Morinda citrifolia Essential Oil in the Control of Banana Anthracnose: Impacts on Phytotoxicity, Preventive and Curative Effects and Fruit Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Pathogen and Molecular Identification
2.2. Extraction and Chemical Characterization of Essential Oil
2.3. In Vitro Fungistatic Action of Morinda citrifolia Essential Oil on Colletotrichum musae
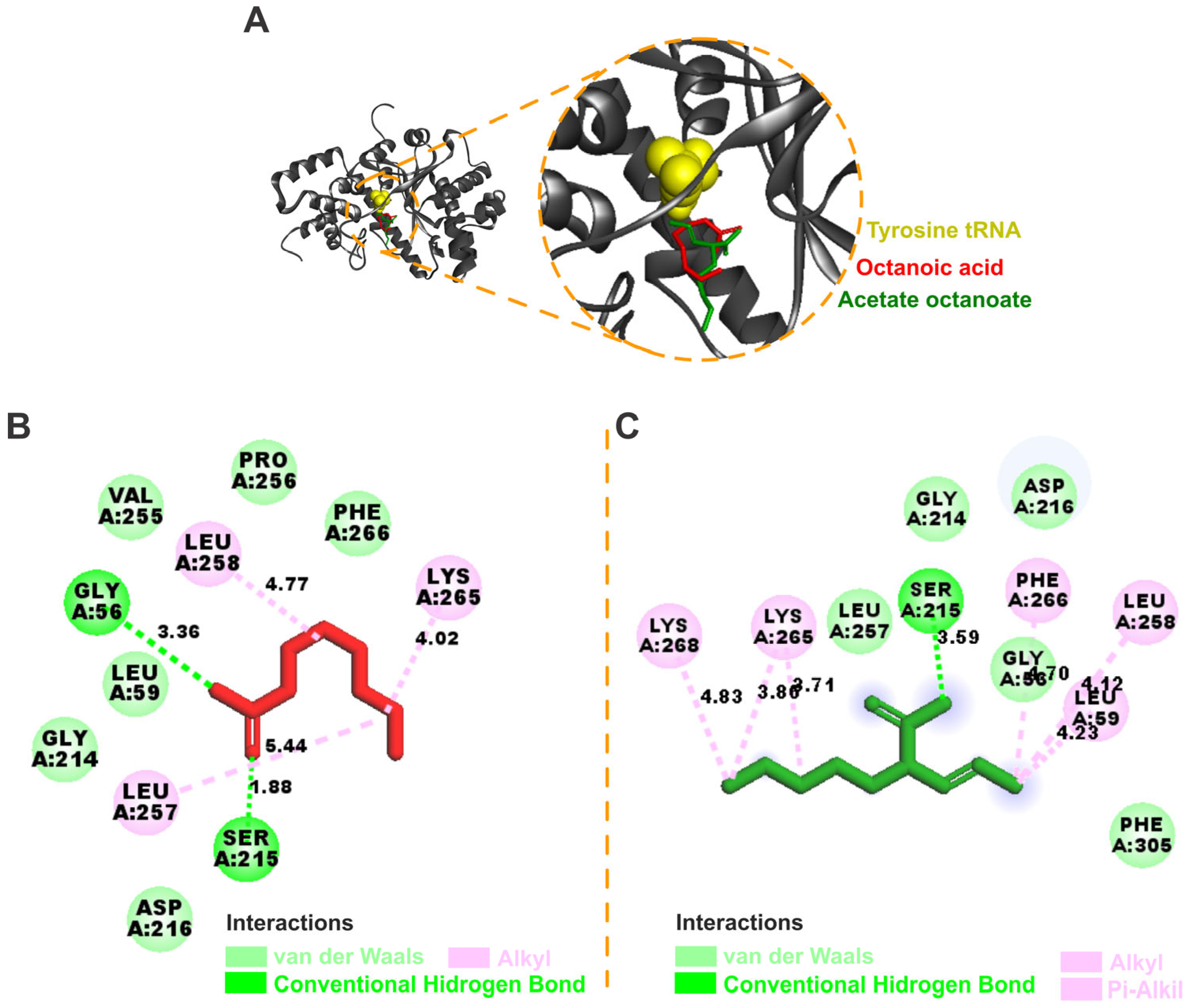
2.4. Molecular Docking
2.4.1. Lingads
2.4.2. Target Modeling
2.4.3. Molecular Docking Calculations
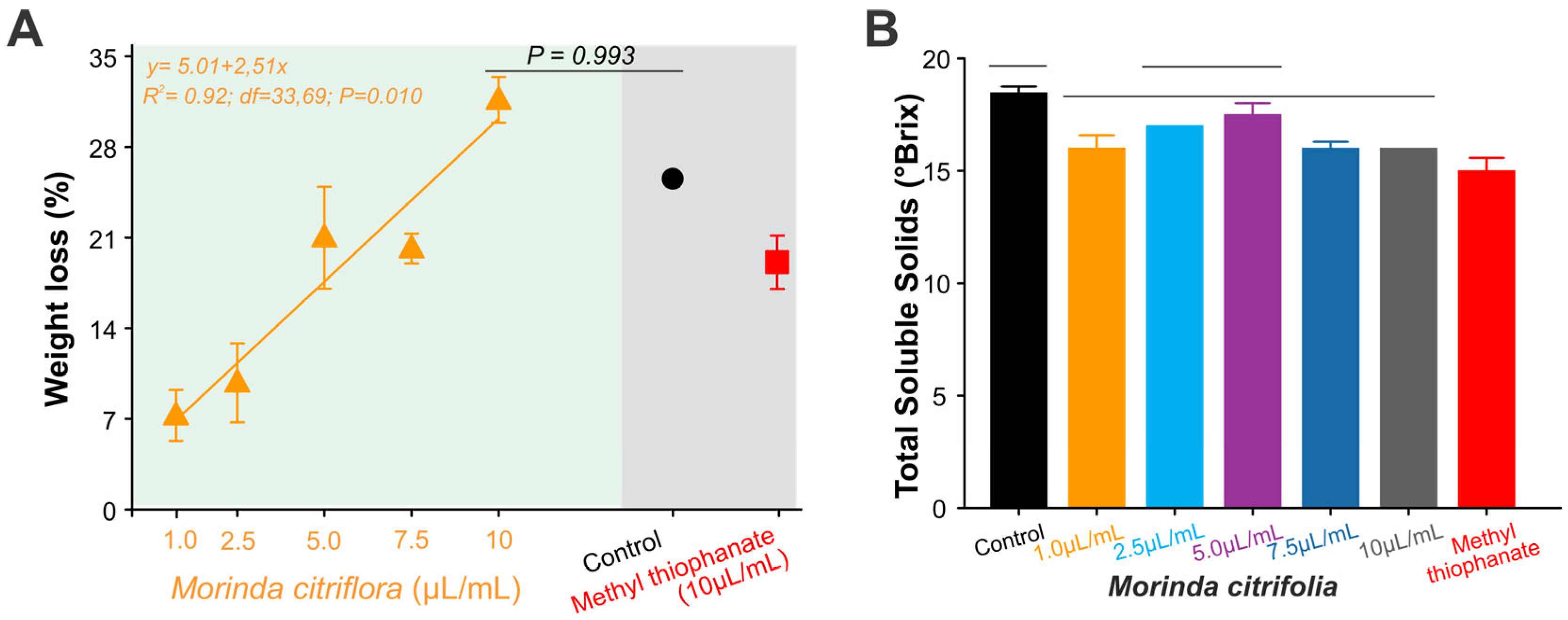
2.5. Phytotoxicity of Morinda citrifolia Essential Oil to Banana Fruit
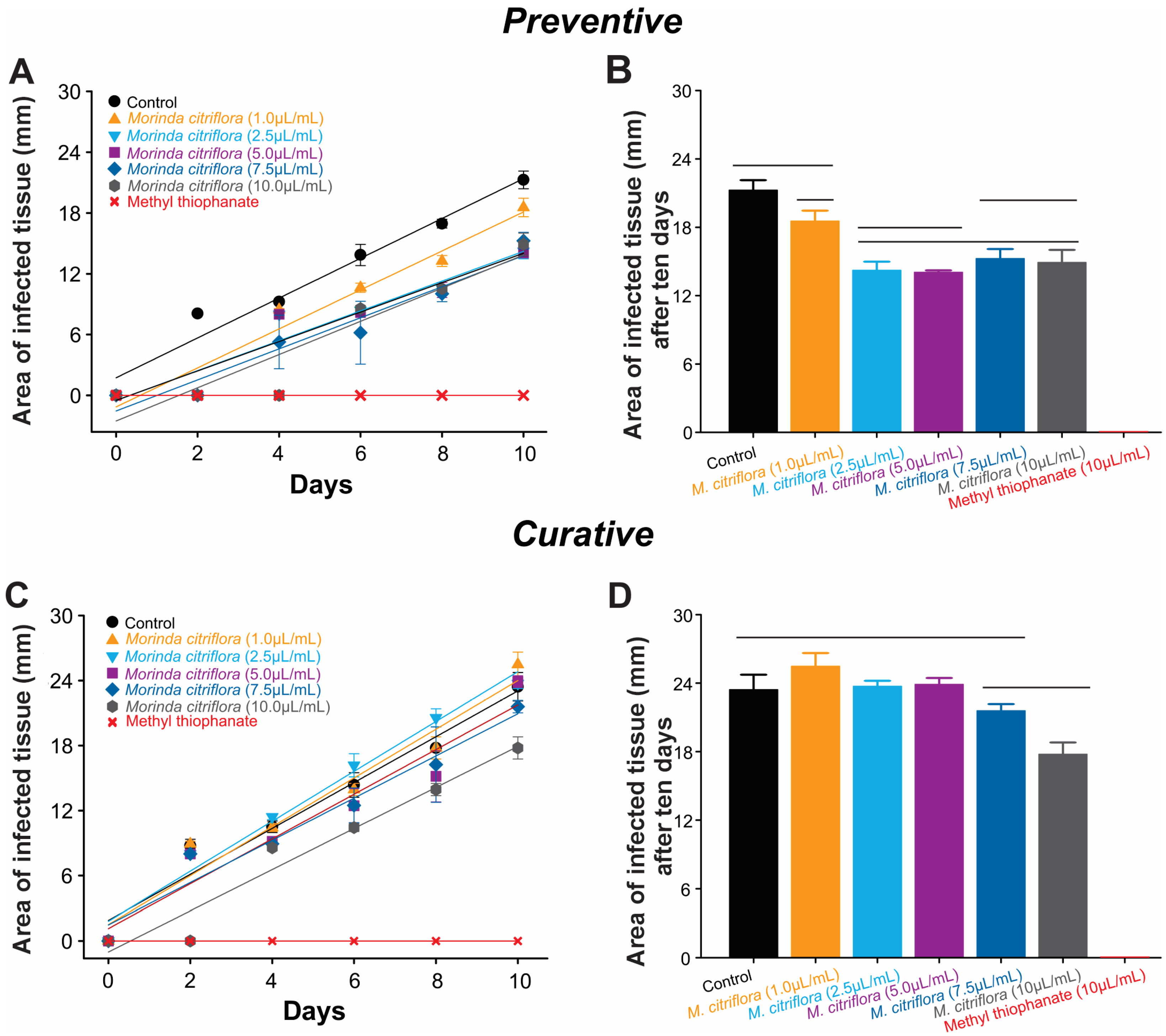
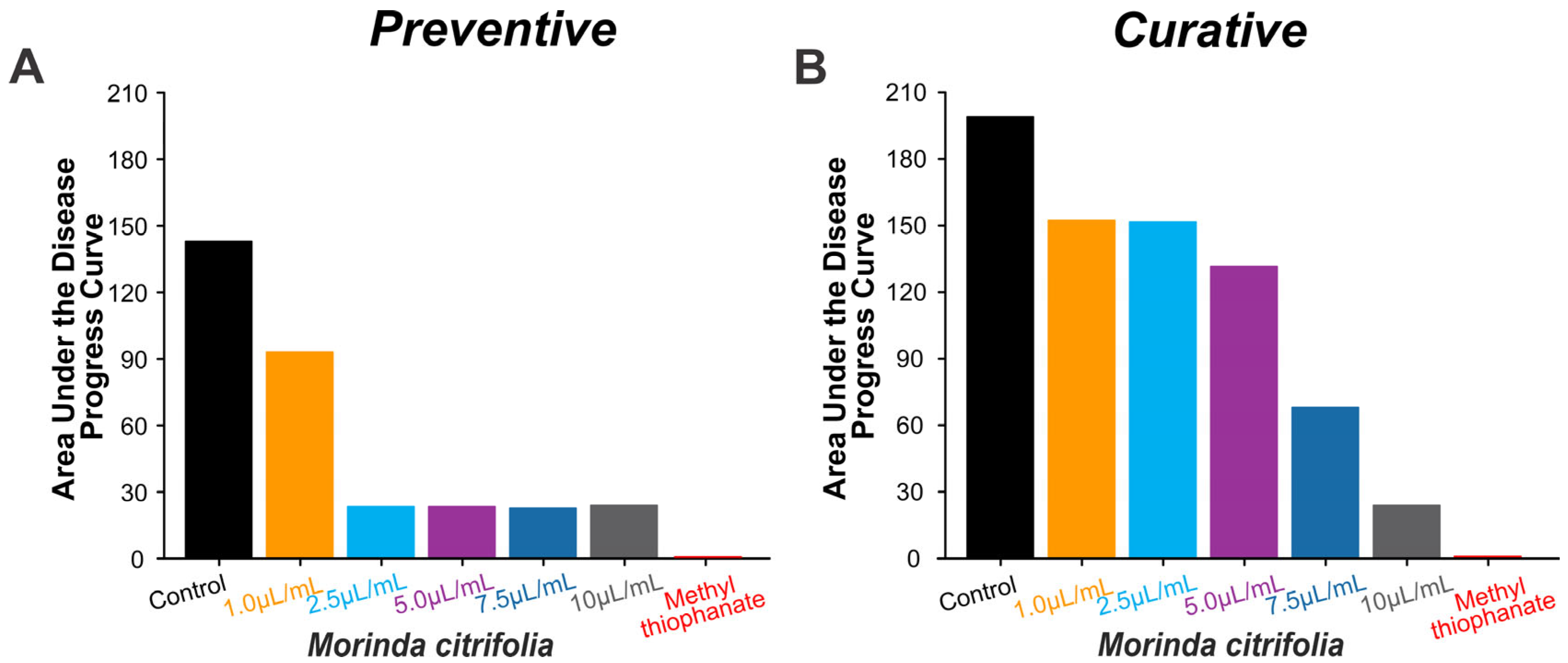
2.6. Preventive and Curative Control of Banana Anthracnose Using Morinda citrifolia Essential Oil
2.7. Effect of Morinda citrifolia Essential Oil on Fruit Quality
2.8. Selectivity of Essential Oil to Microorganism Trichoderma asperellum and Ladybugs Coleomegilla maculata and Eriopis connexa
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition of Morinda citrifolia Essential Oil
3.2. In Vitro Fungistatic Action of Morinda citrifolia Essential Oil on Colletotrichum musae
3.3. Molecular Docking
3.4. Phytotoxicity of Morinda citrifolia Essential Oil to Banana Fruit
3.5. Preventive and Curative Control of Banana Anthracnose Using Morinda citrifolia Essential Oil
3.6. Effect of Morinda citrifolia Essential Oil on the Fruit Quality
3.7. Selectivity of Noni Essential Oil to Non-Target Organisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A Review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Udayanga, D.; Manamgoda, D.S.; Liu, X.; Chukeatirote, E.; Hyde, K.D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013, 61, 165–179. [Google Scholar] [CrossRef]
- Santos, M.C.; Viteri, L.O.; Araujo, S.H.; Mourão, D.C.; Câmara, M.P.; Amaral, A.G.; Oliveira, E.E.; dos Santos, G.R. Molecular characterization and pathogenicity of Colletotrichum on banana fruits: Wound effects on virulence and cross-infection. Microbiol. Res. 2025, 16, 4. [Google Scholar] [CrossRef]
- Alvindia, D.G. Revisiting hot water treatments in controlling crown rot of banana cv. Buñgulan. Crop Prot. 2012, 33, 59–64. [Google Scholar] [CrossRef]
- Alemu, K. Importance and pathogen spectrum of crown rot of banana in Jimma Town, Southwestern Ethiopia. Biol. Agric. Hortic. 2014, 4, 106. [Google Scholar]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Vilaplana, R.; Pazmiño, L.; Valencia-Chamorro, S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol. Technol. 2018, 138, 56–63. [Google Scholar] [CrossRef]
- Lima Oliveira, P.D.; de Oliveira, K.Á.R.; Vieira, W.A.d.S.; Câmara, M.P.S.; de Souza, E.L. Control of anthracnose caused by Colletotrichum species in guava, mango and papaya using synergistic combinations of chitosan and Cymbopogon citratus (D.C. ex Nees) Stapf. essential oil. Int. J. Food Microbiol. 2018, 266, 87–94. [Google Scholar] [CrossRef]
- Gasca, C.A.; Dassoler, M.; Dotto Brand, G.; de Medeiros Nóbrega, Y.K.; Gomes, S.M.; Masrouah Jamal, C.; de Oliveira Magalhães, P.; Fonseca-Bazzo, Y.M.; Silveira, D. Chemical composition and antifungal effect of ethanol extract from Sapindus saponaria L. fruit against banana anthracnose. Sci. Hortic. 2020, 259, 108842. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Li, F.; Zhang, H.; Chen, J.; Yuan, D. Phenyllactic acid treatment for controlling anthracnose disease (Colletotrichum musae) and preserving banana fruit quality during storage. Physiol. Mol. Plant Pathol. 2024, 129, 102181. [Google Scholar] [CrossRef]
- Shan, Y.; Li, F.; Lian, Q.; Xie, L.; Zhu, H.; Li, T.; Zhang, J.; Duan, X.; Jiang, Y. Role of apyrase-mediated eATP signal in chilling injury of postharvest banana fruit during storage. Postharvest Biol. Technol. 2022, 187, 111874. [Google Scholar] [CrossRef]
- Drenth, A.; Kema, G. The vulnerability of bananas to globally emerging disease threats. Phytopathology® 2021, 111, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Noireung, P.; Liu, F.; Hyde, K.D.; Moslem, M.A.; Bahkali, A.H.; Abd-Elsalam, K.A.; Cai, L. Epitypification of Colletotrichum musae, the causative agent of banana anthracnose. Mycoscience 2011, 52, 376–382. [Google Scholar] [CrossRef]
- Conway, B.-A.D.; Venu, V.; Speert, D.P. Biofilm formation and acyl homoserine lactone production in the Burkholderia cepacia complex. J. Bacteriol. 2002, 184, 5678–5685. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of post-harvest anthracnose: Current approaches and future perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef]
- da Costa, A.C.; de Miranda, R.F.; Costa, F.A.; Ulhoa, C.J. Potential of Trichoderma piluliferum as a biocontrol agent of Colletotrichum musae in banana fruits. Biocatal. Agric. Biotechnol. 2021, 34, 102028. [Google Scholar] [CrossRef]
- Siguemoto, E.; Collombel, I.; Hatchy, C.-G.; Delpech, C.; Grabulos, J.; Brat, P.; Hubert, O.; Meot, J.-M. Control of banana anthracnose by hot water dip: A semi-empirical model coupling heat transfer and Colletotrichum musae inactivation. Postharvest Biol. Technol. 2023, 195, 112139. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol. Technol. 2011, 62, 71–76. [Google Scholar] [CrossRef]
- Kumar, A.; Kudachikar, V.B. Antifungal properties of essential oils against anthracnose disease: A critical appraisal. J. Plant Dis. Prot. 2018, 125, 133–144. [Google Scholar] [CrossRef]
- Gazi, S. Entomofauna of Agricultural Crops: Roles, Impacts, and Ecological Significance. Nat. Sci. 2024, 6, 15–19. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, S. Entomopathogenic potential of Trichoderma longibrachiatum and its comparative evaluation with malathion against the insect pest Leucinodes orbonalis. Environ. Monit. Assess. 2015, 188, 37. [Google Scholar] [CrossRef]
- Gabardo, G.; Dalla Pria, M.; Prestes, A.M.C.; da Silva, H.L. Trichoderma asperellum e Bacillus subtilis como antagonistas no crescimento de fungos fitopatogênicos in vitro. Braz. J. Dev. 2020, 6, 55870–55885. [Google Scholar] [CrossRef]
- Andrade-Hoyos, P.; Rivera-Jiménez, M.N.; Landero-Valenzuela, N.; Silva-Rojas, H.V.; Martínez-Salgado, S.J.; Romero-Arenas, O. Beneficios ecológicos y biológicos del hongo cosmopolita Trichoderma spp. en la agricultura: Una perspectiva en el campo mexicano. Rev. Argent. Microbiol. 2023, 55, 366–377. [Google Scholar] [CrossRef]
- Dias, B.L.; de Souza Ferreira, T.P.; Dalcin, M.S.; de Souza Carlos Mourão, D.; de Sena Fernandes, P.R.; Neitzke, T.R.; de Almeida Oliveira, J.V.; Dias, T.; Jumbo, L.O.V.; de Oliveira, E.E.; et al. Lippia sidoides Cham. compounds induce biochemical defense mechanisms against Curvularia lunata sp. in maize plants. Multidiscip. Sci. J. 2025, 8, 7. [Google Scholar] [CrossRef]
- Damascena, J.F.; Viteri, L.O.; Souza, M.H.P.; Aguiar, R.W.; Camara, M.P.; Moura, W.S.; Oliveira, E.E.; Santos, G.R. The preventive and curative potential of Morinda citrifolia essential oil for controlling anthracnose in cassava plants: Fungitoxicity, Phytotoxicity and Target Site. Stresses 2024, 4, 663–675. [Google Scholar] [CrossRef]
- Holanda, L.; Bezerra, G.B.; Ramos, C.S. Potent antifungal activity of essential oil from Morinda Citrifolia fruits rich in short-chain fatty acids. Int. J. Fruit Sci. 2020, 20, S448–S454. [Google Scholar] [CrossRef]
- Dalcin, M.S.; Dias, B.L.; Viteri Jumbo, L.O.; Oliveira, A.C.S.S.; Araújo, S.H.C.; Moura, W.S.; Mourão, D.S.C.; Ferreira, T.P.S.; Campos, F.S.; Cangussu, A.S.R.; et al. Potential action mechanism and inhibition efficacy of Morinda citrifolia essential oil and octanoic acid against Stagonosporopsis cucurbitacearum infestations. Molecules 2022, 27, 5173. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Kanisha Chelliah, C.; Ramachandran, G.; Chackaravarthi, G.; Narayanan, M.; Maruthupandy, M.; Quero, F.; Arunachalam, A.; Ramalinga Viswanathan, M.; Khaled, J.M.; et al. Time dependent inhibition of Morinda citrifolia essential oils against multi drug resistant bacteria and A549 lung cancer cells. J. King Saud. Univ. Sci. 2024, 36, 103023. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef] [PubMed]
- Kovendan, K.; Murugan, K.; Shanthakumar, S.P.; Vincent, S. Evaluation of larvicidal and pupicidal activity of Morinda citrifolia L. (Noni) (Family: Rubiaceae) against three mosquito vectors. Asian Pac. J. Trop. Dis. 2012, 2, S362–S369. [Google Scholar] [CrossRef]
- Torres, M.A.O.; de Fátima Braga Magalhães, I.; Mondêgo-Oliveira, R.; de Sá, J.C.; Rocha, A.L.; Abreu-Silva, A.L. One plant, many uses: A review of the pharmacological applications of Morinda citrifolia. Phytother. Res. 2017, 31, 971–979. [Google Scholar] [CrossRef]
- Dias, B.L.; Sarmento, R.A.; Venzon, M.; Jumbo, L.O.V.; dos Santos, L.S.S.; de Souza Moura, W.; de Souza Carlos Mourão, D.; de Sena Fernandes, P.R.; Neitzke, T.R.; de Almeida Oliveira, J.V.; et al. Morinda citrifolia essential oil: A plant resistance biostimulant and a sustainable alternative for controlling phytopathogens and insect pests. Biology 2024, 13, 479. [Google Scholar] [CrossRef]
- Osorio, P.R.A.; Dias, F.R.; Mourão, D.S.C.; Araujo, S.H.C.; Toledo, P.F.S.; Silva, A.C.F.; Viera, W.A.S.; Câmara, M.P.S.; Moura, W.S.; Aguiar, R.W.A.; et al. Essential oil of Noni, Morinda citrifolia L., fruits controls the rice stem-rot disease without detrimentally affect beneficial fungi and ladybeetles. Ind. Crops. Prod. 2021, 170, 113728. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Ismail, S.; Man, C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012, 92, 593–597. [Google Scholar] [CrossRef]
- Costa, L.T.M.; Smagghe, G.; Viteri Jumbo, L.O.; Santos, G.R.; Aguiar, R.W.S.; Oliveira, E.E. Selective actions of plant-based biorational insecticides: Molecular mechanisms and reduced risks to non-target organisms. Curr. Opin. Environ. Sci. Health 2025, 44, 100601. [Google Scholar] [CrossRef]
- Pimentel-Farias, A.; Vieira-Teodoro, A.; dos Passos, E.M.; de Sena-Filho, J.G.; dos Santos, M.C.; Rabelo-Coelho, C.; Viteri-Jumbo, L. Bioactividad de aceites vegetales a Orthezia praelonga (Hemiptera: Sternorrhyncha: Orthezidae) y selectividad a su predador Ceraeochrysa caligata (Neuroptera: Chrysopidae). Rev. Prot. Veg. 2018, 33. [Google Scholar]
- Barnett, H.L. Illustrated Genera of Imperfect Fungi; Burgess Publishing Company: Minneapolis, MN, USA, 1998; 218p, Available online: http://apspress.edu.com (accessed on 24 May 2025).
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 5 online ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Oliveira, J.A. Efeito do Tratamento Fungicida em Sementes no Controle de Tombamento de Plântulas de Pepino (Cucumis sativus L.) e pimentão (Capsicum annum L.); Escola Superior de Agricultura de Lavras: Lavras, Minas Gerais, Brazil, 1991. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Sasisekharan, V. Conformation of Polypeptides and Proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Anson, M.L., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 23, pp. 283–437. [Google Scholar]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous Automated Model Evaluationn (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins Struct. Funct. Bioinf. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2010, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph Model 1999, 17, 57–61. [Google Scholar] [PubMed]
- Moura, W.; de Souza, S.R.; Campos, F.S.; Sander Rodrigues Cangussu, A.; Macedo Sobrinho Santos, E.; Silva Andrade, B.; Borges Gomes, C.H.; Fernandes Viana, K.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crops. Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, L. The PyMOL molecular graphics system. Version 2018, 1, 8. [Google Scholar]
- Dassault, S.B. Discovery Studio Visualizer; Accelrys Software Inc.: San Diego, CA, USA, 2017. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons.: New York, NY, USA, 1990. [Google Scholar]
- Toledo, P.F.S.; Viteri Jumbo, L.O.; Rezende, S.M.; Haddi, K.; Silva, B.A.; Mello, T.S.; Della Lucia, T.M.C.; Aguiar, R.W.S.; Smagghe, G.; Oliveira, E.E. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Ferreira, T.P.; Bastos, I.M.A.S.; Rezende, S.M.; Viteri Jumbo, L.O.; Didonet, J.; Andrade, B.S.; Melo, T.S.; Smagghe, G.; Oliveira, E.E.; et al. Essential oil from Negramina (Siparuna guianensis) plants controls aphids without impairing survival and predatory abilities of non-target ladybeetles. Environ. Pollut. 2019, 255, 113153. [Google Scholar] [CrossRef]
- de Goes, A.; Martins, R.D.; dos Reis, R.F. Efeito de fungicidas cúpricos, aplicados isoladamente ou em combinação com mancozeb, na expressão de sintomas de fitotoxicidade e controle da ferrugem causada por Puccinia psidii em goiabeira. Rev. Bras. Frutic. 2004, 26, 237–240. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Gonçalves, D.d.C.; Ribeiro, W.R.; Gonçalves, D.C.; Menini, L.; Costa, H. Recent advances and future perspective of essential oils in control Colletotrichum spp.: A sustainable alternative in postharvest treatment of fruits. Food Res. Int. 2021, 150, 110758. [Google Scholar] [CrossRef] [PubMed]
- Rabari, V.P.; Chudashama, K.S.; Thaker, V.S. In vitro screening of 75 essential oils against Colletotrichum gloeosporioides: A causal agent of anthracnose disease of mango. Int. J. Fruit Sci. 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Idris, F.M.; Ibrahim, A.M.; Forsido, S.F. Essential oils to control Colletotrichum musae in vitro and in vivo on banana fruits. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 291–302. [Google Scholar] [CrossRef]
- Nosé, N.P.; Dalcin, M.S.; Dias, B.L.; Toloy, R.S.; Mourão, D.S.C.; Giongo, M.; Cangussu, A.; da Cruz Araujo, S.H.; dos Santos, G.R. Noni essential oil associated with adjuvants in the production of phytoalexins and in the control of soybean anthracnosis. J. Med. Plant Res. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Dias, B.L.; Costa, P.F.; Dakin, M.S.; Dias, F.R.; de Sousa, R.R.; de Souza Ferreira, T.P.; Campos, F.S.; rigues Dos Santos, G.R. Control of papaya fruits anthracnose by essential oils of medicinal plants associated to different coatings. J. Med. Plant Res. 2020, 14, 239–246. [Google Scholar] [CrossRef]
- Veloso, R.A.; de Souza Ferreira, T.P.; Dias, B.L.; De Souza, D.; Mourao, C.; de Araujo Filho, R.N.; Sales, R.; Gloria, L.; Barros, A.M.; de Souza Ferreira, T.P.; et al. Chemical composition and bioactivity of essential oil from Morinda citrifolia L. fruit. J. Med. Plant Res. 2020, 14, 208–214. [Google Scholar] [CrossRef]
- Stevens, S.; Hofmeyr, J.-H.S. Effects of ethanol, octanoic and decanoic acids on fermentation and the passive influx of protons through the plasma membrane of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 656–663. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Elkolli, M.; Elkolli, H.; Alam, M.; Benguerba, Y. In silico study of antibacterial tyrosyl-tRNA synthetase and toxicity of main phytoconstituents from three active essential oils. J. Biomol. Struct. Dyn. 2024, 42, 1404–1416. [Google Scholar] [CrossRef]
- Pang, L.; Weeks, S.D.; Van Aerschot, A. Aminoacyl-tRNA Synthetases as Valuable Targets for Antimicrobial Drug Discovery. Int. J. Mol. Sci. 2021, 22, 1750. [Google Scholar] [CrossRef]
- Solgi, M.; Ghorbanpour, M. Application of essential oils and their biological effects on extending the shelf-life and quality of horticultural crops. Trakia J. Sci. 2014, 12, 198–210. [Google Scholar]
- da Costa Gonçalves, D.; Ribeiro, W.R.; Gonçalves, D.C.; Dian, V.S.; da Silva Xavier, A.; de Oliveira, Á.A.; Menini, L.; Costa, H. Use of Melaleuca alternifolia essential oil as an efficient strategy to extend the shelf life of banana fruits. Biochem. Syst. Ecol. 2023, 108, 104641. [Google Scholar] [CrossRef]
- Mangoba, M.A.A.; de Guzman Alvindia, D. Fungicidal activities of Cymbopogon winterianus against anthracnose of banana caused by Colletotrichum musae. Sci. Rep. 2023, 13, 6629. [Google Scholar] [CrossRef]
- Silva, J.C.E.; Mourão, D.D.S.C.; de Oliveira Lima, F.S.; Sarmento, R.D.A.; Dalcin, M.S.; de Souza Aguiar, R.W.; dos Santos, G.R. The efficiency of Noni (Morinda citrifolia L.) essential oil on the control of leaf spot caused by Exserohilum turcicum in maize culture. Medicines 2017, 4, 60. [Google Scholar] [CrossRef]
- Dalcin, M.S.; CafÃ, A.C.Ã.; de Almeida Sarmento, R.; do Nascimento, I.R.; de Souza Ferreira, T.P.; de Sousa Aguiar, R.W.; dos Santos, G.R. Evaluation of essential oils for preventive or curative management of melon gummy stem blight and plant toxicity. J. Med. Plant Res. 2017, 11, 426–432. [Google Scholar] [CrossRef]
- Walters, D. Plant Defense: Warding off Attack By Pathogens, Herbivores and Parasitic Plants; John Wiley & Sons: London, UK, 2011. [Google Scholar]
- Hartmann, T.; Ober, D. Defense by pyrrolizidine alkaloids: Developed by plants and recruited by insects. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 213–231. [Google Scholar]
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Springer Science & Business Media: New York, NY, USA, 2007; Volume 2. [Google Scholar]
- Elsayed, M.I.; Al-Qurashi, A.D.; Almasaudi, N.M.; Abo-Elyousr, K.A.M. Efficacy of essential oils against gray mold and effect on fruit quality during cold storage in table grapes. S. Afr. J. Bot. 2022, 146, 481–490. [Google Scholar] [CrossRef]
- Sefu, G.; Satheesh, N.; Berecha, G. Effect of essential oils treatment on anthracnose (Colletotrichum gloeosporioides) disease development, quality and shelf life of mango fruits (Mangifera indica L). Am. Eurasian J. Agric. Environ. Sci 2015, 15, 2160–2169. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Fatemi, S.; Jafarpour, M.; Eghbalsaied, S.; Rezapour, A.; Borji, H. Effect of essential oils of Thymus vulgaris and Mentha piperita on the control of green mould and postharvest quality of Citrus Sinensis cv. Valencia. Afr. J. Biotechnol. 2011, 10, 14932–14936. [Google Scholar] [CrossRef]
- Mohammadi, S.; Aminifard, M.H. Effect of essential oils on postharvest decay and some quality factors of peach (Prunus persica var. Redhaven). J. Biol. Environ. Sci. 2012, 6, 147–153. [Google Scholar]


| Compounds | RT | IR | (%) |
|---|---|---|---|
| 2-Heptanone | 5.013 | 927 | 0.14 |
| Methyl hexanoate | 5.787 | 944 | 1.08 |
| Ethyl hexanoate | 7.996 | 991 | 0.5 |
| Hexanoic acid | 8.688 | 1006 | 8.64 |
| Butanoic acid, 4-pentenyl ester | 10.395 | 1043 | 0.19 |
| Methyl octanoate | 12.755 | 1094 | 5.35 |
| Octanoate acetate | 15.846 | 1163 | 3.58 |
| Octanoic acid | 17.413 | 1198 | 64.03 |
| 4-Pentenyl hexanoate | 18.602 | 1225 | 4,3 |
| Decanoic acid, methyl ester | 21.390 | 1288 | 0,19 |
| Butanoic acid | 26.995 | 1420 | 10.16 |
| Hexyl octanoate | 31.939 | 1540 | 0.37 |
| Others | - | - | 1.47 |
| Total | - | - | 100 |
| Ligand | Affinity Energy (kcal/mol) |
|---|---|
| Octanoic acid | −4.8 |
| Hexanoic acid | −4.4 |
| Butanoic acid | −4.5 |
| Methyl octanoate | −4.4 |
| Acetate octanoate | −4.7 |
| 4-Pentene octanoate | −4.5 |
| Treatment (µL/mL) | Scale (24 h) | Observed Phytotoxicity | Scale (48 h) | Observed Phytotoxicity |
|---|---|---|---|---|
| Control (H2O + tween-80) | 0 | Absent | 0 | Absent |
| Methyl tiophanate 10 | 0 | Absent | 0 | Absent |
| 10 | 0 | Absent | 0 | Absent |
| 20 | 0 | Absent | 1 | Light chlorosis |
| 30 | 0 | Absent | 2 | Moderate chlorosis |
| 40 | 1.67 | Light chlorosis | 3 | Severe chlorosis |
| 50 | 1.67 | Light chlorosis | 3 | Severe chlorosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.C.; Viteri, L.O.; Fernandes, P.R.; Carvalho, R.C.; Gonzalez, M.A.; Herrera, O.M.; Osório, P.R.; Mourão, D.S.C.; Araujo, S.H.; Moraes, C.B.; et al. Morinda citrifolia Essential Oil in the Control of Banana Anthracnose: Impacts on Phytotoxicity, Preventive and Curative Effects and Fruit Quality. Microbiol. Res. 2025, 16, 149. https://doi.org/10.3390/microbiolres16070149
Santos MC, Viteri LO, Fernandes PR, Carvalho RC, Gonzalez MA, Herrera OM, Osório PR, Mourão DSC, Araujo SH, Moraes CB, et al. Morinda citrifolia Essential Oil in the Control of Banana Anthracnose: Impacts on Phytotoxicity, Preventive and Curative Effects and Fruit Quality. Microbiology Research. 2025; 16(7):149. https://doi.org/10.3390/microbiolres16070149
Chicago/Turabian StyleSantos, Maysa C., Luis O. Viteri, Paulo R. Fernandes, Rosilene C. Carvalho, Manuel A. Gonzalez, Osmany M. Herrera, Pedro R. Osório, Dalmarcia S. C. Mourão, Sabrina H. Araujo, Cristiano B. Moraes, and et al. 2025. "Morinda citrifolia Essential Oil in the Control of Banana Anthracnose: Impacts on Phytotoxicity, Preventive and Curative Effects and Fruit Quality" Microbiology Research 16, no. 7: 149. https://doi.org/10.3390/microbiolres16070149
APA StyleSantos, M. C., Viteri, L. O., Fernandes, P. R., Carvalho, R. C., Gonzalez, M. A., Herrera, O. M., Osório, P. R., Mourão, D. S. C., Araujo, S. H., Moraes, C. B., Giongo, M. V., Moura, W. S., Camara, M. P., Cangussu, A. S. R., Aguiar, R. W. S., Oliveira, E. E., & Santos, G. R. (2025). Morinda citrifolia Essential Oil in the Control of Banana Anthracnose: Impacts on Phytotoxicity, Preventive and Curative Effects and Fruit Quality. Microbiology Research, 16(7), 149. https://doi.org/10.3390/microbiolres16070149










