Abstract
This study evaluated the acaricidal effects of biosurfactants produced by Serratia ureilytica against the two-spotted spider mite Tetranychus urticae and their compatibility with the predatory mite Ambliseus swirski. The biosurfactants were obtained via liquid cultures of the bacterial strains. In the laboratory, T. urticae was exposed via acaricide-immersed leaves and A. swirskii via acaricide-coated glass vials. In the greenhouse, mite-infested plants were sprayed with the biosurfactants. In the laboratory, biosurfactants produced by S. ureilytica NOD-3 and UTS exhibited strong acaricidal activity, causing 95% mortality in adults and reducing egg viability by more than 60%. In the greenhouse trial, all biosurfactants significantly suppressed T. urticae populations at all evaluated periods (7, 14, and 21 days post-application). Gas chromatography–mass spectrometry (GC-MS) analysis of the biosurfactants identified several fatty acids, including hexadecanoic acid, pentanoic acid, octadecanoic acid, decanoic acid, and tetradecanoic acid, as well as the amino acids L-proline, L-lysine, L-valine, and glutamic acid. These fatty acids and amino acids are known structural components of lipopeptides. Furthermore, the bioinformatic analysis of the genomes of the three S. ureilytica strains revealed nonribosomal peptide synthetase (NRPS) gene clusters homologous to those involved in the biosynthesis of lipopeptides. These findings demonstrate that S. ureilytica biosurfactants are promising eco-friendly acaricides, reducing T. urticae populations by >95% while partially sparing A. swirskii.
1. Introduction
The genus Serratia (Enterobacteriaceae) comprises 32 identified species of Gram-negative bacteria, known for their adaptability to diverse environments, including water, soil, small mammals, and insects [1,2,3]. In recent years, several Serratia species have been investigated for their potential as biocontrol agents against agricultural pests [4,5,6]. Species like Serratia liquefaciens, S. marcescens, and S. entomophila have shown high effectiveness in controlling insect pest species from various groups, such as Orthoptera, Coleoptera, Hemiptera, Lepidoptera, and Diptera [2,7,8]. Building on these findings, recent attention has turned to other lesser-known species within the genus, such as S. ureilytica, which has demonstrated antibacterial, antifungal, nematocidal, and insecticidal activity [2,9,10]. Despite its potential, research on its application as a microbial insecticide or acaricide remains limited. Notably, some S. ureilytica strains have shown high effectiveness (over 50% mortality) against the dust mite Tyrophagus putrescentiae [11]. The activity of S. ureilytica against insects and mites is likely driven by its enzymatic activity (e.g., proteases, chitinases, and collagenases) and the action of secondary metabolites, as observed in other species of Serratia. These mechanisms underscore its potential for biological control [3,12]. A recent study highlights the nematocidal and antifungal properties of metabolites produced by S. ureilytica, demonstrating more than 90% mortality on Nacobbus aberrans and 70–80% inhibition of the mycelial growth of Fusarium oxysporum strains. These effects are attributed to amino acids and fatty acids produced by the liquid culture of this bacterium [10].
The two-spotted spider mite (Tetranychus urticae) is a major polyphagous pest, impacting over 1100 economically important plant species in greenhouse and field crops [13,14]. When feeding, T. urticae causes chlorotic spots by perforating plant cells and extracting their contents [15]. Under high population densities, this mite can infest flowers and fruits, significantly reducing stomatal resistance, the photosynthetic rate, and transpiration and ultimately lowering crop yields [16,17]. The frequent use of chemical acaricides has led to the development of acaricide-resistant populations [18], which has raised interest in the use of environmentally friendly control methods. Among these, bacteria have shown strong potential for agricultural pest management. In this sense, Serratia species exhibit significant potential for the biocontrol of pest insects and mites [4,11,19,20]. Bacterial derivatives offer a promising alternative to chemical pesticides because they are safer for humans, reduce the resistance risks, and minimize environmental residues [21]. In this context, this study evaluated the acaricidal potential of biosurfactants produced by S. ureilytica as a control agent against the two-spotted spider mite Tetranychus urticae and their compatibility with the predatory mite Amblyseius swirskii. In addition, the chromatographic profiles of the biosusfactants were also assessed via gas chromatography–mass spectrometry.
2. Materials and Methods
2.1. Bacterial Strains
Serratia strains were isolated from Mimosa pudica nodules in the Municipality of Ocosingo, Chiapas, Mexico and preserved in 20% glycerol at −80 °C [22]. Taxonomic identification was performed by genomic sequencing, and the strains were classified as S. ureilytica UTS, S. ureilytica NOD-3, and S. ureilytica NOD-7. Their genomes were deposited in GenBank under the BioProject IDcorrect PRJNA998017. The BioSample numbers are SAMN21162916 for S. ureilytica UTS, SAMN36717748 for S. ureilytica NOD-3, and SAMN36717746 for S. ureilytica NOD-7 [11].
2.2. Production of Biosurfactants
Bacterial strains were individually precultured in 100 mL of Luria–Bertani medium for 24 h at 30 °C and 140 rpm. A 40 mL sample of each preculture was taken and added to 400 mL of sterile PG culture medium (4 g/L casein peptone, 14 g/L glycerol, and 1.50 g/L (NH4)2SO4), resulting in a final inoculum concentration of 10% (v/v). The cultures were incubated in beakers at 30 °C for 168 h at 140 rpm [23].
After incubation, the culture medium of each strain was centrifuged at 6000 rpm for 25 min at 4 °C. The cell-free supernatant obtained was acidified to pH 2 using a 1 M HCl solution [24]. Subsequently, two volumes of cold ethanol were added [25], and the mixture was stirred for 30 min and kept at 4 °C for 72 h. The formed precipitate was removed by centrifugation at 6000 rpm for 20 min at 4 °C. Finally, the supernatant was evaporated in a rotary evaporator until a brown and viscous solution was obtained [26]. The biosurfactants were stored at room temperature (30–32 °C) for 24 to 48 h and were subsequently used for bioassays
2.3. Characterization of Biosurfactants
The characterization of the biosurfactants produced by the bacterial strains included both functional assays and chemical analysis. Two tests were performed to detect the surfactant activity of the biosurfactant produced by the bacterial strains in the culture medium: the dispersal test and the emulsification index test. For the dispersal test, the methodologies of Sharma et al. [27] and García-Reyes and Yañez-Ocampo [28] were followed. The oil dispersion test consisted of adding 20 mL of water to a Petri dish, followed by 200 µL of used motor oil to the center of the dish. Finally, 20 µL of the crude extract of each strain was gently added to the center of the oil drop. The presence of biosurfactants in the crude extract was indicated by the formation of a transparent halo, whose diameter correlated with the surfactant activity.
The emulsification rates of the culture samples were determined by adding 2 mL of cell-free supernatant to 2 mL of soybean oil in a test tube, followed by vortexing for 2 min and allowing the mixture to stand for 24 h at room temperature. The resulting emulsion was compared with those formed by sodium dodecyl sulfate (SDS) and 0.5% Tween 80, which served as positive controls. The emulsification index was calculated by dividing the height of the emulsion layer (HE) by the total height of the mixture (HT) and multiplying it by 100, namely EI24 (%) = (HE/HT) × 100, according to standard protocols [29,30].
Additionally, the chemical compositions of the biosurfactants were analyzed by gas chromatography–mass spectrometry (GC-MS) using a TRACE GC system coupled to an ITQ900 ion trap mass detector (Thermo Electron Corporation, Milan, Italy). The carrier gas was helium, with a flow rate of 1 mL/min. The column used was a TRACE-5MS column (30 m length, 0.25 μm film thickness, and 0.25 mm internal diameter). The GC was equipped with a split–splitless injector maintained at 270 °C. The oven temperature was programmed as follows: initially set at 50 °C for 1 min and then increased in three steps from 50 to 300 °C at a rate of 7 °C/min and finally held at 300 °C for 5 min, resulting in a total run time of 56 min. Mass spectra were acquired by electron impact ionization at 70 eV, and the detector was operated in total ion current (TIC) scan mode, ranging from 50 to 650 m/z, with a scanning rate of 0.2 scans/s (dwell time). The transfer line and ion source temperatures were maintained at 270 °C and 200 °C, respectively. The raw data were processed using the Xcalibur™ software version 4.0 (Thermo Scientific, San Jose, CA, USA).
2.4. Colony Establishment of T. urticae
The mite colony was established on Solanum melongena L. (eggplant) plants at Tecnológico Nacional de México, Conkal campus, in Yucatán, México (21.07° N, 89.52° W; 8 m.a.s.l.). The colony was maintained on healthy 2–4-month-old plants in a rustic greenhouse under fluctuating temperature and humidity conditions (23–33 °C and 70–85% relative humidity), with a photoperiod of 12 h light–12 h dark. The predatory mite Ablyseius swirskii was purchased from Koppert Biological Systems (Querétaro, Mexico).
2.5. Preparation of Biosurfactants for Bioassays
For the bioassays, the biosurfactant solutions were prepared by dissolving 50 mg of each biosurfactant in 100 mL of distilled water, resulting in a final concentration of 0.5% (w/v). The chemical acaricide abamectin was used as a positive control at a concentration of 18 mg/L (Abakrone 1 mL/L, Biokrone S.A. de C.V, Celaya, Gto, México.), while distilled water was used as a negative control.
2.6. Evaluation of Mortality of T. urticae and A. swirskii in the Laboratory
For the Tetranychus urticae adult mortality assays, the foliar dip method was used [31]. Solanum melongena leaf discs (5 cm in diameter) were immersed for 5 s in the biosurfactant solutions, which were prepared individually in 250 mL beakers. After air-drying at room temperature for 30 min, the discs were placed adaxial side up on moistened absorbent cotton inside 9-cm-diameter Petri dishes. The edges were lined with moistened cotton to prevent the mites from escaping. Fifteen T. urticae adults were transferred onto each disc, and mortality was recorded at 24, 48, and 72 h post-application. The Petri dishes were kept at 24 ± 3 °C under a 14 h light–10 h dark photoperiod. Mites were considered dead if they showed no movement when gently stimulated with a fine brush. Each biosurfactant was tested with ten replicates, with each Petri dish serving as an independent replicate.
The effects of the biosurfactants on Amblyseius swirskii mortality were assessed in the laboratory following the methodology of Nexticapan-Garcéz et al. [32]. A 5 mL sample of each biosurfactant solution was added to a 20 mL glass vial and agitated to ensure uniform coating of the inner surface. Excess solution was removed, and the vials were air-dried for 3 h. Fifteen A. swirskii adults were then introduced into each vial, which was securely capped. Mortality was recorded at 24, 48, and 72 h post-exposure. Each vial served as an independent replicate, with ten replicates evaluated per biosurfactant.
2.7. Evaluation of Egg Mortality of T. urticae in the Laboratory
For the egg mortality assay, 20 adult females were placed on 5-cm-diameter eggplant (Solanum melongena) leaf discs positioned on moistened cotton inside 9-cm-diameter Petri dishes. After 24 h, all adults were removed, leaving only the deposited eggs. The number of eggs was standardized to 20 per disc by removing any excess. Leaf discs were carefully grasped with forceps and immersed for 5 s in the biosurfactant solutions and then air-dried at room temperature for 30 min before being returned to their original Petri dishes. The dishes were maintained at 24–30 °C for 10 days. Egg mortality was recorded based on the number of unhatched eggs. Each Petri dish served as an independent replicate, with ten replicates per biosurfactant compound [31].
2.8. Evaluation of Population Suppression of T. urticae in Greenhouse
Two-month-old eggplant plants were used for greenhouse evaluations. The experiment was conducted in a greenhouse with a plastic roof and lateral anti-aphid netting, under fluctuating environmental conditions (23–33 °C temperature, 70–85% relative humidity). Eggplant plants were infested with Tetranychus urticae adults, and, three weeks later, once the mite colony was established, the biosurfactants were applied with a hand sprayer (Klintek ATO-100, México) to both sides of the leaves, ensuring full coverage up to the drip point. The mite population density was assessed before treatment and at 1, 7, 14, and 21 days post-treatment. One young leaf per plant was collected and taken to the laboratory, where the number of mites (adults, nymphs, and eggs) was counted using a stereomicroscope (40× magnification). The leaf area was measured with a leaf area integrator (LI-COR®, USA, model LI-300C). Each plant served as an independent replicate, with ten replicates evaluated per treatment.
2.9. Bioinformatic Analysis of Genes Related to Biosurfactant Biosynthesis
For the genome analysis of the bacterial strains, genes associated with biosurfactant biosynthesis were identified using the AntiSMASH bioinformatics tool available at https://antismash.secondarymetabolites.org/#!/start (accesed on 6 January 2025). Default parameters were applied, and additional features were enabled, including MiBiG cluster comparison, Pfam cluster analysis, cluster explosion, Pfam-based GO term annotation, and TIGRFam classification.
2.10. Statistical Analysis
A completely randomized design was used for all experiments. Data were subjected to analysis of variance (ANOVA) after verifying the assumptions of normality and homoscedasticity. Significant differences between means (p < 0.05) were determined using Tukey’s test. All statistical analyses were performed using the InfoStat software (2017) package.
3. Results
3.1. Toxicity of Biosurfactants in T. urticae Adults and Eggs in the Laboratory
All biosurfactants caused significant mortality on T. urticae adults at 24 h (F = 150.3, gl = 4, 45, p < 0.0001), 48 h (F = 228.2, gl = 4, 45, p < 0.0001), and 72 h post-treatment (F = 1175.8, gl = 4, 45, p < 0.0001). At 24 h, the most effective treatments were S. ureilytica NOD-3 and UTS, which caused 62–68% mortality, while abamectin induced 91% mortality (Figure 1). At 48 h, S. ureilytica UTS achieved 92% mortality, comparable to abamectin (98%), whereas S. ureilytica NOD-3 and NOD-7 caused 84% mortality (Figure 1). At 72 h post-treatment, S. ureilytica NOD-3 and UTS achieved 99% mortality, comparable to abamectin (Figure 1).
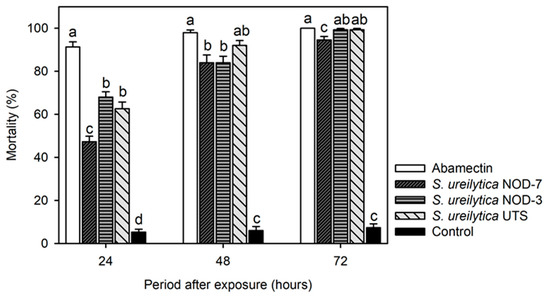
Figure 1.
Adult mortality (mean ± standard error) of Tetranychus urticae treated with biosurfactants obtained from three strains of S. ureilytica cell-free culture medium. Means with different letters indicate statistically significant differences (Tukey’s test, p < 0.05).
The biosurfactants also exhibited significant ovicidal activity against T. urticae eggs (F = 67.64, gl = 4, 45, p < 0.001). The most effective treatments were S. ureilytica NOD-3 and UTS, which induced mortality rates exceeding 60%. In contrast, NOD-7 showed moderate efficacy, causing 45% mortality (Figure 2).
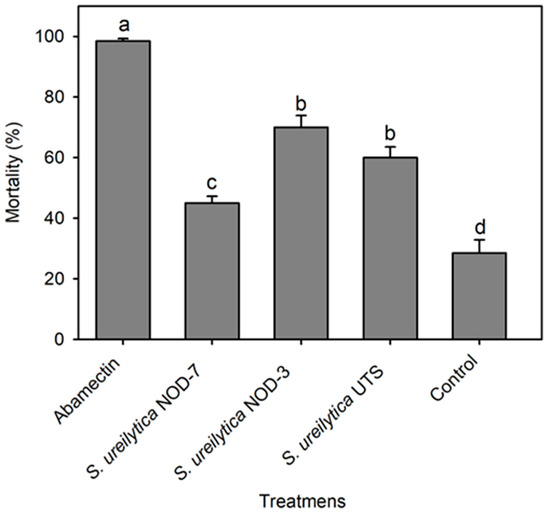
Figure 2.
Egg mortality (mean ± standard error) of Tetranychus urticae 10 days after treatment with biosurfactants obtained from three strains of S. ureilytica cell-free culture medium. Means with different letters indicate statistically significant differences (Tukey’s test, p < 0.05).
3.2. Toxicity of Biosurfactants in Predatory Mite Amblyseius swirskii in the Laboratory
The exposure of Amblyseius swirskii adults to the biosurfactants caused significant mortality, with statistically significant differences among treatments (Figure 3). At 24 h post-exposure, S. ureilytica NOD-3 and NOD-7 induced the highest mortality rates, reaching 17.3% and 14.6%, respectively (F = 383.3, gl = 4, 45, p < 0.0001). Mortality increased at 48 h, reaching 61.3% and 67.3% for S. ureilytica NOD-3 and NOD-7, respectively (F = 163.9, df = 4, 45, p < 0.001). By 72 h, S. ureilytica UTS, NOD-3, and NOD-7 caused mortality rates ranging from 76.6% to 82%. In contrast, abamectin caused 100% mortality as early as 48 h post-treatment (Figure 3).
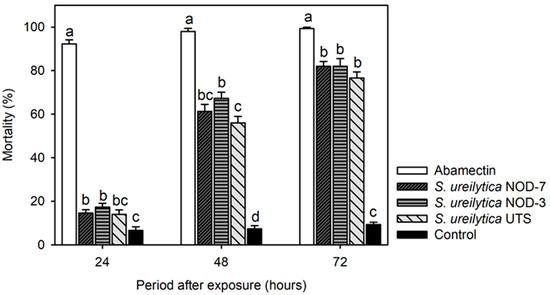
Figure 3.
Adult mortality (mean ± standard error) of Amblyseius swirskii treated with biosurfactants obtained from three strains of S. ureilytica cell-free culture medium. Means with different letters indicate statistically significant differences (Tukey’s test, p < 0.05).
3.3. Suppression of T. urticae Population Density in Greenhouse
Overall, the biosurfactants derived from S. ureilytica strains NOD-7 and UTS were the most effective in suppressing T. urticae populations under greenhouse conditions, consistently maintaining low densities of adults, nymphs, and eggs across the 21-day evaluation period. All biosurfactants significantly reduced the mite population densities at 7, 14, and 21 days post-application. For adults, NOD-7 and UTS were most effective, maintaining densities between 4.0 and 12.4 individuals per cm2 throughout the evaluation. NOD-3 also showed considerable efficacy, with densities ranging from 10.8 to 15.5 individuals per cm2 (Figure 4A). For nymphs, NOD-7 and UTS again performed the best, with densities between 3.8 and 13.2 individuals per cm2 (Figure 4B). Although initial egg suppression was limited, significant reductions were observed by day 7, with NOD-7 and UTS reducing the egg densities to 9.6–16.2 eggs per cm2. NOD-3 also reduced the egg density to 22.1 eggs per cm2 (Figure 4C).
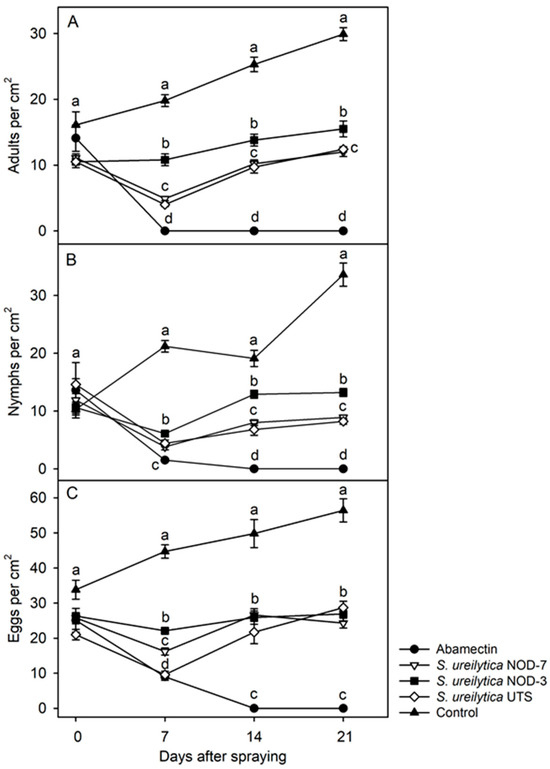
Figure 4.
Suppression of population density (mean ± standard error) of Tetranychus urticae adults (A), nymphs (B), and eggs (C) treated with biosurfactants obtained from three strains of S. ureilytica cell-free culture medium under greenhouse conditions. Means for the same time point (day) post-exposure that do not share the same letter are significantly different (Tukey’s test, p < 0.05).
3.4. Analysis of Biosurfactants by GC-MS
The crude extracts obtained from the bacterial culture medium were positive in the drop collapse test, as well as in the oil diffusion test, with 53.0 (mm) for S. ureilytica UTS, 58.0 (mm) for S. ureilytica NOD-3, and 51.0 (mm) for S. ureilytica NOD-7. The emulsification index at 24 h was 42.85% for S. ureilytica UTS, 42.98% for S. ureilytica NOD-3, and 44.28% for S. ureilytica NOD-7.
The GC-MS analysis of crude extract samples obtained from the bacterial culture medium detected signals for the amino acids L-proline (7.66 min RT), glutamic acid (19.77 min RT), L-lysine (22.64 min RT), and L-valine (22.74 min RT) (Table 1). The amino acid L-proline was present in the biosurfactants obtained from the three S. ureilytica strains, while glutamic acid and L-valine were only present in those obtained from S. ureilytica NOD-3 and S. ureilytica NOD-7, respectively. L-Lysine was present in biosurfactants obtained from S. ureilytica UTS and S. ureilytica NOD-7.

Table 1.
Isoforms detected by GC-MS of crude biosurfactants extracted from S. urielytica strains (https://pubchem.ncbi.nlm.nih.gov/ (5 May 2025)).
The GC-MS analysis revealed fatty acids including hexadecanoic, pentanoic, octadecanoic, decanoic, and tetradecanoic acids. Among these, hexadecanoic and octadecanoic acids were present in all three strains. Notably, metabolites such as hexadecanamide and octadecanamide were also detected. The amino acid c-guanidinobutyric acid was exclusively present in the UTS strain.
3.5. Identification of Metabolites in S. ureilytica Genomes by Bioinformatics
The bioinformatic analyses of the genomes of the three S. urielytica strains with the anti-SMASH program detected various metabolites. The metabolites had similarity with prodigiosin, lankacidin C, viobactin, O-antigen, yersinopine, xenotetrapeptide, rhizomideA/rhizomideB/rhizomideC, trichrysobactin/cyclictrichrysobactin/chrysobactin/dichrysobactin, microcin E492, and kolossin, which are encoded by nonribosomal peptide synthetases (NRPSs) (Table 2).

Table 2.
Results of the identification of the secondary metabolite synthesis regions in three strains of S. ureilytica.
4. Discussion
This study evaluated the lethal toxicity of biosurfactants derived from liquid cultures of Serratia ureilytica against adults and eggs of Tetranychus urticae under laboratory conditions, as well as their efficacy in suppressing mite populations in greenhouse trials. Additionally, the nontarget effects on the predatory mite Amblyseius swirskii were assessed. The result showed that the biosurfactants S. ureilytica NOD-3 and UTS induced high mortality in T. urticae, exceeding 95% in adults and more than 60% in eggs. These effects are comparable to those produced by spiromesifen and bifenazate [33]. The biosurfactants caused also considerable mortality in A. swirskii (75% to 85%). This strong acaricidal activity is consistent with the mechanisms of action described for microbial biosurfactants, which primarily disrupt cell membranes [34] and cause cuticular dehydration [35], tegument lysis [36], and histological damage to epithelial tissue, particularly in the midgut [37,38]. The cytolytic effects of biosurfactants are attributed to their amphiphilic nature, enabling them to be inserted into lipid bilayers, increase membrane permeability, and cause cellular collapse [39]. In mites such as T. urticae, biosurfactants may penetrate through the cuticles or intersegmental membranes, leading to dehydration and cell death [40]. The significant mortality observed in A. swirskii suggests potential selectivity issues, likely due to similarities in lipid composition between target and nontarget species. This underscores the need to optimize formulations or application strategies, such as through spot treatment, timing, or controlled-release systems, to minimize the impacts on nontarget organisms within integrated pest management programs. The biosurfactants produced by S. ureilytica may represent promising alternatives for mite control in agriculture, offering a novel property as hydrolytic enzymes, which may be related to chitin degradation in mites in this bacterial species, as previous studies have documented the efficacy of cell suspensions against nematodes and phytopathogenic fungi [10,12], as well as against phytopathogenic bacteria and insect pests [2,9].
These results show that biosurfactants produced in liquid cultures of S. ureilytica have high effectiveness regarding the suppression of T. urticae population densities in greenhouse trials. To our knowledge, the existing literature has only reported on the lethal effects of S. ureilytica cell suspensions in the dust mite Tyrophagus putrescentiae in the laboratory [11]. In other studies, it has been documented that the species Serratia marcescens has potential as a biocontrol agent against insect pests [41]; however, at present, no studies have reported the acaricidal effects of biosurfactants derived from liquid cultures of this bacterial species. The acaricidal activity of S. ureilytica biosurfactants against T. urticae may be attributed to the presence of lytic enzymes and specific fatty acids detected in biosurfactant-rich crude extracts [10]. Biosurfactants produced by other bacterial species are recognized as biologically active compounds [41]. Active fractions of extracts from liquid cultures of Bacillus velezensis and B. amyloliquefaciens have shown that the effects against T. urticae are partially mediated by the presence of cyclodipeptides, like bacillomycin, macrolactin, and surfactins [42,43]. In the present work, the GC-MS analysis of biosurfactants derived from S. ureilytica showed the presence of fatty acids and amino acids. Notably, the biosurfactant produced by the S. ureilytica UTS strain exhibited the highest number of fatty acids compared with the other S. ureilytica strains, and this biosurfactant also displayed outstanding acaricidal effects on T. urticae. This suggests that these fatty acids are likely responsible for the acaricidal activity—particularly hexadecanoic, octadecanoic, and tetra-decanoic acids, which have been reported to have insecticidal effects [44]. In addition, the GC-MS analysis in the present study identified the amino acids L-proline, L-lysine, L-valine, and glutamic acid in the biosurfactants. These amino acids are known constituents of lipopeptides, which have been associated with biocontrol activity against various agricultural pests [45].
The bioinformatic analysis of bacterial genomes offers valuable insights into the potential repertoire of bioactive compounds that a microorganism may produce. In this study, the analysis of the genomes of S. ureilytica strains via computational tools revealed the presence of nonribosomal peptide synthetase (NRPS) gene clusters with homology to known bioactive metabolites. One of the detected NRPSs showed similarity to 5-dimethylallylindole-3-acetonitrile, an antibacterial compound [46]. Another NRPS exhibited structural homology to xenotetrapeptide, a metabolite with insecticidal properties [47,48]. Additionally, a third NRPS cluster displayed homology to kolossin, a compound reported with bioactivity against unicellular protists [49,50].
Taken together, our results suggest that secondary metabolites derived from liquid cultures of Serratia species have great potential against plant pest mites. Our results are aligned with those of previous studies that have highlighted the potential of S. ureilytica as a plant protection agent [10,51]. These results, in conjunction with the existing literature, provide compelling evidence that the secondary metabolites produced by S. ureilytica exhibit a broad spectrum of biological activity against diverse phytoparasites.
5. Conclusions
The biosurfactants produced by S. ureilytica strains showed significant acaricidal activity against T. urticae, affecting both adults and eggs in the laboratory, and effectively suppressed the populations of eggs, nymphs, and adults in greenhouse trials. However, the adverse effects observed on the predatory mite Amblyseius swirskii emphasize the importance of improving the selectivity through formulation adjustments or targeted application strategies. Although the laboratory and semi-field results are encouraging, the broader applicability and environmental safety of these compounds remain to be confirmed. The gas chromatography–mass spectrometry (GC-MS) analysis confirmed the presence of fatty acids and amino acids that are characteristic components of lipopeptides—metabolites with known biological activity. Additionally, the bioinformatic analysis of the bacterial genomes revealed nonribosomal peptides (NRPs) with homology to compounds previously associated with antibacterial and insecticidal properties. These findings highlight the potential of S. ureilytica-derived biosurfactants as promising candidates for the control of pests in agriculture.
Author Contributions
Conceptualization, A.W.-V. and E.R.-S.; methodology, A.W.-V., E.R.-S., M.C.-B., S.E.-Z., A.A.G.-S., E.R.-S. and C.G.-G.; validation, A.W.-V., E.R.-S., M.C.-B., S.E.-Z., A.A.G.-S., E.R.-C., C.G.-G., R.G.-H., R.R.-T., G.M.-B. and E.P.P.-R.; formal analysis, A.W.-V., E.R.-S. and M.C.-B.; investigation, A.W.-V., E.R.-S., M.C.-B., S.E.-Z., A.A.G.-S., E.R.-C., C.G.-G., R.G.-H., R.R.-T., G.M.-B. and E.P.P.-R.; resources, A.W.-V., E.R.-S., M.C.-B., S.E.-Z., A.A.G.-S., E.R.-C., C.G.-G., R.G.-H., R.R.-T., G.M.-B. and E.P.P.-R.; writing—original draft preparation, A.W.-V. and E.R.-S.; writing—review and editing, A.W.-V., E.R.-S., M.C.-B., S.E.-Z., A.A.G.-S., E.R.-C., C.G.-G., R.G.-H., R.R.-T., G.M.-B. and E.P.P.-R.; visualization, A.W.-V., E.R.-S., M.C.-B., S.E.-Z., A.A.G.-S., E.R.-C., C.G.-G., R.G.-H., R.R.-T., G.M.-B. and E.P.P.-R.; supervision, A.W.-V. and E.R.-S.; project administration, A.W.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Universidad Tecnológica de la Selva, with the approval of the project in the FOMENTO A LA FORMACIÓN DE RECURSOS HUMANOS DE ALTA CALIDAD Y DESARROLLO DE PROYECTOS DE INVESTIGACIÓN EN LA UNIVERSIDAD TECNOLÓGICA DE LA SELVA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors acknowledge the support received from the Universidad Tecnológica de la Selva and Laboratorio Nacional Conahcyt Análisis Genético, Agropecuarioy Forestal.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kshetri, L.; Naseem, F.; Pandey, P. Role of Serratia sp. as Biocontrol Agent and Plant Growth Stimulator, with Prospects of Biotic Stress Management in Plant. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Microorganisms for Sustainability; Sayyed, R., Ed.; Springer: Singapore, 2019; Volume 13. [Google Scholar] [CrossRef]
- Allen, J.L.; Doidge, N.P.; Cheng, C.; Lynch, M.; Crabb, H.K.; Scheerlinck, J.-P.; Bushell, R.; Browning, G.F.; Marenda, M.S.; Kuo, C.-H. Genomic characterisation of an entomopathogenic strain of Serratia ureilytica in the critically endangered phasmid Dryococelus australis. PLoS ONE 2022, 17, e0265967. [Google Scholar] [CrossRef]
- Moreno-Castillo, B.; Ortiz-Barrios, H.; Salvador-Figueroa, M.; Adriano-Anaya, M.L. Serratia marcescens (Enterobacteriaceae): Promotora de la salud vegetal. Biociencias 2022, 5, 1–9. [Google Scholar]
- Bidari, F.; Shams-Bakhsh, M.; Mehrabadi, M. Isolation and characterization of a Serratia marcescens with insecticidal activity from Polyphylla olivieri (Col.: Scarabaeidae). J. Appl. Èntomol. 2017, 142, 162–172. [Google Scholar] [CrossRef]
- Ruiu, L.; Virdis, B.; Mura, M.E.; Floris, I.; Satta, A.; Tarasco, E. Oral insecticidal activity of new bacterial isolates against insects in two orders. Biocontrol Sci. Technol. 2017, 27, 886–902. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, L.; Feng, K.; Lu, X.; Luo, J.; Tang, F. Effects of Serratia marcescens (SM1) and its interaction with common biocontrol agents on the termite, Odontotermes formosanus (Shiraki). J. For. Res. 2021, 32, 1263–1267. [Google Scholar] [CrossRef]
- Niu, H.; Sun, Y.; Zhang, Z.; Zhao, D.; Wang, N.; Wang, L.; Guo, H. The endophytic bacterial entomopathogen Serratia marcescens promotes plant growth and improves resistance against Nilaparvata lugens in rice. Microbiol. Res. 2022, 256, 126956. [Google Scholar] [CrossRef]
- Mai, A.-G.M. Serratia A Novel Source of Secondary Metabolites. Adv. Biotechnol. Microbiol. 2018, 11, 555814. [Google Scholar] [CrossRef]
- Cao, X.; Ye, Y.; Li, P. Genome Sequence Resource of Serratia ureilytica HNU47: A Strain with Biocontrol Potential Against Bacterial Wilt Pathogen Ralstonia solanacearum. Plant Dis. 2023, 107, 919–921. [Google Scholar] [CrossRef]
- Zamorano-González, C.A.; Ramírez-Trujillo, J.A.; Pilotzi-Xahuentitla, H.; Yáñez-Ocampo, G.; Hernández-Nuñéz, E.; Suárez-Rodríguez, R.; Orea-Flores, M.L.A.; Gómez-Rodríguez, O.; Espinosa-Zaragoza, S.; Rangel-Zaragoza, J.L.; et al. In Vitro Evaluation of the Biosurfactant Produced by Serratia ureilytica UTS with Antifungal and Nematicidal Activity Against Nacobbus aberrans. Curr. Microbiol. 2025, 82, 63. [Google Scholar] [CrossRef]
- Espinosa-Zaragoza, S.; Domínguez-Liévano, A.; Gómez-Gutiérrez, J.A.; Wong-Villarreal, A.; Aguilar-Marcelino, L.; Cerqueda-García, D.; Rangel-Zaragoza, J.L.; Sanzón-Gómez, D.; Mireles-Arriaga, A.I.; Sachman-Ruíz, B. In vitro Acaricidal Activity of Serratia Ureilytica Against the Dust Mite Tyrophagus Putrescentiae and Identification of Genes Related to Biocontrol. Curr. Microbiol. 2024, 81, 199. [Google Scholar] [CrossRef]
- Wong-Villarreal, A.; Méndez-Santiago, E.W.; Gómez-Rodríguez, O.; Aguilar-Marcelino, L.; García, D.C.; García-Maldonado, J.Q.; Hernández-Velázquez, V.M.; Yañez-Ocampo, G.; Espinosa-Zaragoza, S.; Ramírez-González, S.I.; et al. Nematicidal Activity of the Endophyte Serratia ureilytica against Nacobbus aberrans in Chili Plants (Capsicum annuum L.) and Identification of Genes Related to Biological Control. Plants 2021, 10, 2655. [Google Scholar] [CrossRef]
- Kumari, S.; Chauhan, U.; Kumari, A.; Nadda, G. Comparative toxicities of novel and conventional acaricides against different stages of Tetranychus urticae Koch (Acarina: Tetranychidae). J. Saudi Soc. Agric. Sci. 2017, 16, 191–196. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Beauchamp, M.J.; Lavine, M.D.; Lavine, L.C.; Zhu, F.; Walsh, D.B. Physiological resistance alters behavioral response of Tetranychus urticae to acaricides. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- A Schmidt-Jeffris, R.; Coffey, J.L.; Miller, G.; A Farfan, M.; Riddick, E. Residual Activity of Acaricides for Controlling Spider Mites in Watermelon and Their Impacts on Resident Predatory Mites. J. Econ. Èntomol. 2021, 114, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Alam, Z.; Miah, R.U.; Mian, I.H.; Mustarin, K.-E. Toxicity of pesticides to Tetranychus urticae Koch (Acari:Tetranychidae) and their side effects on Neoseiulus californicus (Acari:Phytoseiidae). Int. J. Acarol. 2015, 41, 688–693. [Google Scholar] [CrossRef]
- Montoya, A.; Galano-Flores, G.; Rodríguez, H.; Franco, A.A.; Zardi, O.Z.; Yamamoto, P.T. Toxicity of acaricides on Tetranychus urticae (Koch) in the laboratory. Rev. Prot. Veg. 2017, 32, 60–67. [Google Scholar]
- Zhao, J.; Wang, Z.; Ji, M.; Cheng, J.; Zhu, G.; Yu, C. Synthesis and bioactivity evaluation of novel spiromesifen derivatives. Pest Manag. Sci. 2011, 68, 10–15. [Google Scholar] [CrossRef]
- Tu, S.; Qiu, X.; Cao, L.; Han, R.; Zhang, Y.; Liu, X. Expression and characterization of the chitinases from Serratia marcescens GEI strain for the control of Varroa destructor, a honey bee parasite. J. Invertebr. Pathol. 2010, 104, 75–82. [Google Scholar] [CrossRef]
- Pritam, C.; Sukanta, K.S. Systemic infestation of Serratia entomophila AB2 through plant tissue inferred protection against insect pest and fungal pathogens. Afr. J. Microbiol. Res. 2013, 7, 2651–2655. [Google Scholar] [CrossRef]
- Ruiu, L. Insect Pathogenic Bacteria in Integrated Pest Management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef]
- Sanchéz-Cruz, R.; Vázquez, I.T.; Batista-García, R.A.; Méndez-Santiago, E.W.; del Rayo, S.C.; Leija, A.; Lira-Ruan, V.; Hernández, G.; Wong-Villarreal, A.; Folch-Mallol, J.L. Isolation and characterization of endophytes from nodules of Mimosa pudica with biotechnological potential. Microbiol. Res. 2019, 218, 76–86. [Google Scholar] [CrossRef]
- Rosas-Galván, N.S.; Martínez-Morales, F.; Marquina-Bahena, S.; Tinoco-Valencia, R.; Serrano-Carreón, L.; Bertrand, B.; León-Rodríguez, R.; Guzmán-Aparicio, J.; Alvaréz-Berber, L.; Trejo-Hernández, M.d.R. Improved production, purification, and characterization of biosurfactants produced by Serratia marcescens SM3 and its isogenic SMRG-5 strain. Biotechnol. Appl. Biochem. 2018, 65, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Phetrong, K.; H-Kittikun, A.; Maneerat, S. Production and characterization of bioemulsifier from a marine bacterium, Acinetobacter calcoaceticus subsp. anitratus SM7. Songklanakarin J. Sci. Technol. 2008, 30, 297–305. [Google Scholar]
- García-Reyes, S.; Yáñez-Ocampo, G.; Wong-Villarreal, A.; Rajaretinam, R.K.; Thavasimuthu, C.; Patiño, R.; Ortiz-Hernández, M.L. Partial characterization of a biosurfactant extracted from Pseudomonas sp. B0406 that enhances the solubility of pesticides. Environ. Technol. 2018, 39, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soni, J.; Kaur, G.; Kaur, J. A study on biosurfactant production in Lactobacillus and Bacillus sp. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 723–733. [Google Scholar]
- García-Reyes, S.; Yañez-Ocampo, G. Microbial Biosurfactants: Methods for Their Isolation and Characterization. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 641–648. [Google Scholar] [CrossRef]
- Dubey, K.V.; Charde, P.N.; Meshram, S.U.; Shendre, L.P.; Dubey, V.S.; Juwarkar, A.A. Surface-active potential of biosurfactants produced in curd whey by Pseudomonas aeruginosa strain-PP2 and Kocuria turfanesis strain-J at extreme environmental conditions. Bioresour. Technol. 2012, 126, 368–374. [Google Scholar] [CrossRef]
- Ben Ayed, H.; Jemil, N.; Maalej, H.; Bayoudh, A.; Hmidet, N.; Nasri, M. Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. Int. Biodeterior. Biodegrad. 2015, 99, 8–14. [Google Scholar] [CrossRef]
- Cua-Basulto, M.E.; Ruiz-Sánchez, E.; Chan-Cupul, W.; Reyes-Ramírez, A.; Ballina-Gómez, H.; Núñez, E.H.; Martin-Mex, R.; Gorocica, Á.M.H.; Ruiz-Jiménez, A.L. Effects of botanical acaricides on Tetranychus urticae and compatibility with the predatory mite Amblyseius swirskii. Arch. Phytopathol. Plant Prot. 2023, 57, 1359–1371. [Google Scholar] [CrossRef]
- Nexticapan-Garcéz, Á.; Cua-Basulto, M.; Martín-Mex, R.; Pérez-Brito, D.; Larqué-Saavedra, A.; Villanueva-Couoh, E.; Pérez-Gutiérrez, A.; Ruiz-Sánchez, E. Effects of botanical acaricides on Raoiella indica and Oligonychus sp. and their toxicity on two species of phytoseiid predatory mites. Arch. Phytopathol. Plant Prot. 2021, 54, 2221–2232. [Google Scholar] [CrossRef]
- Cua-Basulto, M.; Ruiz-Sánchez, E.; Chan-Cupul, W.; Reyes-Ramírez, A.; Ballina-Gómez, H.; Hernández-Nuñez, E. Effects of chemical acaricides on the mortality of the two spotted spider mite Tetranychus urticae KOCH (Acari: Tetranychidae). Trop. Subtrop. Agroecosyst. 2022, 25, 1–9. [Google Scholar] [CrossRef]
- Buchoux, S.; Lai-Kee-Him, J.; Garnier, M.; Tsan, P.; Besson, F.; Brisson, A.; Dufourc, E.J. Surfactin-Triggered Small Vesicle Formation of Negatively Charged Membranes: A Novel Membrane-Lysis Mechanism. Biophys. J. 2008, 95, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.C.; Yang, S.Y.; Kim, Y.C.; Kim, I.S.; Kim, Y.H. Identification of surfactin as an aphicidal metabolite produced by Bacillus amyloliquefaciens G1. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 751–753. [Google Scholar] [CrossRef]
- Mnif, I.; Elleuch, M.; Chaabouni, S.E.; Ghribi, D. Bacillus subtilis SPB1 biosurfactant: Production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae. Crop Prot. 2013, 50, 66–72. [Google Scholar] [CrossRef]
- Ghribi, D.; Abdelkefi-Mesrati, L.; Boukedi, H.; Elleuch, M.; Ellouze-Chaabouni, S.; Tounsi, S. The impact of the Bacillus subtilis SPB1 biosurfactant on the midgut histology of Spodoptera littoralis (Lepidoptera: Noctuidae) and determination of its putative receptor. J. Invertebr. Pathol. 2012, 109, 183–186. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Boukedi, H.; Laarif, A.; Tounsi, S. Biosurfactant produced by Bacillus subtilis V26: A potential biological control approach for sustainable agriculture development. Org. Agric. 2020, 10, 117–124. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Cuatlayotl-Cottier, R.; la Peña, A.H.-D.; Peña-Chora, G.; Salazar-Magallón, J.A. Insecticidal activity of industrial by-products fermented by Bacillus thuringiensis strain GP139 against Mites (Prostigmata: Tetranychidae) and Aphids (Hemiptera: Aphidoidea). Biocontrol Sci. Technol. 2020, 32, 103–109. [Google Scholar] [CrossRef]
- Gómez-Gutiérrez, J.A.; Wong-Villarreal, A.; Aguilar-Marcelino, L.; Yañez-Ocampo, G.; Hernández-Nuñéz, E.; Caspeta-Mandujano, J.M.; García-Flores, A.; Cruz-Arévalo, J.; Vargas-Uriostegui, P.; Gomez-Rodríguez, O. In vitro nematicidal and acaricidal effect of biosurfactants produced by Bacillus against the root-knot nematode Nacobbus aberrans and the dust mite Tyrophagus putrescentiae. Braz. J. Microbiol. 2023, 54, 1127–1136. [Google Scholar] [CrossRef]
- Li, X.-Y.; Wang, Y.-H.; Yang, J.; Cui, W.-Y.; Munir, S.; He, P.-F.; Wu, Y.-X.; He, Y.-Q. Acaricidal Activity of Cyclodipeptides from Bacillus amyloliquefaciens W1 against Tetranychus urticae. J. Agric. Food Chem. 2018, 66, 10163–10168. [Google Scholar] [CrossRef]
- Li, X.; Munir, S.; Xu, Y.; Wang, Y.; He, Y. Combined mass spectrometry-guided genome mining and virtual screening for acaricidal activity in secondary metabolites of Bacillus velezensis W1. RSC Adv. 2021, 11, 25441–25449. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, N.; Bahador, N.; Hesami, S. A Study on Larvicidal Activity and Phylogenetic Analysis of Staphylococcus epidermidis as a Biosurfactant-Producing Bacterium. Pol. J. Environ. Stud. 2021, 30, 4511–4519. [Google Scholar] [CrossRef]
- Penha, R.O.; Vandenberghe, L.P.S.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 70. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Zhao, W.; He, L.; Khan, T.A.; Lai, X.; Sun, Y.; Huang, W.; Yi, G.; Xia, L. Streptomyces enissocaesilis L-82 has broad-spectrum antibacterial activity and promotes growth for Carassius auratus. Appl. Microbiol. Biotechnol. 2024, 108, 220. [Google Scholar] [CrossRef]
- Mahar, A.N.; Munir, M.; Elawad, S.; Gowen, S.R.; Hague, N.G.M. Pathogenicity of bacterium, Xenorhabdus nematophila isolated from entomopathogenic nematode (Steinernema carpocapsae) and its secretion against Galleria mellonella larvae. J. Zhejiang Univ. B 2005, 6, 457–463. [Google Scholar] [CrossRef]
- Kegler, C.; Nollmann, F.I.; Ahrendt, T.; Fleischhacker, F.; Bode, E.; Bode, H.B. Rapid Determination of the Amino Acid Configuration of Xenotetrapeptide. ChemBioChem 2014, 15, 826–828. [Google Scholar] [CrossRef]
- Bode, H.B.; Brachmann, A.O.; Jadhav, K.B.; Seyfarth, L.; Dauth, C.; Fuchs, S.W.; Kaiser, M.; Waterfield, N.R.; Sack, H.; Heinemann, S.H.; et al. Structure Elucidation and Activity of Kolossin A, the D-/L-Pentadecapeptide Product of a Giant Nonribosomal Peptide Synthetase. Angew. Chem. Int. Ed. Engl. 2015, 54, 10352–10355. [Google Scholar] [CrossRef]
- Tobias, N.J.; Linck, A.; Bode, H.B. Natural Product Diversification Mediated by Alternative Transcriptional Starting. Angew. Chem. Int. Ed. Engl. 2018, 57, 5699–5702. [Google Scholar] [CrossRef]
- Clements, T.; Ndlovu, T.; Khan, S.; Khan, W. Biosurfactants produced by Serratia species: Classification, biosynthesis, production and application. Appl. Microbiol. Biotechnol. 2019, 103, 589–602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).