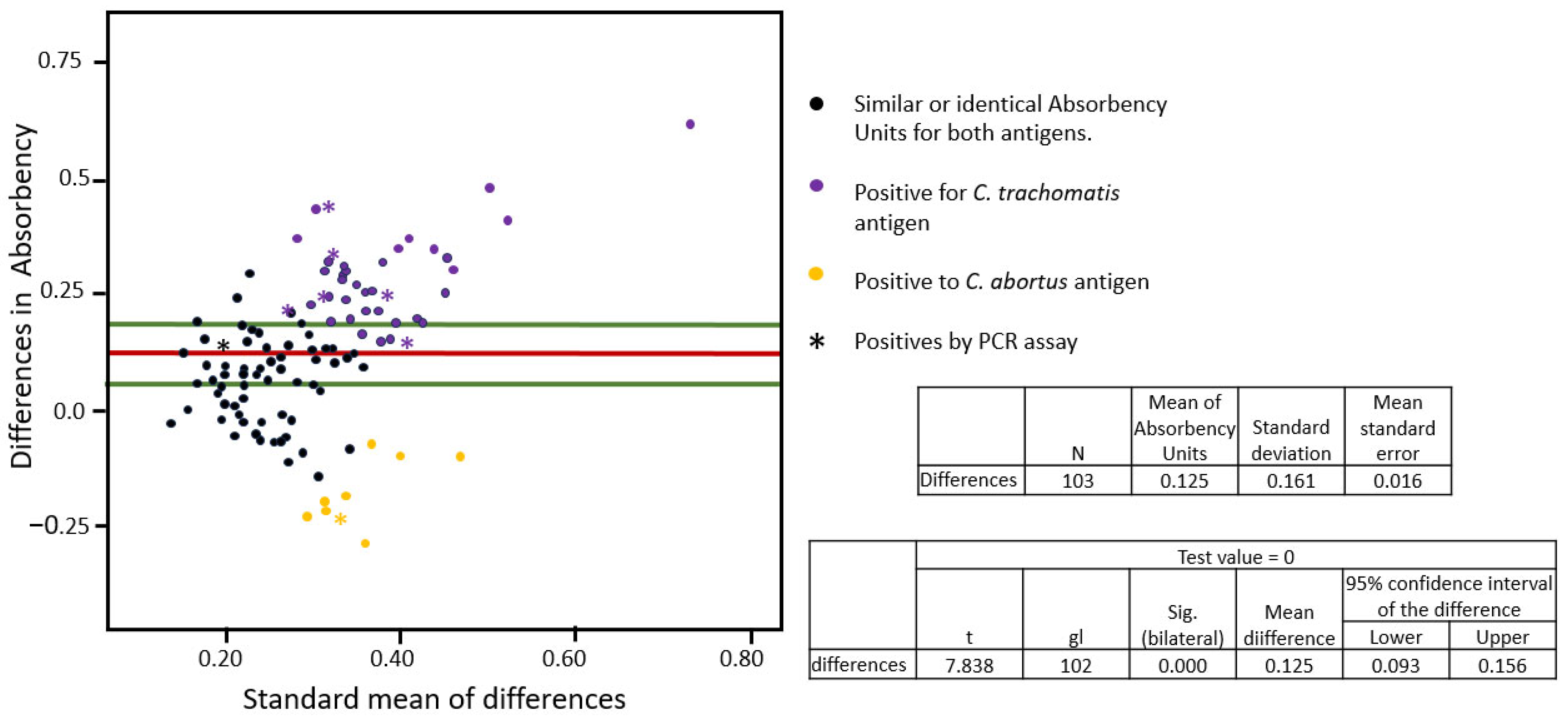
3.3. The Cross-Reactivity Analyses Between the Recombinant MOMPs
3.4. Association Between Antibodies Against C. trachomatis and Gynecology or Obstetric Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Chlamydia trachomatis |
| CA | Chlamydia abortus |
| CTrMOMP | Chlamydia trachomatis recombinant MOMP |
| CArMOMP | Chlamydia abortus recombinant MOMP |
| MOMP | Major outer membrane protein |
References
- World Health Organization. Sexually Transmitted Infections (STIs). Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 15 May 2025).
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Dielissen, P.W.; Teunissen, D.A.M.; Lagro-Janssen, A.L.M. Chlamydia prevalence in the general population: Is there a sex difference? a systematic review. BMC Infect. Dis. 2013, 13, 534. [Google Scholar] [CrossRef]
- World Health Organization. Chlamydia. Available online: https://www.who.int/news-room/fact-sheets/detail/chlamydia (accessed on 15 May 2025).
- Alexiou, Z.W.; Hoenderboom, B.M.; Hoebe, C.J.; Dukers-Muijrers, N.H.; Götz, H.M.; van der Sande, M.A.; de Vries, H.J.; den Hartog, J.E.; Morré, S.A.; van Benthem, B.H. Reproductive tract complication risks following Chlamydia trachomatis infections: A long-term prospective cohort study from 2008 to 2022. Lancet Reg. Health Eur. 2024, 45, 101027. [Google Scholar] [CrossRef]
- Escarcega-Tame, M.A.; López-Hurtado, M.; Escobedo-Guerra, M.R.; Reyes-Maldonado, E.; Castro-Escarpulli, G.; Guerra-Infante, F.M. Co-infection between genotypes of the human papillomavirus and Chlamydia trachomatis in Mexican women. Int. J. STD AIDS 2020, 31, 1255–1262. [Google Scholar] [CrossRef]
- Herrmann, B.; Malm, K. Comparison between Abbott m2000 RealTime and Alinity m STI systems for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2217–2220. [Google Scholar] [CrossRef]
- Villagrana, Z.J.R.; López, H.M.; Flores, S.V.R.; de Haro, C.M.J.; Escobedo, G.M.R.; Guerra, I.F.M. Persistence of Chlamydia trachomatis in the endometrium and peritoneal fluid of patients with infertility but negative cervical cultures. Ginecol. Obstet. Mex. 2013, 81, 23–28. [Google Scholar]
- Puolakkainen, M. Laboratory diagnosis of persistent human chlamydial infection. Front. Cell. Infect. Microbiol. 2013, 3, 99. [Google Scholar] [CrossRef][Green Version]
- Hepler, R.W.; Nahas, D.D.; Lucas, B.; Kaufhold, R.; Flynn, J.A.; Galli, J.D.; Swoyer, R.; Wagner, J.M.; Espeseth, A.S.; Joyce, J.G.; et al. Spectroscopic analysis of chlamydial major outer membrane protein in support of structure elucidation. Protein Sci. 2018, 27, 1923–1941. [Google Scholar] [CrossRef] [PubMed]
- Horner, P. Can Chlamydia Serology Be Used to Help Inform a Potential Future Chlamydia Vaccination Strategy? Sex. Transm. Dis. 2017, 44, 722–724. [Google Scholar] [CrossRef]
- Rahman, K.S.; Darville, T.; Russell, A.N.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Lee, D.A.E.; Kaltenboeck, B. Comprehensive molecular serology of human Chlamydia trachomatis infections using peptide enzyme-linked immunosorbent assays. mSphere 2018, 3, e00253-18. [Google Scholar] [CrossRef]
- Bas, S.; Muzzin, P.; Ninet, B.; Bornand, J.E.; Scieux, C.; Vischer, T.L. Chlamydial serology: Comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J. Clin. Microbiol. 2001, 39, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, M.R. Pneumonia due to Chlamydia pneumoniae in children: Epidemiology, diagnosis, and treatment. Pediatr. Pulmonol. 2003, 36, 384–390. [Google Scholar] [CrossRef]
- Rahman, K.S.; Kaltenboeck, B. Multipeptide Assays for Sensitive and Differential Detection of Anti-Chlamydia trachomatis Antibodies. J. Infect. Dis. 2021, 24, S86–S95. [Google Scholar] [CrossRef]
- Turin, L.; Surini, S.; Wheelhouse, N.; Rocchi, M.S. Recent advances and public health implications for environmental exposure to Chlamydia abortus: From enzootic to zoonotic disease. Vet. Res. 2022, 53, 37. [Google Scholar] [CrossRef]
- Alhajj, M.; Zubair, M.; Farhana, A. Interfering Factors. In Enzyme-Linked Immunosorbent Assay; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555922/#article-21178.s5 (accessed on 17 May 2025).
- Cortés-Rivas, A.; López-Hurtado, M.; Guerra-Infante, F. Seroprevalence of Chlamydia trachomatis infection using an ELISA test sensitized with a recombinant protein. JBBR 2025, 9, 9–16. [Google Scholar] [CrossRef]
- de Haro-Cruz, M.J.; Guadarrama-Macedo, S.I.; López-Hurtado, M.; Escobedo-Guerra, M.R.; Guerra-Infante, F.M. Obtaining an ELISA test based on a recombinant protein of Chlamydia trachomatis. Int. Microbiol. 2019, 22, 471–478. [Google Scholar] [CrossRef]
- Broeze, K.A.; Opmeer, B.C.; Coppus, S.F.P.J.; Van Geloven, N.; Alves, M.F.C.; Ånestad, G.; Bhattacharya, S.; Allan, J.; Guerra-Infante, M.F.; Den Hartog, J.E.; et al. Chlamydia antibody testing and diagnosing tubal pathology in subfertile women: An individual patient data meta-analysis. Hum. Reprod. Update 2011, 17, 301–310. [Google Scholar] [CrossRef]
- Akabueze, J.C.; Agu, P.U.; Ugwu, E.O.; Obi, S.N.; Aniebue, U.U.; Eleje, G.U.; Ugwu, A.O.; Anigbo, C.S.; Ekwueme, P.C.; Eze, M.I.; et al. Association Between Anti-Chlamydial Antibodies and Tubal Factor Infertility in South Eastern Nigeria. West Afr. J. Med. 2024, 41, 1091–1096. [Google Scholar]
- López-Hurtado, M.; García-Romero, S.; Escobedo-Guerra, M.R.; Bustos-López, D.; Guerra-Infante, F.M. Prevalence of genital Chlamydia trachomatis infection in women attending in the National Institute of Perinatology from Mexico City. Rev. Chil. Infectol. 2018, 35, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef]
- Connolly, S.; Kilembe, W.; Inambao, M.; Visoiu, A.M.; Sharkey, T.; Parker, R.; Wall, K.M.; Tichacek, A.; Hunter, E.; Allen, S. A Population-Specific Optimized GeneXpert Pooling Algorithm for Chlamydia trachomatis and Neisseria gonorrhoeae to Reduce Cost of Molecular Sexually Transmitted Infection Screening in Resource-Limited Settings. J. Clin. Microbiol. 2020, 58, e00176-20. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Management of Symptomatic Sexually Transmitted Infections. Available online: https://www.who.int/publications/i/item/9789240024168 (accessed on 15 May 2025).
- Hubacher, D.; Lara-Ricalde, R.; Taylor, D.J.; Guerra-Infante, F.; Guzmán-Rodríguez, R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N. Engl. J. Med. 2001, 345, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Öhman, H.; Rantsi, T.; Joki-Korpela, P.; Tiitinen, A.; Surcel, H.-M. Prevalence and persistence of Chlamydia trachomatis-specific antibodies after occasional and recurrent infections. Sex. Transm. Infect. 2020, 96, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, J.B.; Simnacher, U.; Longbottom, D.; Livingstone, M.; Maile, J.; Soutschek, E.; Walder, G.; Boden, K.; Sachse, K.; Essig, A. Analysis of Humoral Immune Responses to Surface and Virulence-Associated Chlamydia abortus Proteins in Ovine and Human Abortions by Use of a Newly Developed Line Immunoassay. J. Clin. Microbiol. 2016, 54, 1883–1890. [Google Scholar] [CrossRef]
- Palomares Reséndiz, E.G.; Mejía Sánchez, P.; Aguilar Romero, F.; Cruz Colín, L.D.L.; Jiménez Severiano, H.; Leyva Corona, J.C.; Morales Pablos, M.I.; Díaz Aparicio, E. Frecuencia y factores de riesgo asociados a la presencia de Chlamydia abortus, en rebaños ovinos en México. Rev. Mex. Cienc. Pecu. 2020, 11, 783–794. [Google Scholar] [CrossRef]
- Hernández-Trejo, M.; Herrera-González, N.; Guerra-Infante, F.M. Serological evidence of infection by three Chlamydia species in pregnant women. Ginecol. Obstet. Mex. 2014, 82, 585–590. [Google Scholar]
- Baud, D.; Regan, L.; Greub, G. Comparison of five commercial serological tests for the detection of anti-Chlamydia trachomatis antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 669–675. [Google Scholar] [CrossRef]
- Jones, C.S.; Maple, P.A.; Andrews, N.J.; Paul, I.D.; Caul, E.O. Measurement of IgG antibodies to Chlamydia trachomatis by commercial enzyme immunoassays and immunofluorescence in sera from pregnant women and patients with infertility, pelvic inflammatory disease, ectopic pregnancy, and laboratory diagnosed Chlamydia psittaci/Chlamydia pneumoniae infection. J. Clin. Pathol. 2003, 56, 225–229. [Google Scholar]
- Chu, J.; Yarrarapu, S.N.S.; Vaqar, S.; Durrani, M.I. Psittacosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538305/ (accessed on 1 July 2025).
- Hou, L.; Jia, J.; Qin, X.; Fang, M.; Liang, S.; Deng, J.; Pan, B.; Zhang, X.; Wang, B.; Mao, C.; et al. Prevalence and genotypes of Chlamydia psittaci in birds and related workers in three cities of China. PLoS ONE. 2024, 19, e0308532. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Cao, H.; Ji, J.; Zhang, R.; Li, W.; Guo, H.; Chen, L.; Ma, C.; Cui, M.; et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: An epidemiological and aetiological investigation. Lancet Microbe 2022, 3, e512–e520. [Google Scholar] [CrossRef]
- Ortega, N.; Caro, M.R.; Gallego, M.C.; Murcia-Belmonte, A.; Álvarez, D.; Del Río, L.; Cuello, F.; Buendía, A.J.; Salinas, J. Isolation of Chlamydia abortus from a laboratory worker diagnosed with atypical pneumonia. Ir. Vet. J. 2015, 69, 8. [Google Scholar] [CrossRef] [PubMed]
- Walder, G.; Meusburger, H.; Hotzel, H.; Oehme, A.; Neunteufel, W.; Dierich, M.P.; Würzner, R. Chlamydophila abortus pelvic inflammatory disease. Emerg. Infect. Dis. 2003, 9, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, D.; Livingstone, M.; Ribeca, P.; Beeckman, D.S.A.; van der Ende, A.; Pannekoek, Y.; Vanrompay, D. Whole genome de novo sequencing and comparative genomic analyses suggests that Chlamydia psittaci strain 84/2334 should be reclassified as Chlamydia abortus species. BMC Genom. 2021, 22, 159. [Google Scholar] [CrossRef]
| Pregnant Women | Infertile Women | Chi Value | p Value | |||
|---|---|---|---|---|---|---|
| N | 23 | 80 | ||||
| Age (years) | <34 | 52 | 16 | 36 | 4.312 | 0.038 Ɨ |
| ≥34 | 51 | 7 | 44 | |||
| Sexual debut | <18 | 48 | 13 | 35 | 1.171 | NS |
| ≥18 | 55 | 10 | 45 | |||
| NSP | <2 | 50 | 7 | 43 | 3.888 | 0.049 Ɨ |
| ≥2 | 53 | 16 | 37 | |||
| Ectopic pregnancies | yes | 3 | 2 | 1 | 3.502 | NS |
| no | 100 | 21 | 79 | |||
| Miscarriage | yes | 6 | 1 | 5 | 0.118 | NS |
| no | 97 | 22 | 75 | |||
| Endocrine–ovarian factors | yes | 94 | 18 | 76 | 6.277 | 0.012 * |
| no | 9 | 5 | 4 | |||
| Uterine factor | yes | 55 | 13 | 42 | 0.116 | NS |
| no | 48 | 10 | 38 | |||
| TFI | yes | 40 | 7 | 33 | 0.88 | NS |
| no | 63 | 16 | 47 | |||
| Masculine factor | yes | 76 | 14 | 62 | 2.554 | NS |
| no | 27 | 9 | 18 | |||
| Chlamydia diagnosis by PCR | yes | 8 | 2 | 6 | 0.036 | NS |
| no | 95 | 21 | 74 |
| (A) | Detection of Chlamydia trachomatis DNA by PCR Test | Total | |||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| Antibodies against CTrMOMP | positive | 6 | 26 | 32 | |||
| Sensitivity = 75% | PPV = 19% | Lr(+) = 2.78 | |||||
| negative | 2 | 69 | 71 | Specificity = 72.6% | NPV = 97% | Lr(−) = 0.34 | |
| Total | 8 | 95 | 103 | ||||
| (B) | Detection of Chlamydia trachomatis DNA by PCR test | Total | |||||
| Positive | Negative | ||||||
| Antibodies against CArMOMP | positive | 1 | 17 | 18 | |||
| Sensitivity = 12.5% | PPV = 6% | Lr(+) = 0.72 | |||||
| negative | 7 | 78 | 85 | Specificity = 82% | NPV = 92% | Lr(−) = 1.06 | |
| Total | 8 | 95 | 103 | ||||
| (C) | Antibodies against CTrMOMP | Total | |||||
| Positive | Negative | ||||||
| Antibodies against CArMOMP | positive | 7 | 11 | 18 | |||
| Sensitivity = 20.6% | PPV = 38.9% | Lr(+) = 1.31 | |||||
| negative | 27 | 58 | 85 | Specificity = 84.1% | NPV = 68.2% | Lr(−) = 0.94 | |
| Total | 34 | 69 | 103 | ||||
| Anti-Chlamydia trachomatis IgG Antibodies | |||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | RR | CI 95% | p Value | |||
| N | 32 | 71 | |||||
| Age (years) | <34 | 52 | 16 | 36 | 1.014 | 0.67–1.54 | NS |
| ≥34 | 51 | 16 | 35 | ||||
| Sexual debut | <18 | 48 | 16 | 32 | 0.9 | 0.59–1.39 | NS |
| ≥18 | 55 | 16 | 39 | ||||
| NSP | <2 | 50 | 15 | 35 | 1.05 | 0.68–1.63 | NS |
| ≥2 | 53 | 17 | 36 | ||||
| Infertile | yes | 80 | 26 | 54 | 0.94 | 0.76–2.93 | NS |
| no | 23 | 6 | 17 | ||||
| Ectopic pregnancies | yes | 3 | 2 | 1 | 0.23 | 0.021–1.16 | NS |
| no | 100 | 30 | 70 | ||||
| Miscarriage | yes | 6 | 1 | 5 | 2.254 | 0.274–15.8 | NS |
| no | 97 | 31 | 66 | ||||
| Endocrine–ovarian factors | yes | 94 | 32 | 62 | 0.87 | 0.8–0.95 | NS |
| no | 9 | 0 | 9 | ||||
| Uterine factor | yes | 55 | 15 | 40 | 1.2 | 0.79–1.83 | NS |
| no | 48 | 17 | 31 | ||||
| TFI | yes | 40 | 16 | 24 | 0.68 | 0.42–1.09 | NS |
| no | 63 | 16 | 47 | ||||
| Masculine factor | yes | 76 | 23 | 53 | 1.04 | 0.46–1.78 | NS |
| no | 27 | 9 | 18 | ||||
| Chlamydia diagnosis by PCR | positive | 8 | 6 | 2 | |||
| negative | 95 | 26 | 69 | 1.2 | 1.01–1.42 | 0.011 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Infante, F.M.; de Haro-Cruz, M.J.; López-Hurtado, M.; De la Rosa-Ramos, M.A.; Díaz-Aparicio, E.; Arellano-Reynoso, B. Comparative Evaluation of Recombinant Chlamydia abortus and Chlamydia trachomatis Major Outer Membrane Proteins for Diagnosing Human Chlamydial Infection. Microbiol. Res. 2025, 16, 159. https://doi.org/10.3390/microbiolres16070159
Guerra-Infante FM, de Haro-Cruz MJ, López-Hurtado M, De la Rosa-Ramos MA, Díaz-Aparicio E, Arellano-Reynoso B. Comparative Evaluation of Recombinant Chlamydia abortus and Chlamydia trachomatis Major Outer Membrane Proteins for Diagnosing Human Chlamydial Infection. Microbiology Research. 2025; 16(7):159. https://doi.org/10.3390/microbiolres16070159
Chicago/Turabian StyleGuerra-Infante, Fernando M., María J. de Haro-Cruz, Marcela López-Hurtado, Miguel A. De la Rosa-Ramos, Efrén Díaz-Aparicio, and Beatriz Arellano-Reynoso. 2025. "Comparative Evaluation of Recombinant Chlamydia abortus and Chlamydia trachomatis Major Outer Membrane Proteins for Diagnosing Human Chlamydial Infection" Microbiology Research 16, no. 7: 159. https://doi.org/10.3390/microbiolres16070159
APA StyleGuerra-Infante, F. M., de Haro-Cruz, M. J., López-Hurtado, M., De la Rosa-Ramos, M. A., Díaz-Aparicio, E., & Arellano-Reynoso, B. (2025). Comparative Evaluation of Recombinant Chlamydia abortus and Chlamydia trachomatis Major Outer Membrane Proteins for Diagnosing Human Chlamydial Infection. Microbiology Research, 16(7), 159. https://doi.org/10.3390/microbiolres16070159








