Microbial Biocontrol Agents Engineer Plant Biometrics and Host Response Against Xanthomonas oryzae pv. oryzae in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Cultures and Rice Cultivar
2.2. Pathogenicity Assay of X. oryzae pv. oryzae Isolate
2.3. In Vitro Compatibility Test of Bioagents
2.4. In Vitro Bio-Efficacy Assay
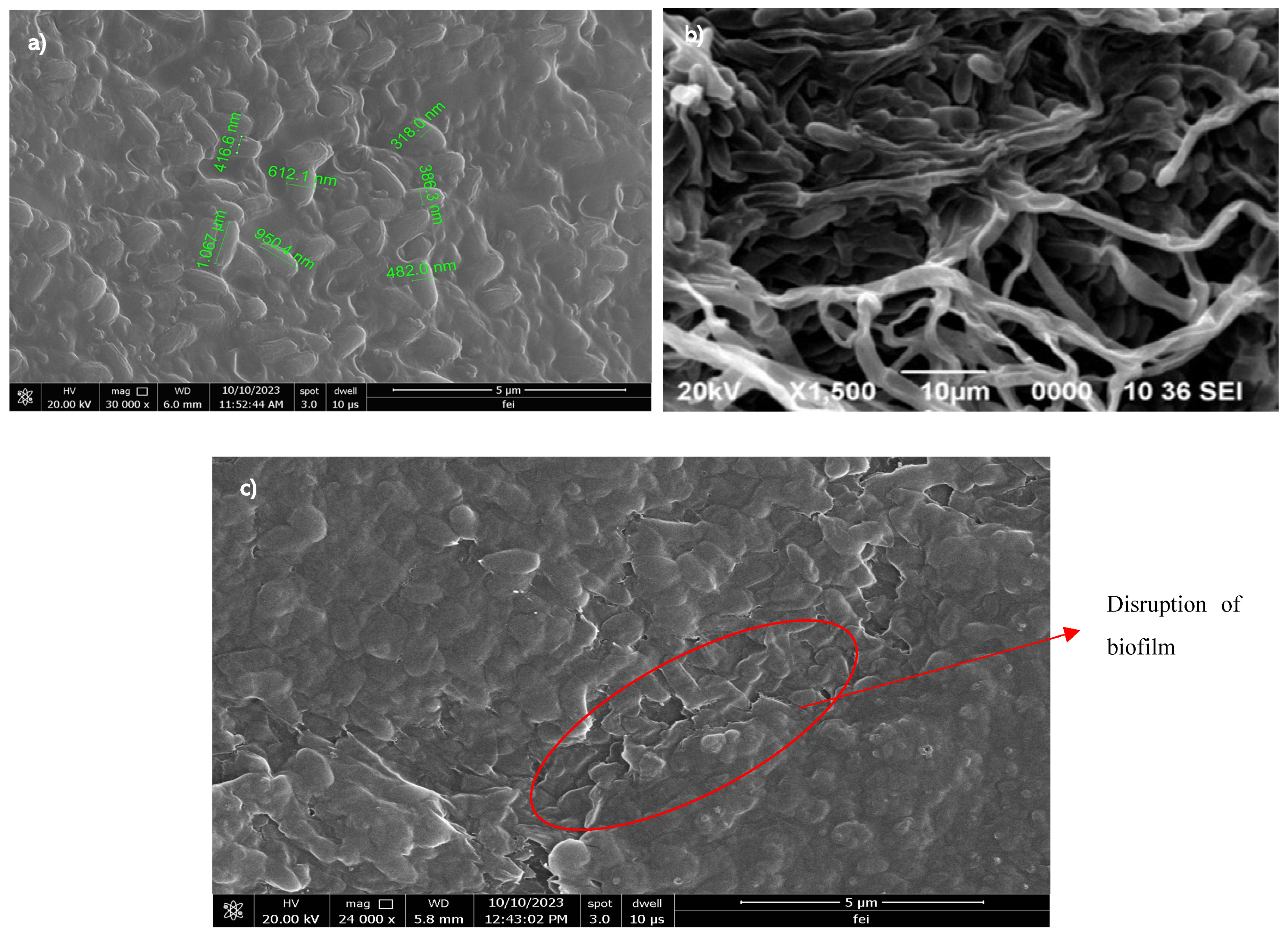
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Pot Experiment Setup
- T1: Positive control (inoculation with Xoo at 108 CFU/mL on the 45th day after transplantation)
- T2: Seed treatment with T. asperellum formulation (1%) for 1 h + two foliar sprays (1%) at 45 and 60 days after transplantation (DAT)
- T3: Treatment of seeds with P. fluorescens formulation (1%) for 1 h + two foliar sprays (1%) at 45 and 60 DAT
- T4: Treatment of seeds with T. asperellum (1%) and P. fluorescens (1%) for 1 h + two foliar sprays carrying T. asperellum + P. fluorescens (1% each) at 45 and 60 DAT
- T5: Negative control (clip inoculated with sterilized water only)
2.6.1. Observations on Percentage Seed Germination and Seedling Vigor
2.6.2. Observations on Plant Growth Parameters and Disease Index
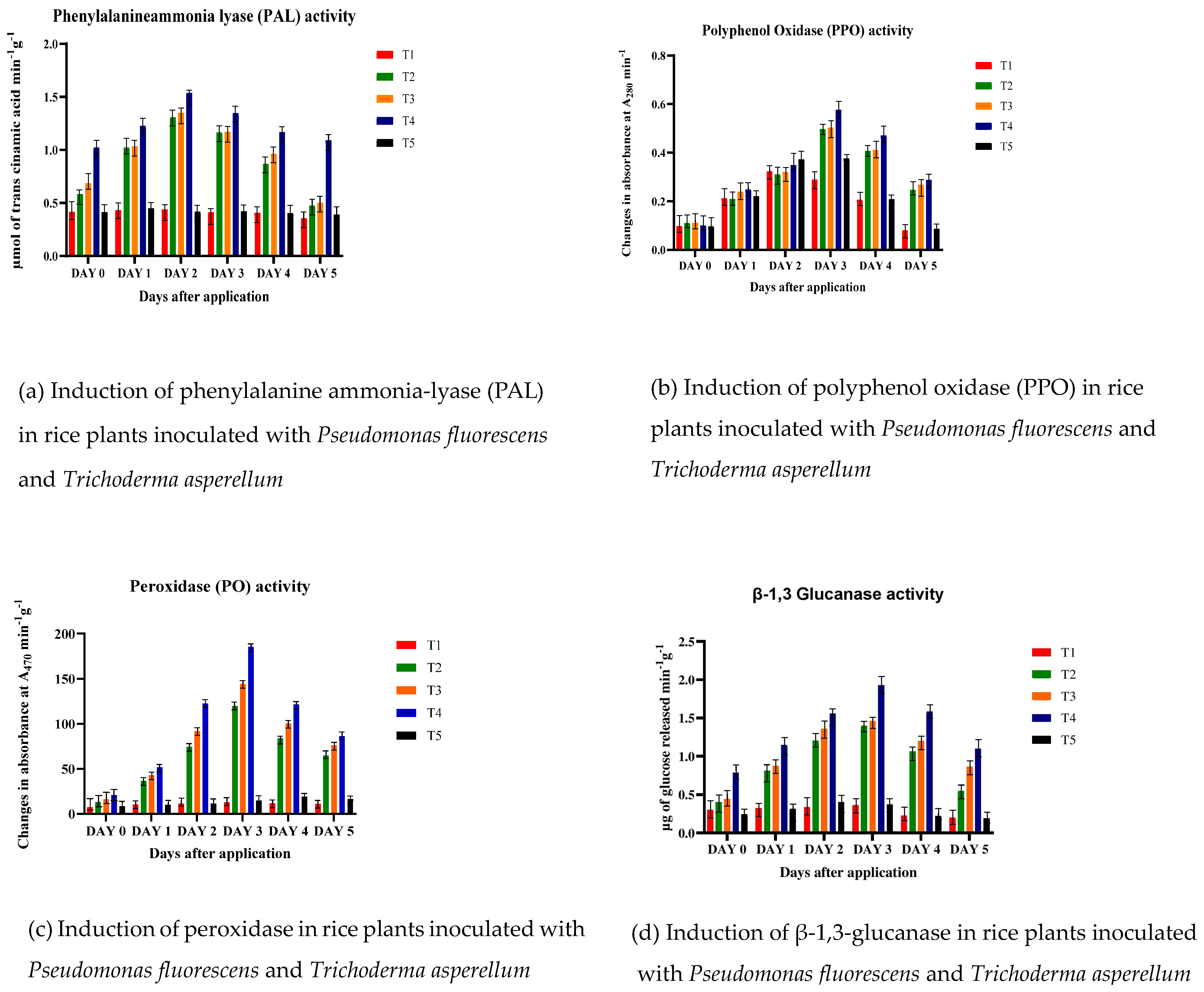
2.6.3. Enzyme Assays
Assay of PAL
Assay of PPO
Assay of ß-1,3 Glucanase
Assay of PO
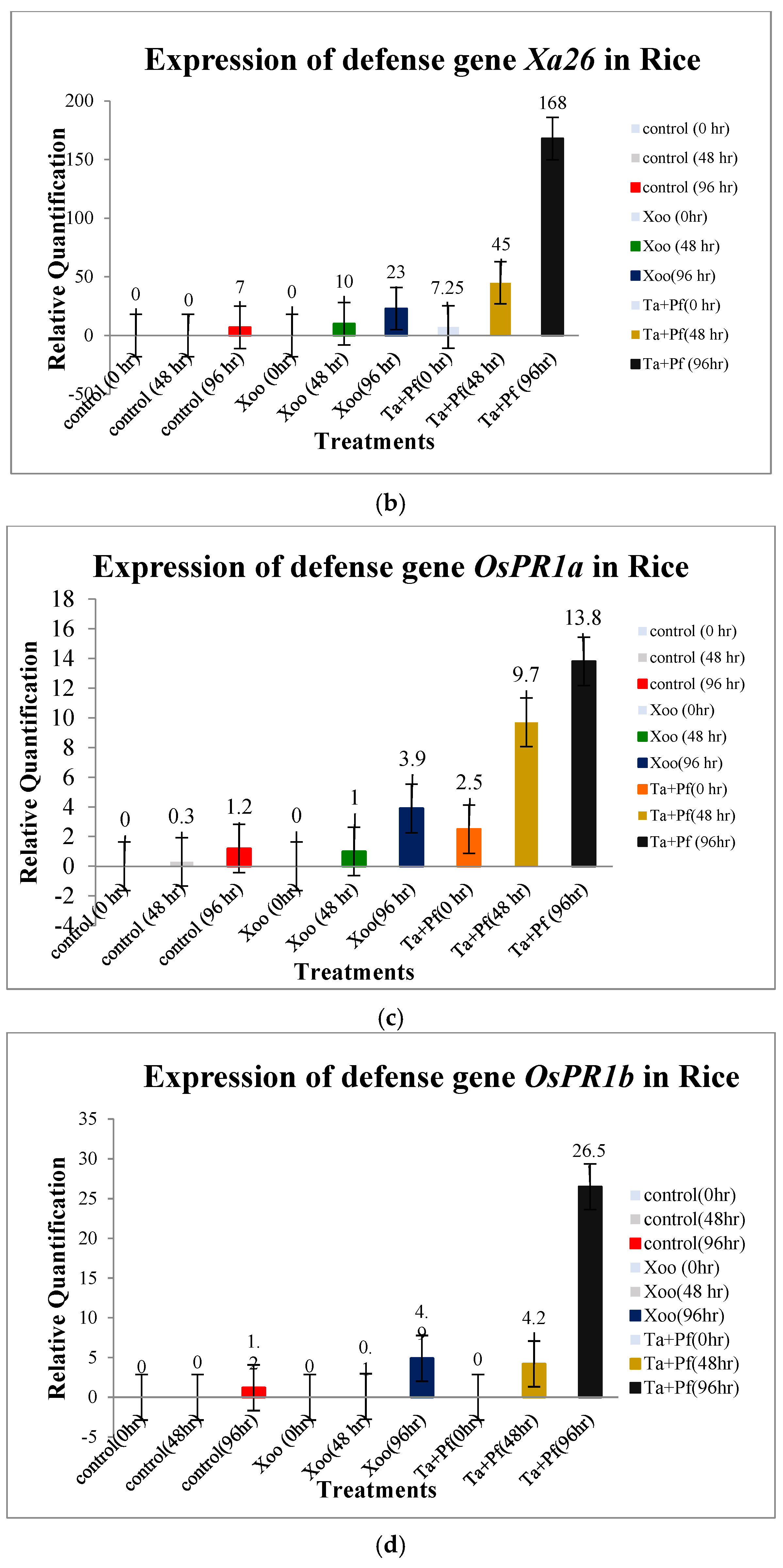
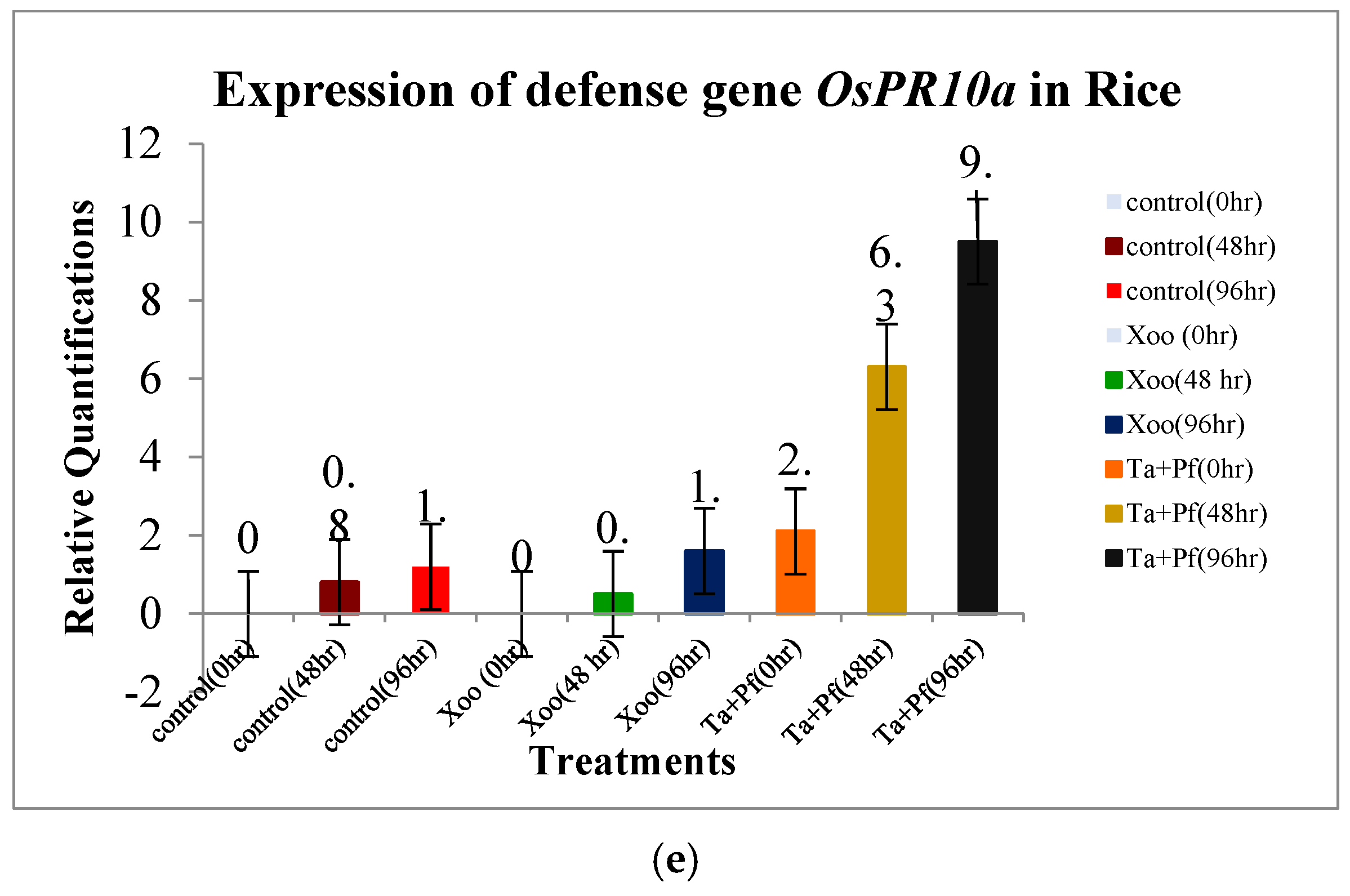
2.7. Gene-Expression Profile, Evaluated Through qRT-PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Confirmation of the Pathogen
3.2. In Vitro Antagonistic Activity
3.3. SEM Analysis
3.4. In Vivo Bio-Efficacy Response
3.4.1. Seed Germination and Plant Growth Parameters
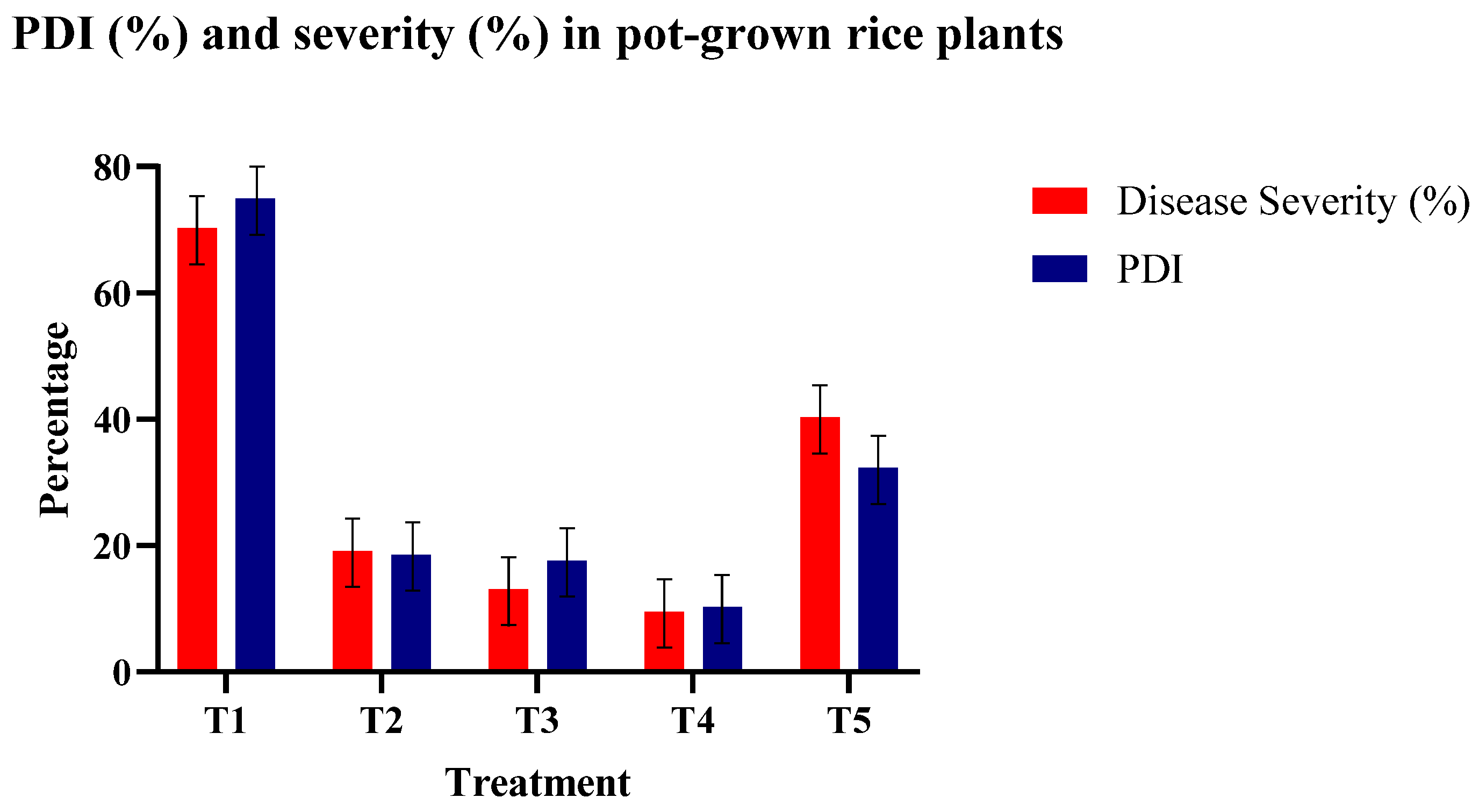
3.4.2. Response with Respect to Disease Index
3.5. Regulation of Defense Enzymes
3.6. Expression of Defense Genes in Rice Due to the Application of T. asperellum + P. fluorescens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nino-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Sanya, D.R.A.; Syed-Ab-Rahman, S.F.; Jia, A.; Onesime, D.; Kim, K.M.; Ahohuendo, B.C.; Rohr, J.R. A review of approaches to control bacterial leaf blight in rice. World J. Microbiol. Biotechnol. 2022, 38, 113. [Google Scholar] [CrossRef]
- Qudsia, H.; Akhter, M.; Riaz, A.; Haider, Z.; Mahmood, A. Comparative efficacy of different chemical treatments for paddy blast, brown leaf spot, and bacterial leaf blight diseases in rice (Oryza sativa L.). Appl. Microbiol. 2017, 3, 1000138. [Google Scholar] [CrossRef]
- Ansari, T.H.; Ahmed, M.; Ara, A.; Khan, M.A.I.; Mian, M.S.; Zahan, Q.S.A.; Tomita, M. Yield loss assessment of rice due to bacterial blight at different resistance levels. Bangladesh J. Plant Pathol. 2018, 34, 71–76. [Google Scholar]
- Sharma, P.; Bora, L.C.; Bora, P. In vitro evaluation on population dynamics of Pseudomonas fluorescens in suppression of bacterial blight of rice (Xanthomonas oryzae pv. oryzae) enriched with micronutrients. J. Soils Crops 2021, 31, 219–224. [Google Scholar]
- Tao, H.; Li, X.; Huo, H.; Cai, Y.; Cai, A. Bacillus velezensis Y6, a Potential and Efficient Biocontrol Agent in Control of Rice Sheath Blight Caused by Rhizoctonia solani. Microorganisms 2024, 12, 1694. [Google Scholar] [CrossRef]
- Moran-Diez, M.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Chetia, S.K.; Kalita Monalisha Ali, M.S.; Das, B.C.; Ahmed, T. Variability of Xanthomonas oryzae pv. oryzae and introgression of BLB resistance in the popular rice varieties–Ranjit and Shraboni of Assam. Res. Crops 2016, 17, 336–341. [Google Scholar] [CrossRef]
- Ritbamrung, O.; Inthima, P.; Ratanasut, K.; Kawee, S.; Tepsuda, R.; Kittisak, B. Evaluating Xanthomonas oryzae pv. oryzae (Xoo) infection dynamics in rice for distribution routes and environmental reservoirs by molecular approaches. Sci. Rep. 2025, 15, 1408. [Google Scholar] [CrossRef]
- Li, Z.; Shen, S.; Xia, K. Integrative genomic and transcriptomic analysis of Xanthomonas oryzae pv. oryzae pathotype IV, V, and IX in China reveals rice defense-responsive genes. Phytopathol. Res. 2024, 6, 28. [Google Scholar] [CrossRef]
- Bora, P.; Gogoi, S.; Deshpande, M.V.; Garg, P.; Bhuyan, R.P.; Altaf, N.; Saha, N.; Borah, S.M.; Phukon, M.; Tanti, N. Rhizospheric Bacillus spp. exhibit miticidal efficacy against Oligonychus coffeae (Acari: Tetranychidae) of Tea. Microorganisms 2023, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- Dugassa, A.; Alemu, T.; Woldehawariat, Y. In-vitro compatibility assay of indigenous Trichoderma and Pseudomonas species and their antagonistic activities against black root rot disease (Fusarium solani) of faba bean (Vicia faba L.). BMC Microbiol. 2021, 21, 115. [Google Scholar] [CrossRef]
- Chowdappa, A.; Kamalakannan, A.; Kousalya, S.; Gopalakrishnan, C.; Venkatesan, K.; Raju, G.S. In vitro evaluation of antagonists against Xanthomonas axonopodis pv. punicae. Int. J. Pure App. Biosci. 2018, 6, 272–280. [Google Scholar] [CrossRef]
- Gashaw, T.; Sitotaw, B.; Yilma, S. Evaluation of Rhizosphere Bacterial Antagonists against Ralstonia solanacearum Causing Tomato (Lycopersicon esculentum) Wilt in Central Ethiopia. Int. J. Agron. 2022, 22, 6341555. [Google Scholar] [CrossRef]
- Das, S.; Kundu, S.; Meena, K.; Jha, R.K.; Varma, A.; Bahuguna, R.N.; Tripathi, S. Seed biopriming with potential bioagents influences physiological processes and plant defense enzymes to ameliorate sheath blight-induced yield loss in rice (Oryza sativa L.). World J. Microbiol. Biotechnol. 2023, 39, 136. [Google Scholar] [CrossRef]
- Mckinney, H.H. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 1923, 26, 195–217. [Google Scholar]
- Wheeler, B.E.J. An Introduction to Plant Disease; John Wiley and Sons Ltd.: London, UK, 1969; p. 301. [Google Scholar]
- Islam, M.R.; Chowdhury, R.; Roy, A.S.; Islam, M.N.; Mita, M.M.; Bashar, S.; Latif, M.A. Native Trichoderma induced the defense-related enzymes and genes in rice against Xanthomonas oryzae pv. oryzae (Xoo). Plants 2023, 12, 1864. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Harel, E.; Ben-Shaul, R. Assay of catechol oxidase—A critical comparison of methods. Phytochemistry 1966, 5, 783–789. [Google Scholar] [CrossRef]
- Pan, S.Q.; Ye, X.S.; Kuc, J. Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol. Mol. Plant Pathol. 1991, 39, 25–39. [Google Scholar] [CrossRef]
- Addy, S.K.; Goodman, R.N. Polyphenol oxidase and peroxidase activity in apple leaves inoculated with a virulent or an avirulent strain of Erwinia amylovora. Indian Phytopathol. 1973, 25, 575–579. [Google Scholar]
- Ponciano, G.; Yoshikawa, M.; Lee, J.L.; Ronald, P.C.; Whalen, M.C. Pathogenesis-related gene expression in rice is correlated with developmentally controlled Xa21-mediated resistance against Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant Pathol. 2006, 69, 131–139. [Google Scholar] [CrossRef]
- Gangwar, G.P.; Sinha, A.P. Comparative antagonistic potential of Trichoderma spp. against Xanthomonas oryzae pv. oryzae. Ann. Plant Prot. Sci. 2010, 18, 458–463. [Google Scholar]
- Arshad, H.M.; Naureen, S.; Saleem, K.; Ali, S.; Jabeen, T.; Babar, M.M. Morphological and biochemical characterization of Xanthomonas oryzae pv. oryzae isolates collected from Punjab in 2013. Adv. Life Sci. 2015, 2, 125–130. [Google Scholar]
- Bora, P.; Bora, L.C.; Bhuyan, R.P.; Hashem, A.; Abd-Allah, E.F. Bioagent consortia assisted suppression in grey blight disease with enhanced leaf nutrients and biochemical properties of tea (Camellia sinensis). Biol. Control 2022, 170, 104907. [Google Scholar] [CrossRef]
- Yang, R.; Li, S.; Li, Y.; Yan, Y.; Fang, Y.; Zou, L.; Chen, G. Bactericidal effect of Pseudomonas oryziphila sp. nov., a novel Pseudomonas species against Xanthomonas oryzae reduces disease severity of bacterial leaf streak of rice. Front. Microbiol. 2021, 12, 759536. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant-pathogen interactions. Soil Bio. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Ann. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; del-Val, E.; Larsen, J. Interactions of Trichoderma with plants, insects, and plant pathogen microorganisms: Chemical and molecular bases. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 263–290. [Google Scholar]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soil-borne pathogens and beneficial microorganisms. Rhizosphere Achiev. Chall. 2010, 104, 536. [Google Scholar]
- Bora, P.; Bora, L.C. Disease management in horticulture crops through microbial interventions: An overview. Indian J. Agric. Sci. 2020, 90, 1389–1396. [Google Scholar] [CrossRef]
- Bora, P.; Bora, L.C. Microbial antagonists and botanicals mediated disease management in tea, Camellia sinensis (L.) O. Kuntze: An overview. Crop Prot. 2021, 148, 105711. [Google Scholar] [CrossRef]
- Appu, M.; Ramalingam, P.; Sathiyanarayanan, A.; Huang, J. An overview of plant defense-related enzymes responses to biotic stresses. Plant Gene 2021, 27, 100302. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Yoshimura, S.; Yamanouchi, U.; Katayose, Y.; Toki, S.; Wang, Z.X.; Kono, I.; Kurata, N.; Yano, M.; Iwata, N.; Sasaki, T. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. USA 1998, 95, 1663–1668. [Google Scholar] [CrossRef]
- Rouina, H.; Tseng, Y.H.; Nataraja, K.N.; Uma Shaanker, R.; Oelmüller, R. Arabidopsis restricts sugar loss to a colonizing Trichoderma harzianum strain by downregulating SWEET11 and-12 and upregulation of SUC1 and SWEET2 in the roots. Microorganisms 2021, 9, 1246. [Google Scholar] [CrossRef]
- Sheikh, T.M.; Zhang, L.; Zubair, M.; Hanif, A.; Li, P.; Farzand, A.; Ali, H.; Bilal, M.S.; Hu, Y.; Chen, X.; et al. The type III accessory protein HrpE of Xanthomonas oryzae pv. oryzae surpasses the secretion role and enhances plant resistance and photosynthesis. Microorganisms 2019, 7, 572. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Inhibition Zone in mm (Mean ± SD) |
|---|---|
| T1: Control (Xoo alone) | 0.00 |
| T2: Ta + Xoo | 11.42 ± 0.48 c |
| T3: Pf + Xoo | 12.74 ± 0.81 b |
| T4: Ta + P. fluorescens + Xoo | 15.77 ± 0.93 a |
| Treatment | Germination (%), GP (7 DAS) | Seedling Vigor Index | No. of Tillers on Different Days After Sowing (DAT) | Plant Height (cm) on Different Days After Sowing (DAT) | Percent Disease Incidence (PDI) (%) | Yield (g/hill) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root Length, (cm) | Shoot Length, (cm) | VI | 45 | 60 | 45 | 60 | ||||
| T1 | 79.87 (63.29) d* | 5.0 | 11.9 | 1349.80 d | 5 | 10 | 39.64 | 56.7 | 74.99 | 20.28 |
| T2 | 84.60 (66.89) c | 6.9 | 21.3 | 2385.72 c | 7 | 13 | 41.43 | 62.2 | 18.57 | 37.86 |
| T3 | 86.80 (68.70) b | 6.9 | 20.6 | 2387.00 b | 6 | 12 | 42.70 | 61.3 | 17.59 | 45.90 |
| T4 | 92.50 (74.11) a | 10.3 | 23.5 | 3126.5 a | 7 | 15 | 45.22 | 65.5 | 10.29 | 50.63 |
| T5 | 79.99 (63.37) d | 5.3 | 11.5 | 1343.83 d | 5 | 11 | 39.69 | 57.5 | 32.29 | 35.04 |
| S.Ed (±) | 1.021 | 0.060 | 0.166 | 5.525 | - | - | 0.694 | 1.021 | 2.46 | 0.4 |
| CD (p = 0.05) | 2.082 | 0.126 | 0.338 | 11.121 | - | - | 1.473 | 2.081 | 5.03 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, P.; Chetia, S.K.; Sharma, A.; Ahmed, S.S.; Sharma, P.; Bhattacharyya, A.; Borgohain, R.; Saikia, M.; Barua, P.; Konwar, M.J.; et al. Microbial Biocontrol Agents Engineer Plant Biometrics and Host Response Against Xanthomonas oryzae pv. oryzae in Rice. Microbiol. Res. 2025, 16, 151. https://doi.org/10.3390/microbiolres16070151
Bora P, Chetia SK, Sharma A, Ahmed SS, Sharma P, Bhattacharyya A, Borgohain R, Saikia M, Barua P, Konwar MJ, et al. Microbial Biocontrol Agents Engineer Plant Biometrics and Host Response Against Xanthomonas oryzae pv. oryzae in Rice. Microbiology Research. 2025; 16(7):151. https://doi.org/10.3390/microbiolres16070151
Chicago/Turabian StyleBora, Popy, Sanjay Kumar Chetia, Anwesha Sharma, Shenaz Sultana Ahmed, Pranamika Sharma, Ashok Bhattacharyya, Rupam Borgohain, Mrinal Saikia, Parinda Barua, Milon Jyoti Konwar, and et al. 2025. "Microbial Biocontrol Agents Engineer Plant Biometrics and Host Response Against Xanthomonas oryzae pv. oryzae in Rice" Microbiology Research 16, no. 7: 151. https://doi.org/10.3390/microbiolres16070151
APA StyleBora, P., Chetia, S. K., Sharma, A., Ahmed, S. S., Sharma, P., Bhattacharyya, A., Borgohain, R., Saikia, M., Barua, P., Konwar, M. J., Ahmed, S. S., Rath, A., Rahman, M., Saikia, B., Taye, T., Rahman, N., Khan, P., Baruah, M., Sakia, R., & Bharali, A. (2025). Microbial Biocontrol Agents Engineer Plant Biometrics and Host Response Against Xanthomonas oryzae pv. oryzae in Rice. Microbiology Research, 16(7), 151. https://doi.org/10.3390/microbiolres16070151







