Comprehensive Analysis of Etiological Agents and Drug Resistance Patterns in Ventilator-Associated Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
- Participant older than 18 yrs
- VAP-diagnosed participants, as per CDC guidelines [8].
- Patients diagnosed with pneumonia before initiation of mechanical ventilation;
- Patients without sufficient data/without a complete medical record;
- Patients without informed consent (or whose guardians did not provide consent if the patient was unable to do so).
2.3. Patient Selection and Sample Collection
2.4. Laboratory Methods
2.4.1. Identification of Bacterial and Fungal Pathogens
2.4.2. Polymerase Chain Reaction
3. Result
3.1. Pathogens Implicated to Both Late-Onset and Early-Onset VAP
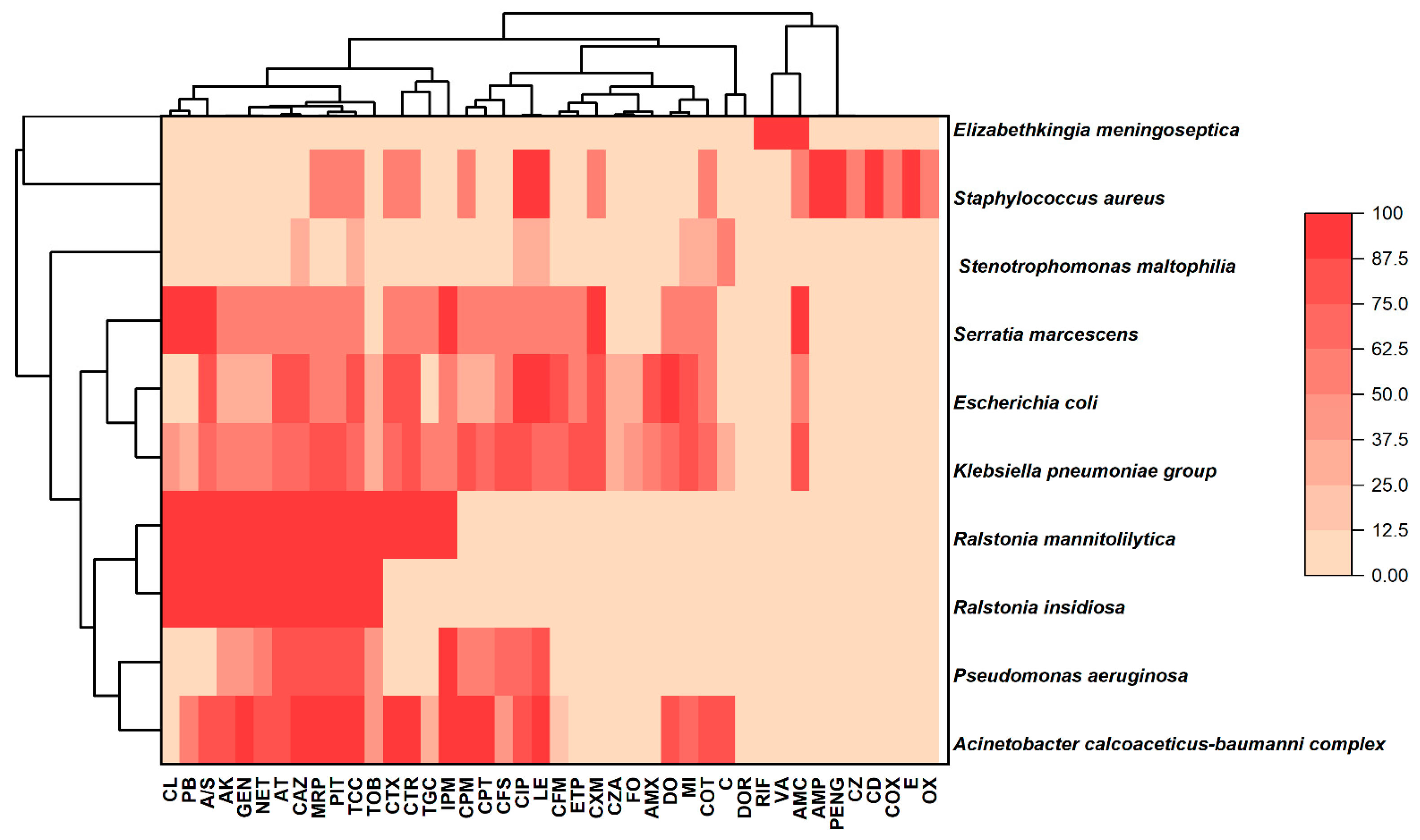
3.2. Pattern of Antibiotic Resistance of Isolated Pathogens Against Different Drugs
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Center for Disease Control and Prevention. Pneumonia (Ventilator-Associated [VAP] and Non-Ventilator-Associated Pneumonia [PNEU]) Event; National Healthcare safety network, Center for Disease Control and Prevention: Atlanta, GA, USA, 2023; pp. 1–19. [Google Scholar]
- Arthur, L.E.; Kizor, R.S.; Selim, A.G.; van Driel, M.L.; Seoane, L. Antibiotics for ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2016, 10, CD004267. [Google Scholar] [CrossRef] [PubMed]
- Mastrogianni, M.; Katsoulas, T.; Galanis, P.; Korompeli, A.; Myrianthefs, P. The Impact of Care Bundles on Ventilator-Associated Pneumonia (VAP) Prevention in Adult ICUs: A Systematic Review. Antibiotics 2023, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Amin, A. Clinical and economic consequences of ventilator-associated pneumonia. Clin. Infect. Dis. 2009, 49 (Suppl. S1), S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Rea-Neto, A.; Youssef, N.C.; Tuche, F.; Brunkhorst, F.; Ranieri, V.M.; Reinhart, K.; Sakr, Y. Diagnosis of ventilator-associated pneumonia: A systematic review of the literature. Crit. Care 2008, 12, R56. [Google Scholar] [CrossRef]
- Rao, S.V.; Thilakchand, K.R.; Boloor, R.; Suresh, S.; George, T.; Pais, M.L.; Jakribettu, R.P.; Baliga, M.S. Antimicrobial resistance pattern in aerobic bacteria isolated from endotracheal aspirate in ventilator-associated pneumonia: Ten years observation from a tertiary care hospital. J. Anaesthesiol. Clin. Pharmacol. 2024, 40, 324–329. [Google Scholar] [CrossRef]
- Kohbodi, G.N.A.; Rajasurya, V.; Noor, A. Ventilator-Associated Pneumonia. In StatPearls [Internet]; 4 September 2023; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507711/ (accessed on 20 October 2024).
- Dey, A.; Bairy, I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: A nine months’ prospective study. Ann. Thorac. Med. 2007, 2, 52–57. [Google Scholar] [CrossRef]
- Dennesen, P.J.; van der Ven, A.J.; Kessels, A.G.; Ramsay, G.; Bonten, M.J. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2001, 163, 1371–1375. [Google Scholar] [CrossRef]
- Edis, E.C.; Hatipoglu, O.N.; Tansel, O.; Sut, N. Acinetobacter pneumonia: Is the outcome different from the pneumonias caused by other agents. Ann. Thorac. Med. 2010, 5, 92–96. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Thakur, H.K.; Tarai, B.; Bhargava, A.; Soni, P.; Rath, P.K.; Mishra, B.P.; Jena, M.K. Pathogenesis, Diagnosis and Therapeutic Strategies for Ventilator-associated Pneumonia. J. Pure Appl. Microbiol. 2024, 18, 772–796. [Google Scholar] [CrossRef]
- Farag, A.M.; Tawfick, M.M.; Abozeed, M.Y.; Shaban, E.A.; Abo-Shadi, M.A. Microbiological profile of ventilator-associated pneumonia among intensive care unit patients in tertiary Egyptian hospitals. J. Infect. Dev. Ctries. 2020, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.S. Strategies to prevent ventilator-associated pneumonia in neonates. Clin. Perinatol. 2010, 37, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111, Erratum in Clin. Infect. Dis. 2017, 64, 1298. https://doi.org/10.1093/cid/ciw799; Erratum in Clin. Infect. Dis. 2017, 65, 1435. https://doi.org/10.1093/cid/cix587; Erratum in Clin. Infect. Dis. 2017, 65, 2161. https://doi.org/10.1093/cid/cix759. [Google Scholar] [CrossRef]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef]
- Dominedò, C.; Ceccato, A.; Torres, A. Ventilator-associated pneumonia: New principles guiding empiric antibiotic therapy. Curr. Opin. Infect. Dis. 2020, 33, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Alnimr, A. Antimicrobial Resistance in Ventilator-Associated Pneumonia: Predictive Microbiology and Evidence-Based Therapy. Infect. Dis. Ther. 2023, 12, 1527–1552. [Google Scholar] [CrossRef]
- Fang, X.; Mei, Q.; Fan, X.; Zhu, C.; Yang, T.; Zhang, L.; Geng, S.; Pan, A. Diagnostic Value of Metagenomic Next-Generation Sequencing for the Detection of Pathogens in Bronchoalveolar Lavage Fluid in Ventilator-Associated Pneumonia Patients. Front. Microbiol. 2020, 11, 599756. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Bouadma, L.; Mathy, V.; Allouche, K.; Patrier, J.; Reboul, M.; Montravers, P.; Timsit, J.-F.; Armand-Lefevre, L. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit. Care 2020, 24, 366. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef]
- CLSI M-38 A Document (Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi), 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology. 8 December 2009. Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf (accessed on 17 March 2025).
- BIOFIRE® FILMARRAY® Pneumonia Plus Panel. 2021. Available online: https://www.biomerieux.com/corp/en/our-offer/clinical-products/biofire-filmarray-pneumonia-panels.html (accessed on 22 March 2025).
- Wałaszek, M.; Kosiarska, A.; Gniadek, A.; Kołpa, M.; Wolak, Z.; Dobroś, W.; Siadek, J. The risk factors for hospital-acquired pneumonia in the Intensive Care Unit. Przegl Epidemiol. 2016, 70, 15–110. [Google Scholar]
- Ibn Saied, W.; Merceron, S.; Schwebel, C.; Le Monnier, A.; Oziel, J.; Garrouste-Orgeas, M.; Marcotte, G.; Ruckly, S.; Souweine, B.; Darmon, M.; et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: Risk factors and outcome. J. Infect. 2020, 80, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ioanas, M.; Ferrer, R.; Angrill, J.; Ferrer, M.; Torres, A. Microbial investigation in ventilator-associated pneumonia. Eur. Respir. J. 2001, 17, 791–801. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Wallet, F.; Loïez, C.; Renaux, E.; Lemaitre, N.; Courcol, R.J. Performances of VITEK 2 colorimetric cards for identification of gram-positive and gram-negative bacteria. J. Clin. Microbiol. 2005, 43, 4402–4406. [Google Scholar] [CrossRef] [PubMed]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.S.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef]
- Charles, M.P.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Kumar, S.; Sivaraman, U. Aetiological agents of ventilator-associated pneumonia and its resistance pattern—A teat for treatment. Australas. Med. J. 2013, 6, 430–434. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Peterson, J.; Fernandez, J.F.; Qin, Z.; Fisher, A.C.; Nicholson, S.C. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir. Care 2013, 58, 1220–1225. [Google Scholar] [CrossRef]
- Ding, X.; Ma, X.; Gao, S.; Su, L.; Shan, G.; Hu, Y.; Chen, J.; Ma, D.; Zhang, F.; Zhu, W.; et al. Effect of ICU quality control indicators on VAP incidence rate and mortality: A retrospective study of 1267 hospitals in China. Crit. Care 2022, 26, 405. [Google Scholar] [CrossRef]
- But, A.; Yetkin, M.A.; Kanyilmaz, D.; Aslaner, H.; Baştuğ, A.; Aypak, A.; Öngürü, P.; Akinci, E.; Mutlu, N.M.; Bodur, H. Analysis of epidemiology and risk factors for mortality in ventilator-associated pneumonia attacks in intensive care unit patients. Turk. J. Med. Sci. 2017, 47, 812–816. [Google Scholar] [CrossRef]
- Garza-González, E.; Camacho-Ortíz, A.; Rodríguez-Noriega, E.; Esparza-Ahumada, S.; Flores-Trevino, S.; Bocanegra-Ibarias, P.; Tijerina-Rodriguez, L.; Morfin-Otero, R. Comparison of Matrix-assisted Laser Desorption Ionization Time-of-flight Mass Spectrometry (MALDI-TOF MS) and the Vitek 2 System for Routine Identification of Clinically Relevant Bacteria and Yeast. Ann. Clin. Lab. Sci. 2020, 50, 119–127. [Google Scholar] [PubMed]
- Hong, H.L.; Hong, S.B.; Ko, G.B.; Huh, J.W.; Sung, H.; Do, K.-H.; Kim, S.-H.; Lee, S.-O.; Kim, M.-N.; Jeong, J.-Y.; et al. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS ONE 2014, 9, e95865. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Voiriot, G.; Houhou-Fidouh, N.; Neuville, M.; Bouadma, L.; Lescure, F.-X.; Descamps, D.; Timsit, J.-F.; Yazdanpanah, Y.; Visseaux, B. Impact of respiratory viruses in hospital-acquired pneumonia in the intensive care unit: A single-center retrospective study. J. Clin. Virol. 2017, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- van Someren Gréve, F.; Juffermans, N.P.; Bos, L.D.J.; Binnekade, J.M.; Braber, A.; Cremer, O.L.; de Jonge, E.; Molenkamp, R.; Ong, D.S.Y.; Rebers, S.P.H.; et al. Respiratory Viruses in Invasively Ventilated Critically Ill Patients-A Prospective Multicenter Observational Study. Crit. Care Med. 2018, 46, 29–36. [Google Scholar] [CrossRef]
- Luyt, C.E.; Combes, A.; Deback, C.; Aubriot-Lorton, M.-H.; Nieszkowska, A.; Trouillet, J.-L.; Capron, F.; Agut, H.; Gibert, C.; Chastre, J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 2007, 175, 935–942. [Google Scholar] [CrossRef]
- Limaye, A.P.; Kirby, K.A.; Rubenfeld, G.D.; Leisenring, W.M.; Bulger, E.M.; Neff, M.J.; Gibran, N.S.; Huang, M.-L.; Hayes, T.K.S.; Corey, L.; et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008, 300, 413–422. [Google Scholar] [CrossRef]
- Papazian, L.; Fraisse, A.; Garbe, L.; Zandotti, C.; Thomas, P.; Saux, P.; Perrin, G.; Gouin, F. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology 1996, 84, 280–287. [Google Scholar] [CrossRef]
- Buchan, B.W.; Windham, S.; Balada-Llasat, J.M.; Leber, A.; Harrington, A.; Relich, R.; Murphy, C.; Bard, J.D.; Naccache, S.; Ronen, S.; et al. Practical Comparison of the BioFire FilmArray Pneumonia Panel to Routine Diagnostic Methods and Potential Impact on Antimicrobial Stewardship in Adult Hospitalized Patients with Lower Respiratory Tract Infections. J. Clin. Microbiol. 2020, 58, e00135-20. [Google Scholar] [CrossRef]
- Foschi, C.; Zignoli, A.; Gaibani, P.; Vocale, C.; Rossini, G.; Lafratta, S.; Liberatore, A.; Turello, G.; Lazzarotto, T.; Ambretti, S. Respiratory bacterial co-infections in intensive care unit-hospitalized COVID-19 patients: Conventional culture vs BioFire FilmArray pneumonia Plus panel. J. Microbiol. Methods 2021, 186, 106259. [Google Scholar] [CrossRef]
- Ginocchio, C.C.; Garcia-Mondragon, C.; Mauerhofer, B.; Rindlisbacher, C.; The EME Evaluation Program Collaborative. Multinational evaluation of the BioFire® FilmArray® Pneumonia plus Panel as compared to standard of care testing. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1609–1622. [Google Scholar] [CrossRef]
- Molina, F.J.; Botero, L.E.; Isaza, J.P.; Cano, L.E.; López, L.; Tamayo, L.; Torres, A. Diagnostic concordance between BioFire® FilmArray® Pneumonia Panel and culture in patients with COVID-19 pneumonia admitted to intensive care units: The experience of the third wave in eight hospitals in Colombia. Crit. Care 2022, 26, 130. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.K.; Hinic, V.; Goldenberger, D.; Gensch, A.; Schweitzer, M.; Bättig, V.; Siegemund, M.; Bassetti, S.; Bingisser, R.; Tamm, M.; et al. Evaluation of the clinical relevance of the Biofire© FilmArray pneumonia panel among hospitalized patients. Infection 2024, 52, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rezia, A.; Vijendra, R. A clinical study on the pattern of antimicrobial drug use and drug resistance in patients with ventilator-associated pneumonia in a tertiary care hospital. Indian J. Pharmacol. 2023, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ojha, A.K.; Dolma, K.G.; Majumdar, T.; Sarmah, P.; Hazarika, S.; Modi, D.; Gogoi, D.; Das, S.; Ramamurthy, T. Monitoring the potential dissemination of antimicrobial resistance in foods, environment, and clinical samples: A one health prospective. Food Sci. Biotechnol. 2024, 34, 803–813. [Google Scholar] [CrossRef]
- Yudhanto, S.; Hung, C.C.; Maddox, C.W.; Varga, C. Antimicrobial Resistance in Bacteria Isolated From Canine Urine Samples Submitted to a Veterinary Diagnostic Laboratory, Illinois, United States. Front. Vet. Sci. 2022, 9, 867784. [Google Scholar] [CrossRef]
| Demographic Profile of Patients with VAP | ||||
|---|---|---|---|---|
| Demography | Bacterial | Fungal | Viral | Total |
| Age | ||||
| <60 yrs | 22 | 3 | 0 | 25 |
| >60 yrs | 44 | 0 | 1 | 45 |
| Total | 66 | 3 | 1 | 70 |
| p value | 0.0247 | 0.0865 | 0.319 | 0.0499 |
| Sex | ||||
| Male | 43 | 1 | 1 | 45 |
| Female | 23 | 2 | 0 | 25 |
| Total | 66 | 3 | 1 | 70 |
| p value | 0.0414 | 0.5678 | 0.319 | 0.0499 |
| Residential | ||||
| Urban | 60 | 2 | 1 | 63 |
| Rural | 6 | 1 | 0 | 7 |
| Total | 66 | 3 | 1 | 70 |
| p value | <0.0001 | 0.5678 | 0.319 | <0.0001 |
| Co-morbidity condition | ||||
| Patients with single co-morbidities | 12 | 0 | 0 | 12 |
| Patients with ≥2 co-morbidities * | 44 | 3 | 0 | 47 |
| Patients with missing data/no co-morbidities | 10 | 0 | 1 | 11 |
| Total | 66 | 3 | 1 | 70 |
| p value | <0.0001 | 0.0541 | 0.371 | <0.0001 |
| Treatment given | ||||
| Intake of steroids | 34 | 3 | 0 | 37 |
| Intake of antibiotics | 67 | 3 | 0 | 70 |
| p value | 0.0115 | >0.9999 | 0.0154 | |
| Pathogens | DM | Kidney Failure | Accidental | Heart Disease | Sepsis | Respiratory Failure | HTN | Immuno-Compromised | Cancer | Thyroid | Motor Neuron Disease | COPD | Surgery | Liver |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | 11 | 7 | 1 | 8 | 4 | 8 | 14 | ND | 3 | 5 | 1 | 2 | 1 | ND |
| Pseudomonas aeruginosa | 10 | 5 | 3 | 4 | 2 | 4 | 10 | ND | 2 | 4 | 1 | 3 | ND | 1 |
| Klebsiella pneumoniae group | 12 | 8 | 1 | 6 | 7 | 10 | 17 | 1 | ND | 3 | ND | 3 | 2 | 1 |
| Escherichia coli | 6 | 1 | ND | 2 | 1 | 1 | 4 | ND | ND | 1 | ND | ND | ND | ND |
| Burkholderia cepacia | 1 | ND | ND | 1 | ND | ND | 1 | ND | ND | 1 | ND | ND | ND | ND |
| Staphylococcus aureus | 5 | 2 | 1 | ND | 1 | 2 | 5 | ND | 1 | 1 | ND | ND | ND | ND |
| Serratia marcescens | 2 | ND | ND | ND | ND | 1 | 1 | ND | 1 | 2 | ND | ND | ND | ND |
| Elizabethkingia meningoseptica | ND | ND | ND | ND | 1 | ND | ND | ND | ND | ND | 1 | ND | ND | ND |
| Candida tropicalis | 3 | 3 | 1 | 2 | 2 | 1 | 3 | ND | ND | ND | ND | ND | ND | ND |
| Candida albicans | ND | ND | ND | ND | ND | 1 | 1 | ND | 1 | ND | ND | ND | ND | ND |
| Ralstonia insidiosa | 1 | ND | ND | ND | ND | ND | ND | ND | 1 | ND | ND | ND | ND | ND |
| Stenotrophomonas maltophilia | ND | ND | ND | 1 | ND | 1 | 1 | ND | ND | ND | ND | ND | ND | ND |
| Ralstonia mannitolilytica | ND | ND | ND | 1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Aspergillus | 1 | 1 | ND | 1 | 1 | 1 | 1 | ND | ND | ND | ND | ND | ND | ND |
| Streptococcus pneumoniae | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Pathogens | Early-Onset-VAP, n (%) | Late-Onset-VAP, n (%) | Chi sq. | p Value |
|---|---|---|---|---|
| Acinetobacter baumannii | 4 (5.71) | 5 (7.14) | 0.1044 | 0.7466 |
| Pseudomonas aeruginosa | 4 (5.71) | 7 (10) | 0.7589 | 0.3837 |
| Klebsiella pneumoniae group | 1 (1.42) | 9 (12.85) | 5.99 | 0.0144 |
| Escherichia coli | 2 (2.85) | 0 | 1.972 | 0.1602 |
| Burkholderia cepacia | 0 | 1 (1.42) | 0.993 | 0.319 |
| Staphylococcus aureus | 0 | 1 (1.42) | 0.993 | 0.319 |
| Serratia marcescens | 1 (1.42) | 0 | 0.993 | 0.319 |
| Elizabethkingia meningoseptica | 0 | 1 (1.42) | 0.993 | 0.319 |
| Candida tropicalis | 2 (2.85) | 0 | 1.972 | 0.1602 |
| Candida albicans | 0 | 1 (1.42) | 0.993 | 0.319 |
| Ralstonia insidiosa | 0 | 1 (1.42) | 0.993 | 0.319 |
| Stenotrophomonas maltophilia | 0 | 1 (1.42) | 0.993 | 0.319 |
| Ralstonia mannitolilytica | 1 (1.42) | 0 | 0.993 | 0.319 |
| Aspergillus | 0 | 1 (1.42) | 0.993 | 0.319 |
| Streptococcus pneumoniae | 1 (1.42) | 0 | 0.993 | 0.319 |
| Polymicrobial infection | 3 (4.28) | 19 (27.14) | 10.16 | 0.0014 |
| Haemophilus influenzae | 1 (1.42) | 1 (1.42) | 0 | >0.9999 |
| Human Rhino/Enterovirus | 1 (1.42) | 0 | 0.993 | 0.319 |
| Mycoplasma pneumoniae | 1 (1.42) | 0 | 0.993 | 0.319 |
| Total | 22 (31.42) | 48 (68.57) | 6.538 | 0.0106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, H.K.; Tarai, B.; Bhargava, A.; Soni, P.; Ojha, A.K.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Jena, M.K. Comprehensive Analysis of Etiological Agents and Drug Resistance Patterns in Ventilator-Associated Pneumonia. Microbiol. Res. 2025, 16, 152. https://doi.org/10.3390/microbiolres16070152
Thakur HK, Tarai B, Bhargava A, Soni P, Ojha AK, Kancharla S, Kolli P, Mandadapu G, Jena MK. Comprehensive Analysis of Etiological Agents and Drug Resistance Patterns in Ventilator-Associated Pneumonia. Microbiology Research. 2025; 16(7):152. https://doi.org/10.3390/microbiolres16070152
Chicago/Turabian StyleThakur, Harendra K., Bansidhar Tarai, Aradhana Bhargava, Pankaj Soni, Anup Kumar Ojha, Sudhakar Kancharla, Prachetha Kolli, Gowtham Mandadapu, and Manoj Kumar Jena. 2025. "Comprehensive Analysis of Etiological Agents and Drug Resistance Patterns in Ventilator-Associated Pneumonia" Microbiology Research 16, no. 7: 152. https://doi.org/10.3390/microbiolres16070152
APA StyleThakur, H. K., Tarai, B., Bhargava, A., Soni, P., Ojha, A. K., Kancharla, S., Kolli, P., Mandadapu, G., & Jena, M. K. (2025). Comprehensive Analysis of Etiological Agents and Drug Resistance Patterns in Ventilator-Associated Pneumonia. Microbiology Research, 16(7), 152. https://doi.org/10.3390/microbiolres16070152







