The Nosocomial Transmission of Carbapenem-Resistant Gram-Negative Bacteria in a Hospital in Baoding City, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Clinical Isolates, Species Identification, and Antimicrobial Susceptibility Testing
2.2. Whole-Genome Sequencing
2.3. Molecular Characterization
2.4. Phylogenetic Analyses
2.5. Induction of Polymyxin-Resistant A. baumannii
3. Results and Discussion
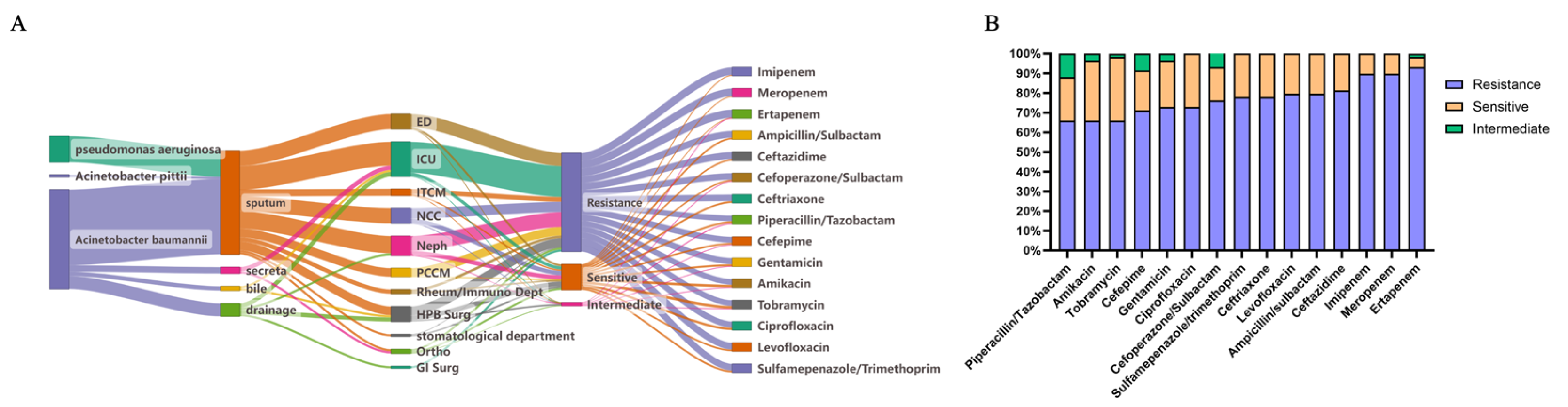
3.1. Epidemiological Feature of MDR Non-Fermenting Gram-Negative Bacteria
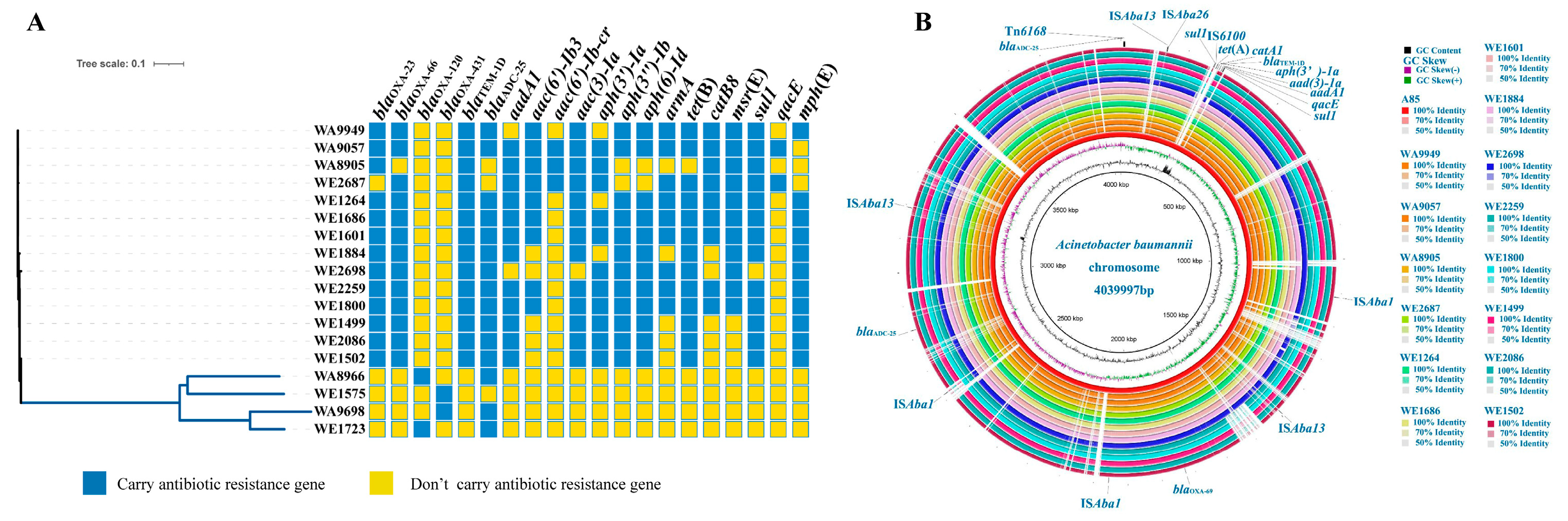
3.2. Antibiotic Resistance, Virulence, and Adaptive Evolution in A. baumannii
3.3. Resistance Mechanisms of Multidrug-Resistant P. aeruginosa
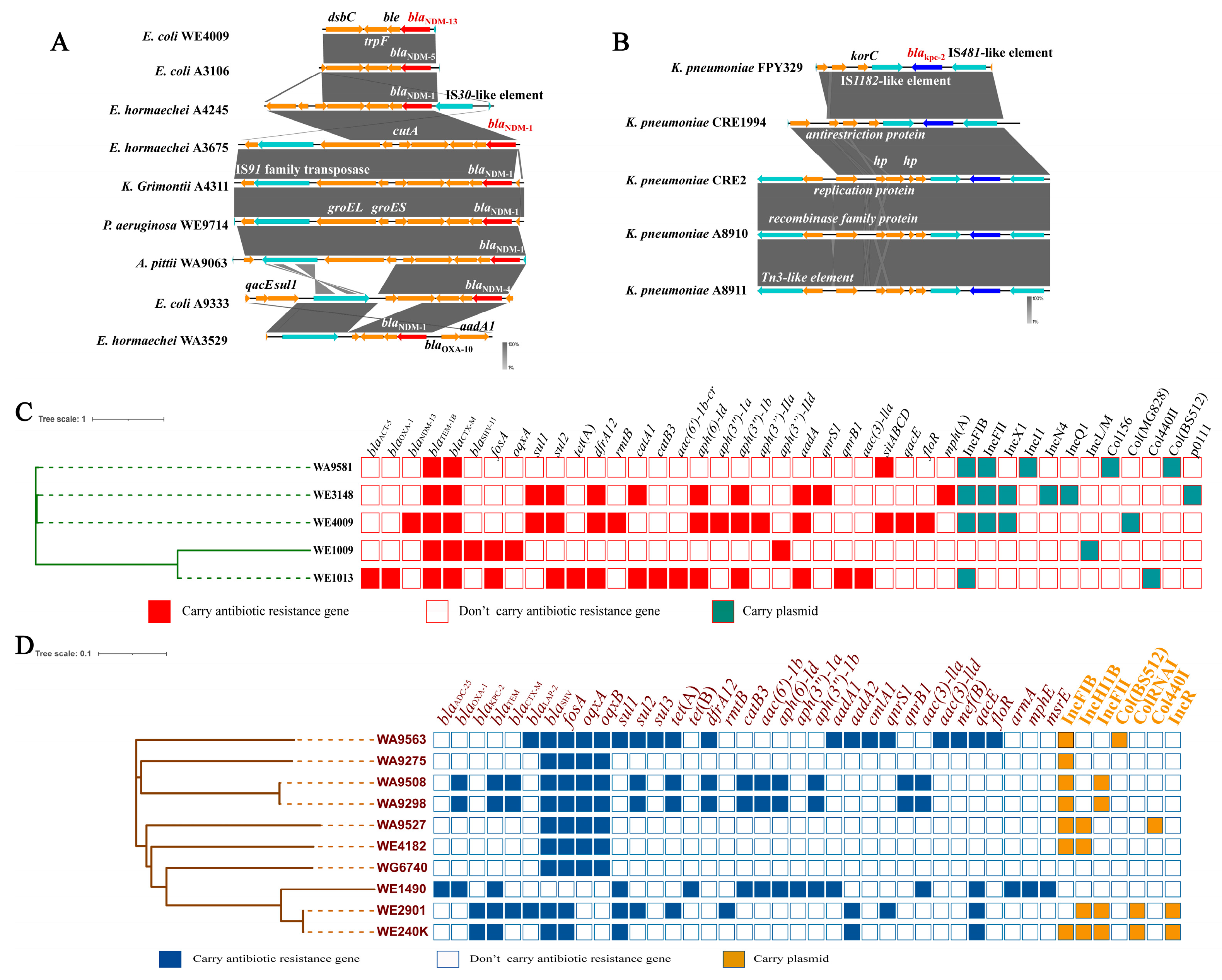
3.4. Genetic Context and Phylogenetic Characteristics of Carbapenem-Resistant Isolates Carrying blaNDM and blaKPC-2 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Oliveira, D.; Forde, B.M.; Kidd, T.J.; Harris, P.; Schembri, M.A.; Beatson, S.A.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed]
- Breidenstein, E.B.; de la Fuente-Nunez, C.; Hancock, R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Carbonara, S.; Marino, A.; Di Caprio, G.; Carretta, A.; Menichetti, F. Mortality Attributable to Bloodstream Infections Caused by Different Carbapenem-Resistant Gram-Negative Bacilli: Results From a Nationwide Study in Italy (ALARICO Network). Clin. Infect. Dis. 2023, 76, 2059–2069. [Google Scholar] [CrossRef]
- Gniadek, T.J.; Carroll, K.C.; Simner, P.J. Carbapenem-Resistant Non-Glucose-Fermenting Gram-Negative Bacilli: The Missing Piece to the Puzzle. J. Clin. Microbiol. 2016, 54, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Reuter, S.; Wille, J.; Xanthopoulou, K.; Stefanik, D.; Grundmann, H.; Seifert, H. A global view on carbapenem-resistant Acinetobacter baumannii. mBio 2023, 14, e02260-23. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 525–545. [Google Scholar] [CrossRef]
- Pelegrin, A.C.; Palmieri, M.; Mirande, C.; Oliver, A.; Moons, P.; Goossens, H.; van Belkum, A. Pseudomonas aeruginosa: A clinical and genomics update. FEMS Microbiol. Rev. 2021, 45, fuab026. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorli, L.; Luque, S.; Gomez-Zorrilla, S.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Iovleva, A.; Mustapha, M.M.; Griffith, M.P.; Komarow, L.; Luterbach, C.; Evans, D.R.; Doi, Y. Carbapenem-Resistant Acinetobacter baumannii in U.S. Hospitals: Diversification of Circulating Lineages and Antimicrobial Resistance. mBio 2022, 13, e02759-21. [Google Scholar] [CrossRef]
- Reyes, J.; Komarow, L.; Chen, L.; Ge, L.; Hanson, B.M.; Cober, E.; Satlin, M.J. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): A prospective cohort study. Lancet Microbe 2023, 4, e159–e170. [Google Scholar] [CrossRef]
- Wang, M.; Ge, L.; Chen, L.; Komarow, L.; Hanson, B.; Reyes, J.; Doi, Y. Clinical Outcomes and Bacterial Characteristics of Carbapenem-Resistant Acinetobacter baumannii Among Patients from Different Global Regions. Clin. Infect. Dis. 2023, 78, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, K.; Wu, Y.; Huang, L.; Fang, Y.; Lu, J.; Zhang, R. Epidemiological and genetic characteristics of clinical carbapenem-resistant Acinetobacter baumannii strains collected countrywide from hospital intensive care units (ICUs) in China. Emerg. Microbes Infect. 2022, 11, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhang, J.; Wang, X.; Zhang, Y.; Wang, H. Identification of a novel plasmid-mediated tigecycline resistance-related gene, tet(Y), in Acinetobacter baumannii. J. Antimicrob. Chemother. 2021, 77, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Nang, S.C.; Azad, M.; Velkov, T.; Zhou, Q.T.; Li, J. Rescuing the Last-Line Polymyxins: Achievements and Challenges. Pharmacol. Rev. 2021, 73, 679–728. [Google Scholar] [CrossRef]
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: First report from Pakistan. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190237. [Google Scholar] [CrossRef]
- Martins-Sorenson, N.; Snesrud, E.; Xavier, D.E.; Cacci, L.C.; Iavarone, A.T.; McGann, P.; Moreira, B.M. A novel plasmid-encoded mcr-4.3 gene in a colistin-resistant Acinetobacter baumannii clinical strain. J. Antimicrob. Chemother. 2020, 75, 60–64. [Google Scholar] [CrossRef]
- Novovic, K.; Jovcic, B. Colistin Resistance in Acinetobacter baumannii: Molecular Mechanisms and Epidemiology. Antibiotics 2023, 12, 516. [Google Scholar] [CrossRef]
- Sharma, S.; Devkota, M.D.; Pokhrel, B.M.; Banjara, M.R. Detection of blaNDM-1, mcr-1 and MexB in multidrug resistant Pseudomonas aeruginosa isolated from clinical specimens in a tertiary care hospital of Nepal. BMC Microbiol. 2023, 23, 153. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Ahn, S.M.; Kim, J.H.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.S.; Song, J.E. Clinical Characteristics and Associated Factors for Mortality in Patients with Carbapenem-Resistant Enterobacteriaceae Bloodstream Infection. Microorganisms 2023, 11, 1121. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.R.; Pimentel, M.I.S.; de Oliveira, É.M.; Jucá, M.B.; Beltrão, E.M.B.; Lopes, A.C.S. Occurrence of blaNDM-1, blaNDM-5, blaNDM-7, and blaKPC-2 genes in clinical isolates of enterobacterales with high genetic variability, from colonization and infection in patients with or without COVID-19, from a hospital in Brazil. J. Appl. Microbiol. 2024, 135, lxae212. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.H.; Xiang, R.; Wang, Y.L.; Wu, S.K.; Lei, C.W.; Kang, Z.Z.; Chen, Y.P.; Ye, X.L.; Lai, Y.; Wang, H.N. Integration of the blaNDM-1 carbapenemase gene into a novel SXT/R391 integrative and conjugative element in Proteus vulgaris. J. Antimicrob. Chemother. 2022, 75, 1439–1442. [Google Scholar] [CrossRef]
- Cai, M.; Song, K.; Wang, R.; Wang, S.; Chen, H.; Wang, H. Tracking intra-species and inter-genus transmission of KPC through global plasmids mining. Cell Rep. 2024, 43, 114351. [Google Scholar] [CrossRef] [PubMed]
- Campana, E.H.; Kraychete, G.B.; Montezzi, L.F.; Xavier, D.E.; Picão, R.C. Description of a new non-Tn4401 element (NTE(KPC)-IIe) harboured on IncQ plasmid in Citrobacter werkmanii from recreational coastal water. J. Glob. Antimicrob. Resist. 2022, 29, 207–211. [Google Scholar] [CrossRef]
- Dortet, L.; Broda, A.; Bernabeu, S.; Glupczynski, Y.; Bogaerts, P.; Bonnin, R.; Larrouy-Maumus, G. Optimization of the MALDIxin test for the rapid identification of colistin resistance in Klebsiella pneumoniae using MALDI-TOF MS. J. Antimicrob. Chemother. 2020, 75, 110–116. [Google Scholar] [CrossRef]
- Dean, C.R.; Visalli, M.A.; Projan, S.J.; Sum, P.E.; Bradford, P.A. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 2003, 47, 972–978. [Google Scholar] [CrossRef]
- Macesic, N.; Uhlemann, A.-C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef]
- Jean, S.-S.; Harnod, D.; Hsueh, P.-R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Teng, J.; Imani, S.; Zhou, A.; Zhao, Y.; Du, L.; Deng, S.; Li, J.; Wang, Q. Combatting resistance: Understanding multi-drug resistant pathogens in intensive care units. Biomed. Pharmacother. 2023, 167, 115564. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Bonomo, R.A. “Airborne assault”: A new dimension in Acinetobacter baumannii transmission. Crit. Care Med. 2013, 41, 2042–2044. [Google Scholar] [CrossRef]
- Boll, J.M.; Crofts, A.A.; Peters, K.; Cattoir, V.; Vollmer, W.; Davies, B.W.; Trent, M.S. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc. Natl. Acad. Sci. USA 2016, 113, E6228–E6237. [Google Scholar] [CrossRef]
- Laborda, P.; Hernando-Amado, S.; Martinez, J.L.; Sanz-Garcia, F. Antibiotic Resistance in Pseudomonas. Adv. Exp. Med. Biol. 2022, 1386, 117–143. [Google Scholar] [PubMed]
- Shu, J.C.; Kuo, A.J.; Su, L.H.; Liu, T.P.; Lee, M.H.; Su, I.N.; Wu, T.L. Development of carbapenem resistance in Pseudomonas aeruginosa is associated with OprD polymorphisms, particularly the amino acid substitution at codon 170. J. Antimicrob. Chemother. 2017, 72, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Willison, E.; Marchese, A. The microbiology and pathogenesis of nonfermenting Gram-negative infections. Curr. Opin. Infect. Dis. 2023, 36, 537–544. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Zhang, P.; Wang, N.; Yuan, Q.; Shi, W.; Zhang, X.; Li, X.; Qu, T. Emergence of carbapenem-resistant Enterobacter hormaechei ST93 plasmids co-harbouring blaNDM-1, blaKPC-2, and mcr-9 in bloodstream infection. J. Glob. Antimicrob. Resist. 2023, 34, 67–73. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, X.; Zeng, S.; Fu, L.; Xu, H.; Li, X. Emergence of a Salmonella Rissen ST469 clinical isolate carrying bla (NDM-13) in China. Front. Cell Infect. Microbiol. 2022, 12, 936649. [Google Scholar] [CrossRef]
- Zou, H.; Berglund, B.; Wang, S.; Zhou, Z.; Gu, C.; Zhao, L.; Meng, C.; Li, X. Emergence of bla(NDM-1), bla(NDM-5), bla(KPC-2) and bla(IMP-4) carrying plasmids in Raoultella spp. in the environment. Environ. Pollut. 2022, 306, 119437. [Google Scholar] [CrossRef]
- Rodrigues, S.H.; Nunes, G.D.; Soares, G.G.; Ferreira, R.L.; Damas, M.S.F.; Laprega, P.M.; Shilling, R.E.; Campos, L.C.; da Costa, A.S.; Malavazi, I.; et al. First report of coexistence of blaKPC-2 and blaNDM-1 in carbapenem-resistant clinical isolates of Klebsiella aerogenes in Brazil. Front. Microbiol. 2024, 15, 1352851. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paredes, D.; Del Canto, F.; Rios, R.; Diaz, L.; Reyes, J.; Arias, C.A.; Zurita, J. Colistin and tigecycline resistance in ESBL-producing E. coli and K. pneumoniae harboring blaKPC genes. Antibiotics 2025, 14, 206. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Su, W.; Li, T.; Li, Z.; Pei, Z.; Zhang, J.; Yin, W. The Nosocomial Transmission of Carbapenem-Resistant Gram-Negative Bacteria in a Hospital in Baoding City, China. Microbiol. Res. 2025, 16, 147. https://doi.org/10.3390/microbiolres16070147
Liao S, Su W, Li T, Li Z, Pei Z, Zhang J, Yin W. The Nosocomial Transmission of Carbapenem-Resistant Gram-Negative Bacteria in a Hospital in Baoding City, China. Microbiology Research. 2025; 16(7):147. https://doi.org/10.3390/microbiolres16070147
Chicago/Turabian StyleLiao, Shengnan, Wei Su, Tianjiao Li, Zeyang Li, Zihan Pei, Jie Zhang, and Wenjuan Yin. 2025. "The Nosocomial Transmission of Carbapenem-Resistant Gram-Negative Bacteria in a Hospital in Baoding City, China" Microbiology Research 16, no. 7: 147. https://doi.org/10.3390/microbiolres16070147
APA StyleLiao, S., Su, W., Li, T., Li, Z., Pei, Z., Zhang, J., & Yin, W. (2025). The Nosocomial Transmission of Carbapenem-Resistant Gram-Negative Bacteria in a Hospital in Baoding City, China. Microbiology Research, 16(7), 147. https://doi.org/10.3390/microbiolres16070147




