3.1. Insects by Demarcated Zone
A total of 353 collected insects were identified as
P. spumarius through morphological examination under a stereomicroscope. Notably, 347 of these individuals (98%) were found in Porto, highlighting a strong concentration of this vector species in that region. These findings align with previous studies conducted in Italy [
23] and Spain [
24], which confirmed the presence of
P. spumarius, indicating a broader European relevance of the vector’s presence.
The high number of insects found in Porto is due to early monitoring efforts starting in 2019 after the first DZ was established there. This longer sampling period, combined with more resources and improved methods, led to more frequent surveys and higher insect detection rates compared to other regions that began monitoring only in 2022.
The sampling effort varied considerably across regions and directly influenced insect detection rates. The Área Metropolitana do Porto, where surveillance began in 2019, encompasses a larger geographical area compared to other DZs and benefited from a longer, more consistent monitoring period. This combination of greater spatial coverage and extended sampling duration allowed for more systematic field activities and improved the chances of detecting P. spumarius, especially across different seasons.
In contrast, the regions of Mirandela I (0.28%), Mirandela II (0.56%), Bougado (0.56%), and Alijó (0.28%) only began surveillance activities in 2022. The delayed implementation in these areas significantly limited the number of observations and reduced opportunities to capture seasonal variation in vector populations. Consequently, these regions recorded much lower detection rates. The complete absence of findings in Sabrosa further highlights the unequal distribution of surveillance effort across DZs.
However, these differences do not necessarily reflect a true absence of the insect vector but are more likely a result of limited spatial and temporal sampling coverage. These findings emphasize the need for harmonized, long-term monitoring across all DZs to provide a reliable assessment of vector presence and to strengthen early detection of potential outbreaks.
Figure 4 provides a breakdown of the results from insect testing, showing that 30 out of the 217 captured
P. spumarius specimens tested positive for
X. fastidiosa, all originating from the DZ in the Área Metropolitana do Porto. These positive results represent 8.49% of the total sampling observations, while 52.9% yielded negative insects (no detection of
X. fastidiosa), and 38.5% of the observations resulted in no insect capture. The fact that all positive cases were concentrated in Porto further reinforces the notion that a higher number of observations and a longer monitoring timeframe increase the likelihood of positive results. In 2019, when knowledge about
X. fastidiosa was still developing, there were no clearly demarcated zones. This lack of formal delineation may have contributed to a high incidence of infected plant material and the establishment of favorable conditions for both the pathogen and its insect vectors.
Additionally, the presence of major transportation hubs within the Porto Demarcated Zone—namely the Port of Leixões and Francisco Sá Carneiro Airport—may have played a critical role in facilitating the unintentional introduction and subsequent spread of insect vectors carrying Xylella fastidiosa. These logistical infrastructures serve as key entry and exit points for international and domestic trade, particularly in agricultural commodities, ornamental plants, and plant propagation materials. As such, they represent high-risk zones for the accidental movement of infected plant material and vector insects. The intense circulation of goods and people in and around these hubs creates numerous opportunities for hitchhiking vectors to be introduced from other regions or countries where the bacterium is already present.
Furthermore, the current phytosanitary regulations and inspection systems in place across parts of the region may exhibit certain structural or operational weaknesses. These vulnerabilities could include insufficient inspection frequency, lack of targeted vector monitoring at critical entry points, and gaps in traceability systems for plant material. Such shortcomings may inadvertently allow the passage of asymptomatic yet infected plants or undetected vectors, thus increasing the risk of local establishment and further transmission of the pathogen [
25]. Given its high connectivity, intense commercial activity, and earlier monitoring start, Porto has emerged as a key location for the detection of
X. fastidiosa and may act as a potential hub for its broader dissemination. This underscores the need for strengthened biosecurity measures and enhanced coordination across sectors for effective surveillance, control, and prevention. Nonetheless, comparisons with other regions should be interpreted with caution, as data collection in those areas began more recently and may not yet reflect the true extent of vector or pathogen presence.
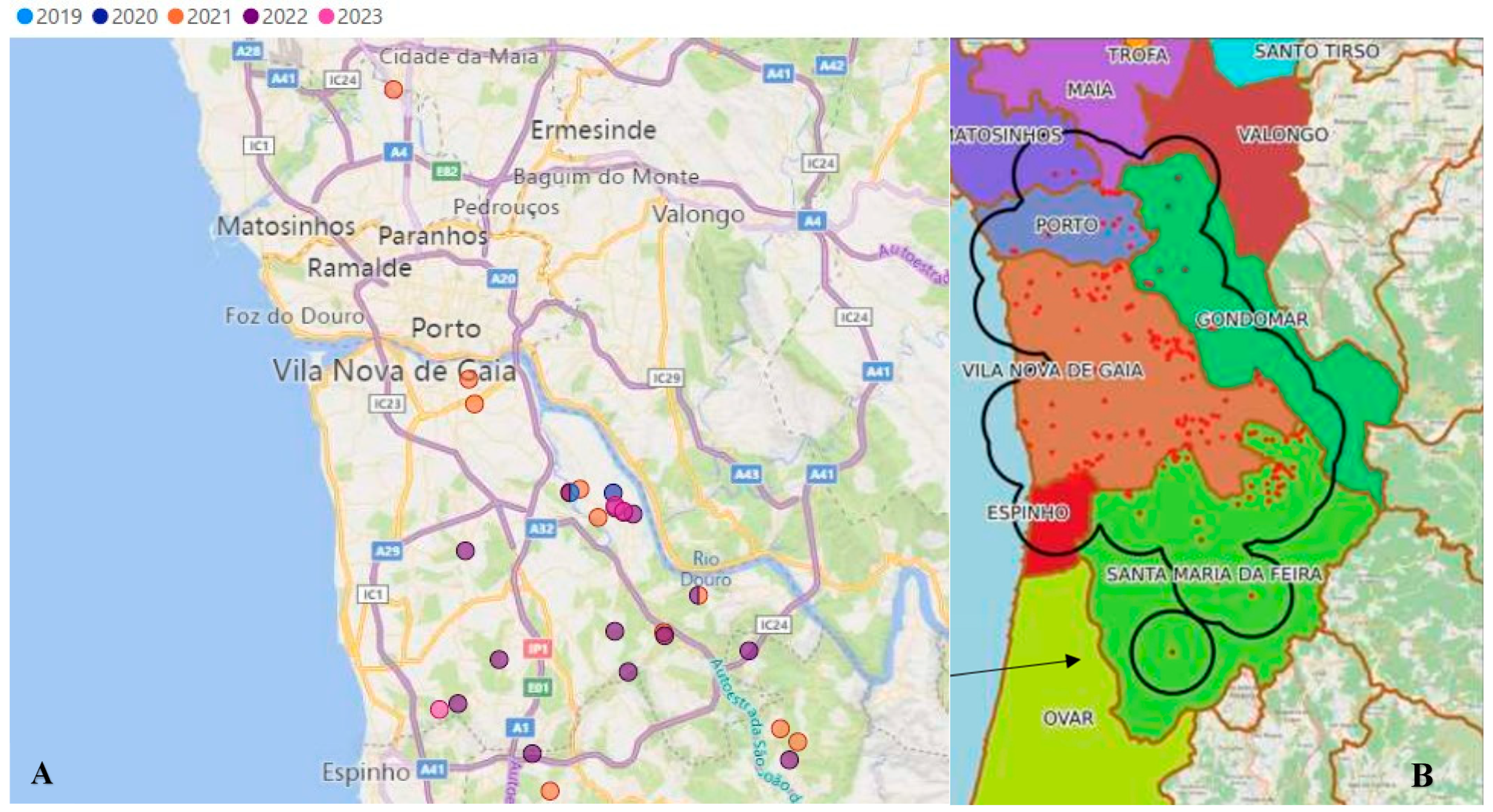
Further insight into the epidemiological dynamics of
X. fastidiosa is provided in
Figure 5, which illustrates the temporal distribution of positive insect cases in Porto (
Figure 5A) and their spatial overlap with the
X. fastidiosa Demarcated Zone within the Área Metropolitana do Porto (
Figure 5B). The strong spatial correlation between the presence of infected insect vectors and the delineated spread of the bacterium provides compelling evidence for the vital role of
P. spumarius in mediating the transmission of
X. fastidiosa. This alignment underscores the importance of the vector in shaping both the distribution and intensity of disease outbreaks and reinforces the need to integrate vector monitoring into disease surveillance frameworks. Understanding these spatial and temporal patterns is crucial for identifying high-risk zones and forecasting potential future outbreaks, particularly in areas that have not yet reported the presence of the bacterium but are ecologically suitable for vector establishment.
Additionally, environmental features and anthropogenic infrastructure within the region appear to contribute to the disease’s dispersal. Major transportation corridors such as roads, as well as natural conduits like the Douro River, may inadvertently facilitate the movement of infected vectors or contaminated plant materials. These landscape elements can function as passive pathways, accelerating the geographic expansion of X. fastidiosa and increasing the vulnerability of surrounding regions. This is particularly concerning for the neighboring Demarcated Douro Region—a vital area for Portuguese viticulture and a globally recognized wine-producing zone. The potential incursion of X. fastidiosa into this area could result in severe economic losses and ecological disturbances, further emphasizing the urgency of preventive action.
A critical factor that exacerbates this risk is the dispersal capacity of the primary vector,
P. spumarius. Although traditionally classified as a weak flyer with limited mobility, recent studies by Bodino et al. demonstrated that individuals can disperse up to 400 m over a two-month period [
26,
27]. While this range may seem modest, it is significant when considered in conjunction with the “hitchhiker” hypothesis. According to this theory, vectors may be inadvertently transported over long distances via human activity, vehicles, or commercial shipments of plant material [
28]. Such unintentional long-range dispersal significantly expands the potential for pathogen spread, particularly across regions with active agricultural trade and movement.
In light of these findings, it becomes evident that stricter biosecurity protocols and enhanced phytosanitary oversight are essential. Comprehensive inspection systems, targeted monitoring at key transit points, and improved traceability mechanisms for plant movement must be prioritized. The data clearly suggest that the distribution of positive insects is not only influenced by the biological behavior of the vector but also by the scale and effectiveness of surveillance efforts. Equally important are the characteristics of the area itself, such as its size and land use patterns, as well as the presence and abundance of suitable host plants, which directly affect the establishment and proliferation of both P. spumarius and X. fastidiosa. Porto’s early designation as a DZ, its strategic location with high commercial connectivity, favorable host plant availability, and the intensity of its monitoring programs have made it an invaluable reference point for understanding the epidemiology of X. fastidiosa in Portugal.
In conclusion, while the data highlight a clear association between the intensity of monitoring and the number of positive insect detections, it is important to recognize that this relationship may be influenced by local infection pressure and vector abundance, rather than solely by sampling effort. The high number of detections in the Porto DZ likely reflects a combination of early surveillance implementation, favorable environmental conditions, higher host plant availability, and established vector populations. As such, Porto serves as a valuable case study for understanding the complex dynamics of X. fastidiosa presence and spread. To improve early detection and control of this pathogen, enhanced and consistent monitoring across all demarcated zones, alongside strengthened phytosanitary measures, will be essential.
3.2. Vector Presence Varies by Location Type
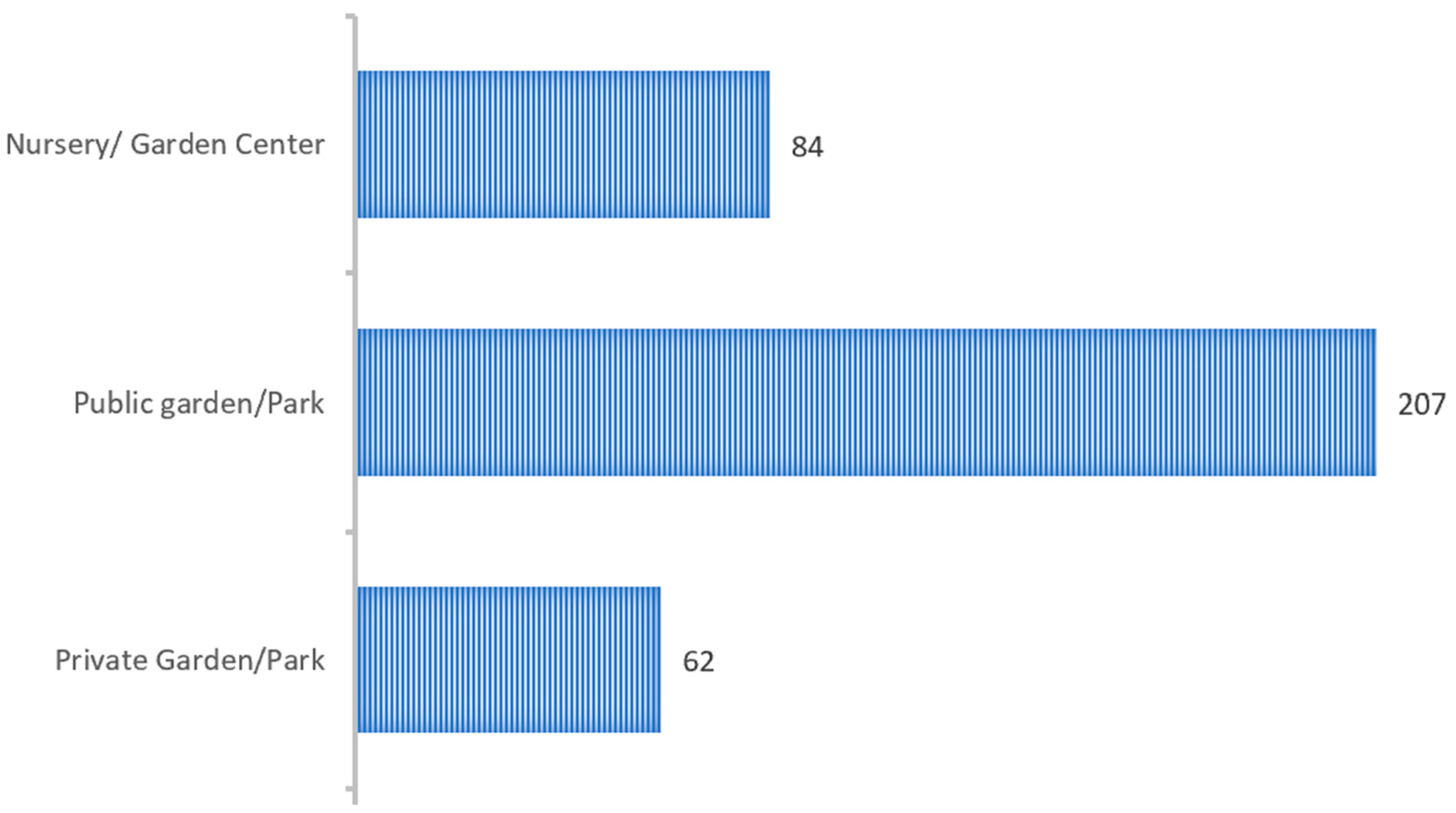
Figure 6 illustrates the distribution of insect detections across different types of prospected sites. The majority of
P. spumarius specimens were found in public gardens (58.6%), followed by nurseries or commercial gardens (23.8%) and private gardens (17.6%). Notably, no detections occurred in forested areas, likely reflecting the very limited sampling effort in these environments during the study period.
This distribution is strongly influenced by the guidelines issued by the national plant health authority (DGAV) [
21], which specify target locations for insect prospection within demarcated zones. Since the establishment of DZs, DGAV has prioritized systematic monitoring in structured environments such as nurseries and public gardens. These areas undergo more frequent inspections and intensified surveillance, contributing to the higher detection rates observed. Additionally, their anthropogenic nature provides favorable microclimatic conditions, such as shade, moisture, and plant diversity, that support the establishment and proliferation of
P. spumarius populations.
Public gardens, in particular, are rich in plant diversity, offering a wide array of nutritional resources, mating sites, and oviposition substrates [
29,
30]. The high number of
P. spumarius detections in these environments likely reflects not only greater monitoring effort but also their intrinsic ecological attractiveness to the species, rather than an unexpected overrepresentation. Public gardens, specifically, present particularly favorable conditions for
P. spumarius presence, including the absence of pesticide use, allowing insect populations to persist; high plant diversity, providing abundant food and shelter; low-disturbance environments, reducing impacts on soil and microhabitats; and presence of organic matter, creating ideal conditions for insect development.
Interestingly, nurseries also recorded significant
P. spumarius populations. This is consistent with findings from agricultural ecosystems such as the pergola-style vineyards in Trentino, Italy, where similar environmental conditions—namely, shaded canopy structures and frequent irrigation—create humid microclimates conducive to sustaining
P. spumarius populations [
31,
32]. However, it is worth considering that the progressive intensification of phytosanitary treatments in nurseries may be contributing to a recent reduction in vector abundance. These practices, although essential for controlling plant pathogens, may inadvertently suppress local spittlebug populations, highlighting the trade-offs between disease management and vector surveillance.
Despite the absence of insect findings in forested areas in our dataset, the literature indicates that such habitats are often associated with higher
P. spumarius presence [
31]. Forest ecosystems serve as refugia for adult spittlebugs, especially during periods of environmental stress or active crop management in more exposed areas. Adults may migrate into woodland zones when conditions in nurseries or gardens become suboptimal, for instance, during heatwaves or post-treatment recovery periods. Our study’s lack of sampling effort in these areas prevents definitive conclusions, but it suggests a crucial gap in current monitoring protocols. Increasing sampling frequency in forested environments could provide a more comprehensive understanding of the vector’s full ecological range and seasonal movement patterns.
Taken together, these results underscore the influence of both ecological and operational factors on vector distribution. The concentration of insect detections in public and cultivated spaces reflects not only favorable habitat conditions but also a targeted surveillance strategy shaped by regulatory guidelines. Nonetheless, expanding future prospection efforts to under-sampled habitats such as forests may yield valuable insights into P. spumarius behavior and population dynamics. This, in turn, would strengthen predictive models for X. fastidiosa spread and improve risk-based surveillance frameworks across all demarcated zones.
3.3. Seasonal Variation in Insect Presence by Trimester
Figure 7 illustrates the seasonal distribution of
P. spumarius individuals captured per trimester over multiple years. The highest number of specimens was recorded during the first trimester (36.5%), followed by the third (23.2%), fourth (23.0%), and second trimesters (17.3%).
According to the known biological cycle of
P. spumarius, adults typically begin to emerge between April and May, with mating occurring in early summer [
18]. During spring, nymphs are found in the herbaceous vegetation, while adults occupy the canopy from May through the summer months. In autumn, adults migrate back to the ground-level weeds or nearby vegetation for oviposition [
19,
33]. Based on the known life cycle of the insect, which includes egg laying near the ground followed by nymphal development and adult emergence in spring and early summer, peak adult activity would typically be expected during the second and third trimesters. These periods align with rising temperatures and increased insect mobility, often associated with feeding, mating, and dispersal across habitats. Surprisingly, our data show a greater number of adult individuals recorded during the first trimester—a pattern that diverges from conventional expectations. This unexpected peak raises several questions regarding the biology and behavior of the insect, especially under specific climatic or ecological conditions. Notably, a substantial proportion of the first-trimester adults were observed in 2019, accounting for 94 out of 133 individuals recorded in that period. Unfortunately, the developmental stage of these individuals was not systematically noted. This gap in data limits our ability to determine whether these were overwintering adults that had survived from the previous year, individuals that emerged prematurely due to unseasonably mild winter conditions or late-stage nymphs misidentified as adults.
Such uncertainty underscores the need for more detailed phenological monitoring, particularly during transitional periods like late winter and early spring. It also suggests that the insect may exhibit greater plasticity in its development and emergence timing than previously understood. Future studies should prioritize accurate stage identification and integrate environmental data to better interpret anomalies in seasonal population dynamics. This approach could enhance our understanding of how climate variability or habitat features influence the timing of adult emergence and, by extension, the insect’s potential as a disease vector.
Several factors may explain the discrepancy between expected and observed distributions. Climatic influences, sampling effort, habitat conditions, and solar radiation all may contribute to the unexpected pattern observed. Temperature and humidity are key environmental factors significantly influencing
P. spumarius development and emergence timing [
34]. Early spring warming or mild winter conditions may trigger premature adult emergence, leading to earlier-than-expected captures during first-trimester surveys. One plausible explanation is the presence of overwintering adults during this trimester. Long-term warming trends and altered precipitation regimes affect insect development, voltinism, and seasonal behavior [
34].
P. spumarius shows a flexible overwintering strategy, with adults capable of surviving winter under mild climatic conditions, particularly in Mediterranean regions where extreme cold is rare. Moreover, the species can delay oviposition until environmental conditions improve, enhancing adaptability to variable seasonal patterns [
32,
35]. Such conditions may have allowed more adults to persist through the first trimester without entering diapause or suffering high mortality.
Solar radiation also plays a crucial role by affecting plant growth, quality, and crop management practices [
36], indirectly impacting
P. spumarius, whose biology is closely linked to temperature and humidity [
32]. Local microclimates, such as south-facing slopes, areas with low canopy cover, or dense shrubland, can receive higher solar radiation and exhibit elevated soil temperatures, accelerating development [
37,
38]. In these habitats, nymphs may develop earlier, resulting in adult emergence prior to the general onset of spring warming. This phenomenon has been observed in Portugal, where first-instar nymphs have been detected as early as February and adult emergence recorded in April [
34].
According to Drosopoulos and Asche in Greece [
39], Godefroid and Durán in southern Spain [
40], and Karban and Strauss in California [
41], humidity and temperature strongly influence the development duration of immature
P. spumarius stages [
42]. Cooler and humid conditions are particularly favorable for egg hatching and larval development, typically between 4 and 10 °C [
42,
43]. For example, in colder regions, development to adulthood takes longer, with egg hatching beginning in April and adults appearing in June [
16]. In Mediterranean climates, nymphal stages are typically detected from the second week of March, with the first adults emerging between late April and early May [
44,
45]. In Portugal, Rodrigues et al. [
30] reported first-instar nymph sightings as early as February, with adult emergence in April. Adequate humidity is essential for hatching and nymph survival, as these stages depend on a consistent supply of xylem sap. Therefore, severe or prolonged summer droughts can significantly reduce population densities [
41,
46]. Neto et al. [
47] reported declines in
P. spumarius during summers with above-average temperatures and below-average precipitation. Under such conditions, the insect may migrate from desiccated vegetation to less water-stressed shrubs and trees or cooler microhabitats such as riparian zones [
46]. Our study’s results support this, as
P. spumarius populations declined markedly during the second and third trimester.
Climatic conditions in Portugal [
38] favor the proliferation of both
P. spumarius and
X. fastidiosa. However, climate change projections forecast rising temperatures, reduced precipitation, and more frequent extreme weather events. These shifts are expected to affect vector distribution and behavior, host plant physiology, pathogen transmission efficiency, and the complex interactions among vectors, hosts, and pathogens [
37].
In addition to climatic influences, variations in sampling effort and methodology likely affected observed patterns. The high number of insects collected in the first trimester of 2019 coincides with intensified sampling following the first X. fastidiosa detection in Portugal. Conversely, in subsequent years, several factors limited sample collection, including weekly collection limits imposed in 2020, COVID-19 lockdown restrictions in early 2021 (which restricted access to private gardens and properties), and delayed authorization of fieldwork in 2023 (only granted in August). Such constraints may have influenced specimen detection, especially during key periods of insect activity.
Changes in local plant communities or habitat conditions might also have impacted insect distribution. Vegetation removal, either due to containment efforts or pesticide application, could have reduced suitable habitats for
P. spumarius, affecting its distribution or detectability. Moreover, prolonged summer droughts may cause population declines, as insects seek refuge in more humid environments such as riverbanks or shaded plant cover [
26,
46]. A decrease in vegetation during the second trimester due to eradication measures in demarcated zones could have altered insect behavior or visibility, resulting in fewer captures.
The presence of
P. spumarius in the first trimester (with higher frequency from January to March compared to June) has epidemiological implications for
X. fastidiosa transmission. Early infections may not benefit from the “winter curing” effect—a phenomenon reported to improve plant recovery when inoculation occurs later in the season [
48]. Consequently, trees are better able to recover if infected later rather than earlier in the year. Thus, earlier inoculation reduces the likelihood of winter curing, increasing the threat posed by this bacterium to crops. Early-season vector presence may increase the risk of persistent infections, especially under changing climatic conditions [
37,
38].
The early-season presence and persistence of P. spumarius across variable climatic conditions highlight the species’ ecological plasticity. This adaptability may be further influenced by land use and agricultural practices modifying habitat structure and microclimates. Practices such as vegetation clearance or altered irrigation may inadvertently create conditions conducive to vector development. Natural refugia, like hedgerows, riparian zones, and unmanaged field margins, may serve as overwintering sites or microhabitats during adverse periods, facilitating seasonal population re-establishment.
Furthermore, climate-induced shifts in voltinism may result in longer activity periods or additional generations per year, substantially altering the seasonal dynamics of X. fastidiosa transmission by increasing temporal overlap between vectors and susceptible hosts. These patterns emphasize the need to integrate climatic, ecological, and phenological data into monitoring frameworks to improve early detection and risk assessment. Adaptive surveillance and habitat management will be essential to mitigate future outbreaks.
In summary, although P. spumarius‘s life cycle suggests peak activity in the second and third trimesters, the presented data reveal a more complex reality influenced by environmental variability, sampling bias, and potentially shifting phenological patterns. Future studies should systematically record developmental stages and expand temporal sampling to better understand these dynamics.
3.4. Presence of Insects by Host Plants
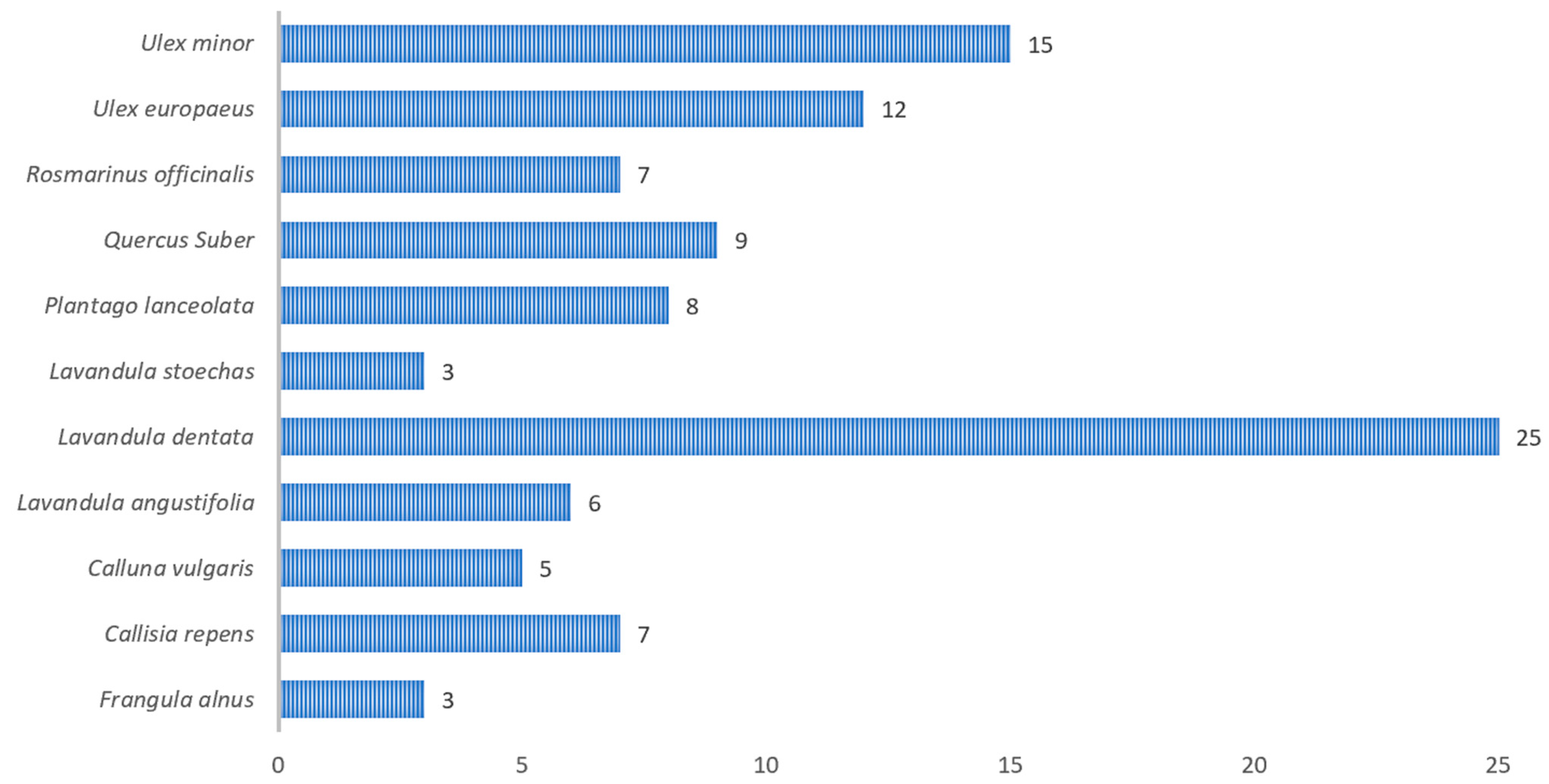
The results presented in
Figure 8 reveal that, out of a total of 353 captures analyzed,
P.spumarius was observed in only 217. These insects were found across different host plant species. This host–plant association data confirmed the extreme polyphagia of
P.spumarius. The most frequent host genders or species where insects were found included
Lavandula dentata (7%),
Ulex minor (4%),
Ulex europaeus (3.4%),
Quercus suber (2.6%),
Plantago lanceolata (2.3%),
Rosmarinus officinalis (1.9%),
Callisia repens (1.9%). This observation is consistent with the guidelines set forth by DGAV, which highlight the importance of monitoring species listed in Annex I and II of Implementing Regulation (EU) 2020/1201, including the species mentioned earlier [
22]. Nearly 65% of host plant species remained unidentified by the prospecting technicians.
Our findings suggest that
P. spumarius shows a marked preference for shrub plants and undergrowth vegetation, which provides important insights into its habitat selection and ecological behavior. Shrubs and undergrowth typically offer ideal conditions for the insect’s life cycle, supplying both shelter and food resources [
26,
49]. These plants, characterized by their compact structure and dense foliage, generate a humid, sheltered microhabitat that is critical for the insect’s survival during its early developmental stages, particularly the nymphal phase.
Shrub and undergrowth vegetation are commonly found across a range of environments, including agricultural lands, natural habitats, and urban areas, facilitating the insect’s ability to thrive in diverse ecological contexts and supporting its broad geographic distribution. The robust architecture of many shrub species, with thick branches and leaves, offers optimal feeding sites for both nymphs and adults while simultaneously providing protection against predators and adverse environmental conditions. Moreover, the undergrowth typically encompasses a high diversity of plant species, offering a variety of potential hosts. This botanical diversity complements the insect’s polyphagous behavior, allowing it to alternate between host plants in response to seasonal changes or nutritional demands.
Shrubs and undergrowth also play a vital role in the insect’s dispersal, serving as staging areas before it transitions into taller vegetation like trees or crops during its adult phase. From a pest management standpoint, this affinity for shrubs and undergrowth creates challenges in controlling insect populations, especially in areas where X. fastidiosa is prevalent. Shrubs commonly grow along field edges, in forests, and even in urban areas, granting the insect easy access to varied environments that are difficult to monitor and manage. Moreover, many shrub species are native or hold significant ecological value, complicating efforts to remove or control them in pest management strategies.
Understanding this preference can inform more effective control measures. Monitoring and pest control efforts should target not only crops or trees affected by X. fastidiosa but also the adjacent shrublands and undergrowth, where insect populations may breed and thrive unnoticed. Implementing vegetation management strategies, such as reducing undergrowth density in critical areas, could help limit suitable habitats for the insect and decrease its role as a vector for plant diseases.
In Italy, arid climates showed reduced spittlebug populations, as did rainfed olive groves in Greece, suggesting that spittlebugs prefer plants subjected to lower water stress [
50]. Popova et al. observed that
P. spumarius exhibited greater abundance on ground vegetation [
37]. In the study conducted by Neto et al., potential vectors were more frequently present on weeds [
51]. In Corsica,
P. spumarius seems to aggregate on
Cistus monspeliensis L. [
41]. Along the coast of California, there is a notable abundance of nymphs found on the seaside daisy,
Erigeron glaucus Ker Gawl [
41]. In Italian olive groves, particularly in the Apulian and Ligurian [
52,
53], as well as in Spain or Portugal [
26,
54], a preference of
P.spumarius for
Apiaceae, as well as for
Asteraceae and
Fabaceae [
50], has been observed. They were reported to preferentially inhabit
Foeniculum vulgare L. and
Galium album Mill [
55]. Our discoveries suggest a correlation among plant communities, ecological circumstances, and insect populations within the undergrowth, especially within the analyzed climatic conditions. This suggests that if
X. fastidiosa strains, which pose a threat to orchards, were introduced into this undergrowth, the transmission probabilities for both crop species would likely be similar.
Conversely, Bodino et al. revealed that populations of
P. spumarius were reduced on
D. viscosa covers, even further diminished on ground vegetation within the grove, and were smallest on crop foliage [
26]. Also, Cornara et al. noted a substantial abundance of the
P.spumarius species, indicating a distinct preference for
Quercus trees [
55]. Out of the total
P.spumarius adults collected, 56.9% were found on
Quercus trees, while
Prunus spp. (
P. avium L.,
P. serotina L.,
P. padus L.),
Cornus sanguinea L., and
Robinia P. spumarius eudoacacia L. each accounted for approximately 5–6% [
26]. Lopes et al. also found
P. spumarius in both
Olea europaea and
Vitis vinifera, in the Andalusia region, where the Mediterranean climate also predominates [
24]. The increased presence of spittlebugs in herbaceous or shrub vegetation during the first and third trimesters may be linked to their oviposition behavior, as they tend to lay their eggs near the ground. This stage is critical in the spittlebug’s biological life cycle, making it an optimal time for implementing control measures, which may prove more effective than at other times of the year [
26]. Emphasis should be placed on monitoring the ground vegetation, particularly plant families housing species susceptible to
X. fastidiosa. Findings from this research underscore the importance of closely monitoring plant families such as
Lavandula dentata,
Ulex minor,
Ulex europaeus, which can serve as favorable sites for oviposition.
Dongiovanni et al. (2019) also indicate that unmonitored nymph populations may lead to a higher incidence of adult emergence, facilitating the acquisition and transmission of
X. fastidiosa. They recommend timely soil tilling during the peak nymphal population to mitigate adult emergence [
54].
In our study, vineyards were not the focus of investigation. However, Rodrigues et al. identified
P. spumarius as the most prevalent spittlebug species in vineyards in Portugal, a finding consistent with other studies conducted in vineyards across Europe and California [
26,
30,
53,
56]. Bodino et al. found that
P. spumarius adults were notably abundant on grapevines during late spring (May–June) [
26]. Furthermore, Avosani et al. and Whittaker et al. argue that
P. spumarius predominates as the primary spittlebug species discovered on grapevines, particularly in mid-June and across both herbaceous and woody plants within and surrounding vineyard areas [
31,
57].
This study did not sample or identify many vegetation types, indicating that crucial associations between insects and various plant species may have been overlooked. This gap suggests that significant interactions that could enhance our understanding of X. fastidiosa vectors in ecosystems might have been missed. Research in this area is still underdeveloped, particularly regarding spittlebug preferences during the summer months.
Our findings highlight specific plants, including Lavandula dentata, Ulex minor, Quercus suber, Plantago lanceolata, and Rosmarinus officinalis, as the most common hosts. These plants offer favorable conditions, such as abundant sap, sufficient shelter, and opportunities for reproduction, making them particularly attractive to P. spumarius. Although each species constitutes only a small fraction of the total, the diversity of host plants underscores the polyphagous nature of P. spumarius.
The wide range of host plant species documented in this study illustrates the extreme polyphagia of P. spumarius, providing valuable insights into its ecological behavior. This adaptability allows it to exploit various plants for nourishment, reproduction, and shelter, enabling it to adjust to different ecological conditions. Its ability to feed on multiple plant species (equi P. spumarius P. spumarius) allows it to thrive in heterogeneous landscapes where the availability of preferred hosts may fluctuate due to seasonal, environmental, or anthropogenic factors. This adaptability is closely tied to its role as a vector for X. fastidiosa. By feeding on a diverse array of plants, P. spumarius facilitates the transmission of the pathogen among different hosts, contributing to the spread of the disease in both cultivated and wild plant communities. The insect’s capacity to switch between various plant species creates an extensive transmission network for X. fastidiosa, complicating containment efforts.
In agricultural settings where X. fastidiosa poses a significant threat, the polyphagous behavior of P. spumarius allows the bacterium to infect a wide range of economically important crops and wild plants, thereby expanding the disease’s reach and impact. Furthermore, P. spumarius’s resilience to environmental changes, stemming from its flexible feeding habits, makes population management particularly challenging. Even when specific plant species are targeted for removal, P. spumarius can easily migrate to other available hosts, ensuring its survival and ongoing role as a vector. This adaptability means that shifts in environmental conditions, such as changes in plant composition due to climate change or habitat alteration, are less likely to disrupt P. spumarius population dynamics, allowing it to persist and spread across various ecosystems, from agricultural fields to natural landscapes, thereby increasing its threat to both cultivated and native plant species.
In conclusion, our study highlights the critical role that shrubs and undergrowth vegetation play in the ecology of P. spumarius and its interaction with Xylella fastidiosa. Understanding the insect’s preference for these habitats and host plants is vital for improving pest management strategies. Targeting these areas, in addition to crops and trees, could significantly reduce the spread of the pathogen, considering the adaptability of P. spumarius to different host plants, its ability to switch between them, and its resilience to environmental changes complicate containment efforts. A more integrated approach to pest management, encompassing a wide range of potential host plants and environmental factors, is essential for mitigating the impact of P. spumarius on agricultural and natural ecosystems.