Transcriptomic Analysis of Macrophages Infected with Mycobacterium smegmatis
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Culture of Cells and Strains
2.2. Mycobacterium smegmatis Infection of Macrophages
2.3. Transcriptomics Assay
2.4. qPCR
2.5. ELISA
2.6. Statistical Analysis
3. Results
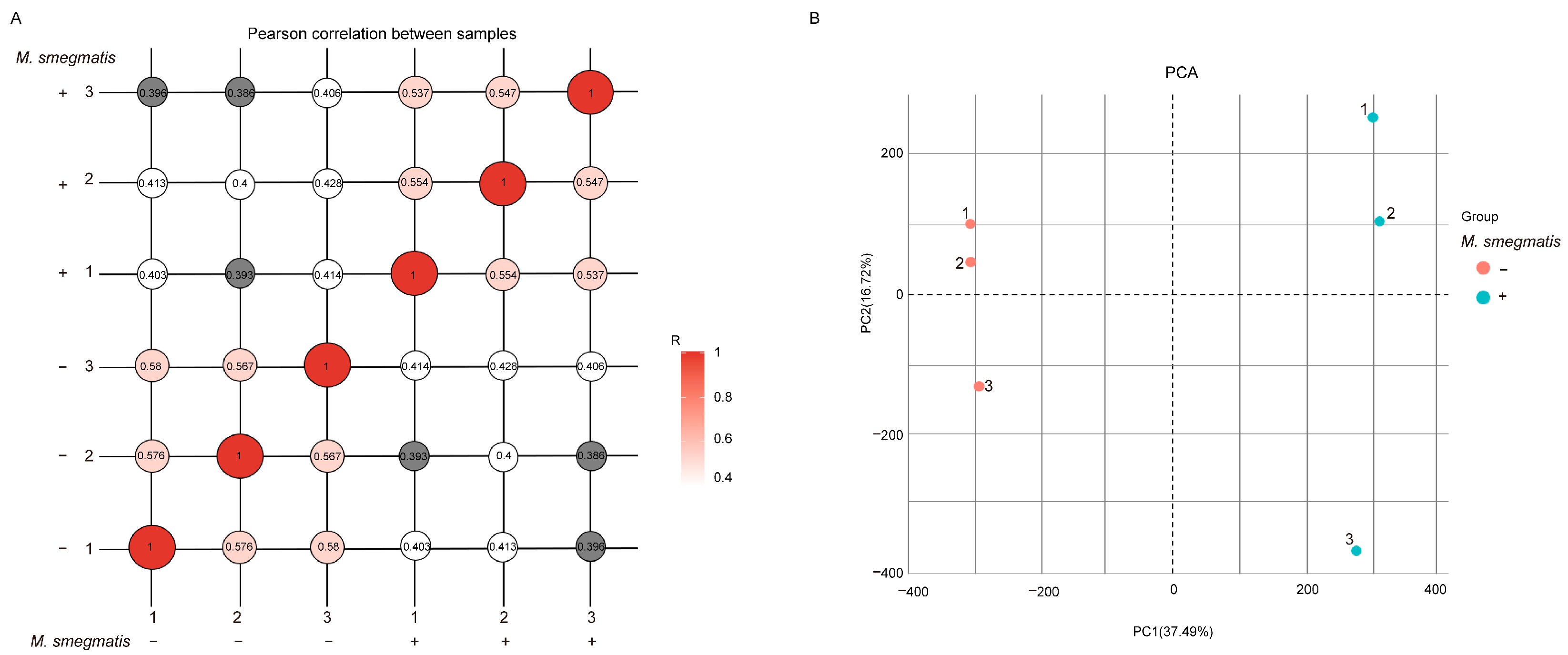
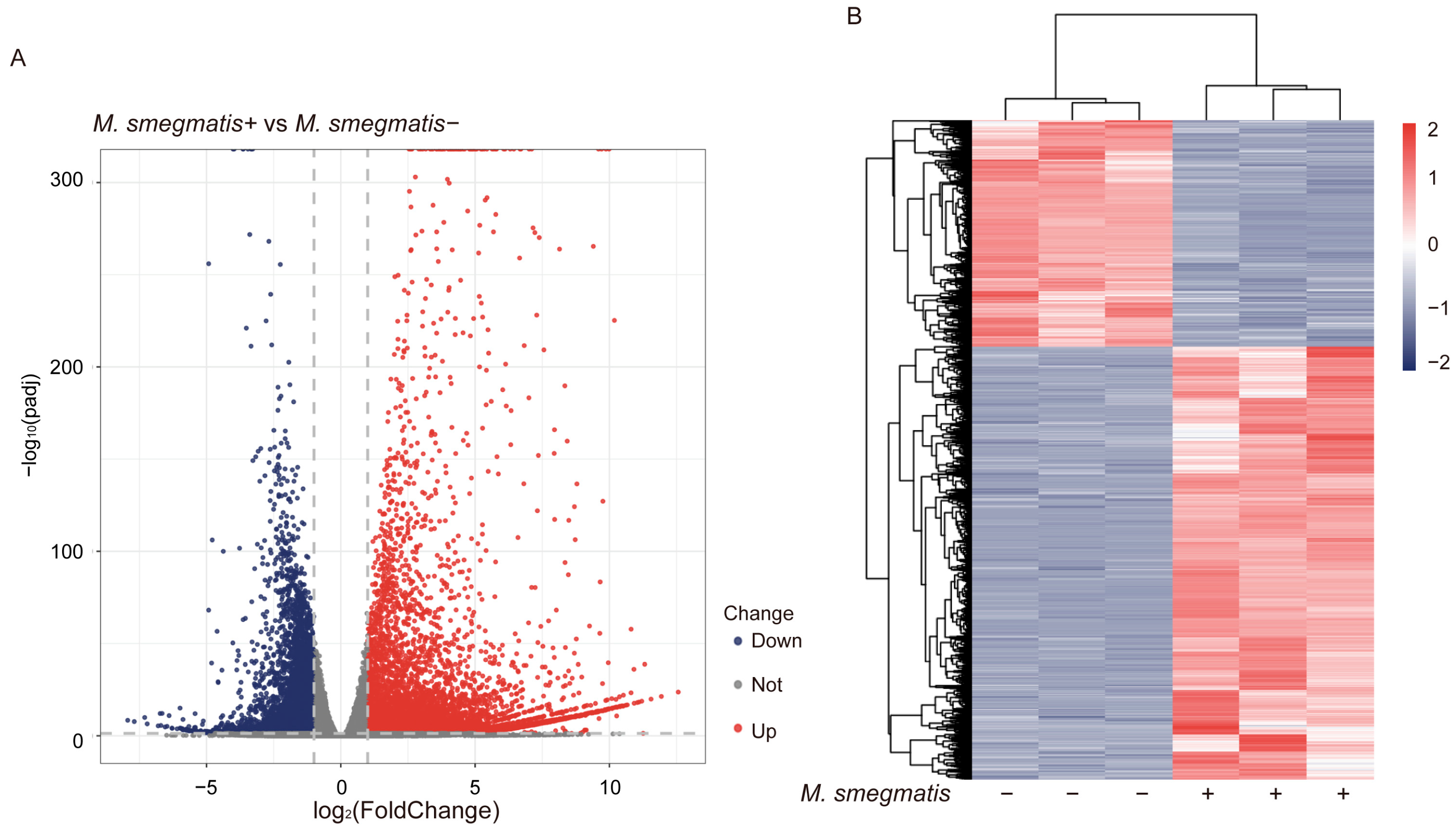
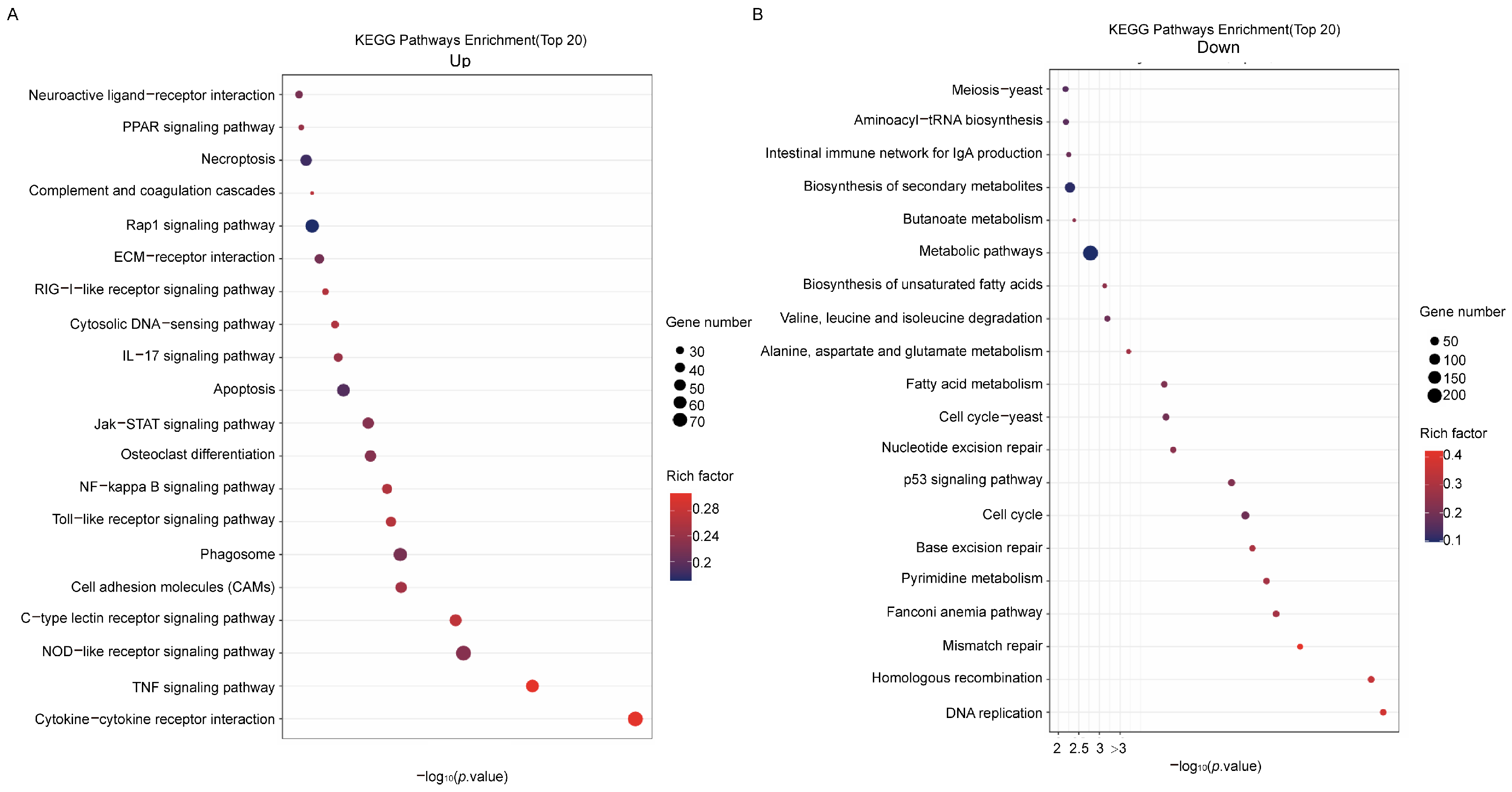
3.1. Mycobacterium smegmatis Can Regulate the Activation of Macrophage Immune Responses
3.2. Mycobacterium smegmatis Regulates Production of IL-6 and TNF-α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTB | Mycobacterium tuberculosis |
| M. smeg | Mycobacterium smegmatis |
| TB | tuberculosis |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-α |
| IRF | Interferon Regulatory Factor |
References
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Koch, A.; Mizrahi, V. Mycobacterium tuberculosis. Trends Microbiol. 2018, 26, 555–556. [Google Scholar] [CrossRef]
- Bañuls, A.L.; Sanou, A.; Van Anh, N.T.; Godreuil, S. Mycobacterium tuberculosis: Ecology and evolution of a human bacterium. J. Med. Microbiol. 2015, 64, 1261–1269. [Google Scholar] [CrossRef]
- Esaulova, E.; Das, S.; Singh, D.K.; Choreño-Parra, J.A.; Swain, A.; Arthur, L.; Rangel-Moreno, J.; Ahmed, M.; Singh, B.; Gupta, A.; et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe. 2021, 29, 165–178.e8. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Moure, U.A.E.; Yang, Y.; Pan, J.; Li, L.; Wang, M.; Ke, X.; Cui, H. Mycobacterium tuberculosis-macrophage interaction: Molecular updates. Front. Cell. Infect. Microbiol. 2023, 13, 1062963. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, X.; Pfau, D.; Ling, Y.; Nathan, C.F. Type I interferon signaling mediates Mycobacterium tuberculosis-induced macrophage death. J. Exp. Med. 2021, 218, e20200887. [Google Scholar] [CrossRef]
- Ahmad, F.; Rani, A.; Alam, A.; Zarin, S.; Pandey, S.; Singh, H.; Hasnain, S.E.; Ehtesham, N.Z. Macrophage: A Cell With Many Faces and Functions in Tuberculosis. Front. Immunol. 2022, 13, 747799. [Google Scholar] [CrossRef]
- Cambier, C.J.; Falkow, S.; Ramakrishnan, L. Host Evasion and Exploitation Schemes of Mycobacterium tuberculosis. Cell 2014, 159, 1497–1509. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Baravikov, D.E.; Koshenskova, K.A.; Kiskin, M.A.; Nelyubina, Y.V.; Primakov, P.V.; Voronina, Y.K.; Garaeva, V.V.; Aleshin, D.A.; Aliev, T.M.; et al. What are the prospects for using complexes of copper(ii) and zinc(ii) to suppress the vital activity of Mycolicibacterium smegmatis? RSC Adv. 2022, 12, 5173–5183. [Google Scholar] [CrossRef]
- Liu, L.; Wen, C.; Cai, X.; Gong, W. A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope. Microorganisms. 2024, 12, 1827. [Google Scholar] [CrossRef]
- Maarsingh, J.D.; Haydel, S.E. Mycobacterium smegmatis PrrAB two-component system influences triacylglycerol accumulation during ammonium stress. Microbiology. 2018, 164, 1276–1288. [Google Scholar] [CrossRef]
- Zarin, S.; Shariq, M.; Rastogi, N.; Ahuja, Y.; Manjunath, P.; Alam, A.; Hasnain, S.E.; Ehtesham, N.Z. Rv2231c, a unique histidinol phosphate aminotransferase from Mycobacterium tuberculosis, supports virulence by inhibiting host-directed defense. Cell. Mol. Life Sci. 2024, 81, 203. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, Y.; Deng, X.; Lu, N.; Li, A.; Gao, S.; He, S.; Wang, Y.; Fu, N.; Wang, Z.; et al. Rv2741 Promotes Mycobacterium Survival by Modulating Macrophage Function via the IL-1α-MAPK Axis. ACS Infect. Dis. 2025, 11, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Abuliken, Y.; Yan, Y.; Gao, C.; Jiang, Z.; Huang, T.; Le, T.T.T.; Xiang, L.; Li, P.; Xie, J. Mycobacterium LacI-type Transcription Regulator Rv3575c Affects Host Innate Immunity by Regulating Bacterial mce4 Operon-Mediated Cholesterol Transport. ACS Infect. Dis. 2024, 10, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, R.; Ramos, E.I.; Vijayaraghavan, M.; Sedano, M.J.; Carmona, A.; Chacon, J.A.; Gadad, S.S.; Dhandayuthapani, S. Mycobacterium smegmatis secreting methionine sulfoxide reductase A (MsrA) modulates cellular processes in mouse macrophages. Biochimie. 2023, 211, 1–15. [Google Scholar] [CrossRef]
- Zormpas, E.; Queen, R.; Comber, A.; Cockell, S.J. Mapping the transcriptome: Realizing the full potential of spatial data analysis. Cell 2023, 186, 5677–5689. [Google Scholar] [CrossRef]
- Martorella, M.; Kasela, S.; Garcia-Flores, R.; Gokden, A.; Castel, S.E.; Lappalainen, T. Evaluation of noninvasive biospecimens for transcriptome studies. BMC Genom. 2023, 24, 790. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Crespo-Castrillo, A.; Arevalo, M.A.; Garcia-Segura, L.M. Aging and sex: Impact on microglia phagocytosis. Aging Cell. 2020, 19, e13182. [Google Scholar] [CrossRef]
- Hu, H.; Xia, H.; Zou, X.; Li, X.; Zhang, Z.; Yao, X.; Yin, M.; Tian, D.; Liu, H. N-acetyl-chitooligosaccharide attenuates inflammatory responses by suppression of NF-κB signaling, MAPK and NLRP3 inflammasome in macrophages. J. Funct. Foods 2021, 78, 104364. [Google Scholar] [CrossRef]
- de Kleijn, S.; Bouwens, M.; Verburg-van Kemenade, B.M.; Cuppen, J.J.; Ferwerda, G.; Hermans, P.W. Extremely low frequency electromagnetic field exposure does not modulate toll-like receptor signaling in human peripheral blood mononuclear cells. Cytokine 2011, 54, 43–50. [Google Scholar] [CrossRef]
- Srivastava, S.; Ernst, J.D.; Desvignes, L. Beyond macrophages: The diversity of mononuclear cells in tuberculosis. Immunol. Rev. 2014, 262, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.C.; Juffermans, N.P.; Florquin, S.; van Rooijen, N.; Vervoordeldonk, M.J.; Verbon, A.; van Deventer, S.J.; van der Poll, T. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 2001, 166, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.C.; Thepen, T.; Weijer, S.; Florquin, S.; van Rooijen, N.; van de Winkel, J.G.; van der Poll, T. Macrophages play a dual role during pulmonary tuberculosis in mice. J. Infect. Dis. 2005, 191, 65–74. [Google Scholar] [CrossRef]
- VanderVen, B.C.; Huang, L.; Rohde, K.H.; Russell, D.G. The Minimal Unit of Infection: Mycobacterium tuberculosis in the Macrophage. Microbiol. Spectr. 2016, 4, 10-1128. [Google Scholar] [CrossRef]
- Elitas, M.; Dhar, N.; McKinney, J.D. Revealing Antibiotic Tolerance of the Mycobacterium smegmatis Xanthine/Uracil Permease Mutant Using Microfluidics and Single-Cell Analysis. Antibiotics 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Szappanos, B.; Buchieri, M.V.; Trauner, A.; Piazza, I.; Picotti, P.; Gagneux, S.; Borrell, S.; Gicquel, B.; Lelievre, J.; et al. High-throughput metabolomic analysis predicts mode of action of uncharacterized antimicrobial compounds. Sci. Transl. Med. 2018, 10, eaal3973. [Google Scholar] [CrossRef]
- Ogbonna, E.C.; Anderson, H.R.; Beardslee, P.C.; Bheemreddy, P.; Schmitz, K.R. Interactome Analysis Identifies MSMEI_3879 as a Substrate of Mycolicibacterium smegmatis ClpC1. Microbiol. Spectr. 2023, 11, e0454822. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, J.; Li, Y.; Zhang, Z.; Chang, L.; Wang, G.; Wu, W.; Yu, L.; Dai, E.; Zhang, L.; et al. Mirror proteases of Ac-Trypsin and Ac-LysargiNase precisely improve novel event identifications in Mycolicibacterium smegmatis MC2 155 by proteogenomic analysis. Front. Microbiol. 2022, 13, 1015140. [Google Scholar] [CrossRef]
- Cordero, P.R.F.; Bayly, K.; Man Leung, P.; Huang, C.; Islam, Z.F.; Schittenhelm, R.B.; King, G.M.; Greening, C. Atmospheric carbon monoxide oxidation is a widespread mechanism supporting microbial survival. ISME J. 2019, 13, 2868–2881. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Hegde, S.R. Integrating multi-omics data to investigate pseudogene expression in Mycolicibacterium smegmatis. Gene 2020, 755, 144908. [Google Scholar] [CrossRef]
- Sakallioglu, I.T.; Maroli, A.S.; De Lima Leite, A.; Marshall, D.D.; Evans, B.W.; Zinniel, D.K.; Dussault, P.H.; Barletta, R.G.; Powers, R. Multi-omics Investigation into the Mechanism of Action of an Anti-tubercular Fatty Acid Analogue. J. Am. Chem. Soc. 2022, 144, 21157–21173. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Hou, Y.; Xu, W.; Wang, W.; Tian, N.; Liu, D.; Sun, Z. Transcriptomic Analysis of Macrophages Infected with Mycobacterium smegmatis. Microbiol. Res. 2025, 16, 146. https://doi.org/10.3390/microbiolres16070146
Sun H, Hou Y, Xu W, Wang W, Tian N, Liu D, Sun Z. Transcriptomic Analysis of Macrophages Infected with Mycobacterium smegmatis. Microbiology Research. 2025; 16(7):146. https://doi.org/10.3390/microbiolres16070146
Chicago/Turabian StyleSun, Hong, Yue Hou, Wenzhao Xu, Wenjing Wang, Na Tian, Dingyi Liu, and Zhaogang Sun. 2025. "Transcriptomic Analysis of Macrophages Infected with Mycobacterium smegmatis" Microbiology Research 16, no. 7: 146. https://doi.org/10.3390/microbiolres16070146
APA StyleSun, H., Hou, Y., Xu, W., Wang, W., Tian, N., Liu, D., & Sun, Z. (2025). Transcriptomic Analysis of Macrophages Infected with Mycobacterium smegmatis. Microbiology Research, 16(7), 146. https://doi.org/10.3390/microbiolres16070146




