Abstract
The COVID-19 pandemic prompted widespread adoption of intensified hand hygiene practices, raising concerns about their medium-term impact on the skin microbiome. This study investigates alterations in the hand microbiome of healthy adults during the pandemic compared to pre-pandemic periods in Majorca, Spain. A total of 30 volunteers (16 women, 14 men; mean age 44.1 ± 8.8 years) were sampled between 2014 and 2021. Palm swabs were collected following WHO guidelines, alongside measurements of skin pH, temperature, and handwashing frequency. Bacterial DNA was extracted and analyzed via 16S rRNA (V3-V4) metagenomic sequencing to assess microbial diversity and composition. Results revealed a significant decline in microbial diversity during the COVID-19 period, accompanied by a marked shift in the community structure. The Firmicutes phylum dominated, with Bacillales increasing from 30.7% to 84.1%, primarily driven by a surge in Staphylococcus species (e.g., S. pasteuri). Conversely, S. hominis and Actinomycetales nearly disappeared. No significant associations were observed with gender or handwashing frequency. The skin temperature increased during the pandemic, while the pH remained stable. The Staphylococcus/Bacillus ratio shifted significantly, favoring Staphylococcus dominance. These findings, derived from a geographically limited population in Majorca, Spain, demonstrate that stringent hygiene measures during COVID-19 reduced microbial diversity and restructured hand microbiome composition. The study underscores the necessity for balanced hygiene strategies that mitigate pathogen transmission while preserving beneficial microbial communities critical to skin health.
1. Introduction
The human skin microbiome plays a crucial role in maintaining skin health, providing a barrier against pathogenic microorganisms, and modulating the host’s immune response [1,2,3]. Skin bacteria can interact with each other in a variety of ways, including the production of chemical signals that can promote or inhibit the growth of other species. These interactions can be both cooperative and competitive and can influence the structure and dynamics of the dermal microbiome.
Such competition between skin bacteria can have implications for skin health. For example, an imbalance in the dermal microbiome, caused by the overgrowth of certain bacterial species or the loss of microbial diversity, can contribute to the development of skin diseases such as acne, dermatitis, psoriasis, and other dysbiosis. Interactions between skin bacteria are an important area of research in the field of the dermal microbiome [4,5,6].
The composition and diversity of the skin microbiome are influenced by various factors, including age, sex, environment, hygiene practices, and health status. Among the different regions of the skin, the hands are of particular interest due to their frequent contact with various surfaces, making them a primary site for microbial exchange and transmission [7,8]. In fact, surfaces in contact with food or those that are critical in the clinical environment are easily colonized by microorganisms from the personnel coming into contact with them. These microorganisms adhere and group together on the surfaces, forming stable biofilms with the consequent risk of harboring pathogens [9,10]. In this context, it is important to consider the specific characteristics of the palmar skin microbiome. Compared to other body sites, the hands harbor a more dynamic and environmentally exposed microbial community, characterized by higher temporal variability and a greater proportion of transient species. In healthy individuals, the predominant skin phyla include Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes [11].
Additionally, during the COVID-19 pandemic, the widespread use of antibiotics, both prescribed and self-administered, may have indirectly affected the composition of the skin microbiome. Although not extensively studied, this factor—combined with the increased frequency of handwashing and sanitizer use—may have altered skin microbial communities in previously unrecognized ways.
The outbreak of the COVID-19 pandemic in late 2019 led to unprecedented global public health measures, including increased hand hygiene practices such as frequent handwashing and the use of alcohol-based hand sanitizers [12,13,14]. These interventions were aimed at reducing the transmission of the SARS-CoV-2 virus but also had potential secondary effects on the skin microbiome. Studies previous to the pandemic had already shown that frequent hand hygiene can significantly alter the microbial communities on the skin, leading to changes in both the diversity and composition of the microbiome [15,16,17,18].
Understanding the impact of these hygiene practices on the skin microbiome is essential, as disruptions in the microbial community can have implications for skin health and disease susceptibility. A few studies revealed intestinal microbiome modifications in the pandemic [19,20]. However, there is a lack of longitudinal studies examining the medium- to long-term effects of these practices on the hand skin microbiome. To our knowledge, the only available information was published this year by Vindenes’ group, with data from the RHINESSA study population in Bergen, Norway [21].
In this study, we aimed to fill this gap by analysing the longitudinal changes in the skin microbiome of the hands from the pre-COVID era (2014–2019) to the COVID period (2020–2021) in Majorca, Spain. Our study provides valuable insights into the effects of prolonged hand hygiene practices on the skin microbiome, highlighting significant changes in microbial diversity and composition during the COVID period. These findings have important implications for understanding the long-term consequences of hygiene interventions and for developing strategies to maintain a healthy skin microbiome in the context of increased hand hygiene.
2. Materials and Methods
A total of 30 students and staff (16 females and 14 males), with a mean age of 44.1 ± 8.8 (age ranging from 24 to 64 years), from the Management Hotel School of the Balearic Islands (EHIB, Spain) participated in the present investigation from 2014 to 2021. The experiments were approved by the Ethics Committee of Research of the University of the Balearic Islands [Comité Ético de Investigación (CER)] and conducted in accordance with the institutional, local and national guidelines and regulations. Written informed consents were obtained from the participants for the publication of the data included in the manuscript. As for the inclusion criteria, while no formal medical screening was conducted, participants self-declared their general health status and were pre-evaluated visually to exclude those with dermatological conditions. All participants were healthy, had not used any antibiotics during the last 15 days, and did not have any skin lesions on their hands at the time of sampling.
2.1. Sample Collection
Before sampling, demographic and individual hygiene-related data were recorded from each participant, including age, gender, and self-reported hand hygiene habits (specifically, the average daily frequency of handwashing). In addition, immediately prior to hand washing, the skin pH and surface temperature of hands were measured using a pH meter equipped with a flat-surface skin electrode (Skincheck, Hanna), and a non-contact infrared thermometer (MiniTemp MTL, Labprocess, Madrid, Spain), respectively.
Samples were taken after explaining the proper handwashing protocol to the participants, always in the same manner. The WHO recommendations were followed during the process [22]. Neutral soap was used, and hands were dried with a sterile gauze. Sterile EUROTUBO Collection swabs (Deltalab, Barcelona, Spain) moistened in PBS were used for sampling. The swabs were rubbed on the palms of both clean hands for 20 s, repeating the moistened smear four times for better sampling. The swab head was aseptically transferred into a 1.5 mL sterile tube using sterile tweezers. To remove as many bacteria as possible from the swab, it was vigorously shaken three times in a vortex, immersing it in a PBS solution. After sampling, the sample was stored at −20 °C with 20% glycerol.
2.2. Sample Processing and Nucleic Acid Extraction
The extraction of bacterial DNA was performed using a Pure Link Microbiome DNA Purification kit (Invitrogen (Carlsbad, CA, USA), Thermo Fisher (Carlsbad, MA, USA)). For sequencing, the bacterial DNA was sent to the Macrogen Inc. Genome Center (Seoul, Republic of Korea). Metagenomic analysis of the 16S amplicon was performed. The primers used were 337F (GACTCCTACGGGAGGCWGCAG) and 800R (TACCAGGGTATCTAATCC), amplifying the V3-V4 regions of the 16S rRNA with paired-end read type on a Miniq Illumina System.
2.3. Bioinformatic Analysis
The raw data sent by Macrogen, in fastq format, was subjected to a data hygiene process. This involved trimming off primer sequences and low-quality bases at read ends, joining paired-end reads, and removing chimeras. These sequence hygiene processes and the taxonomic classification of the amplicon sequence variants were carried out on an MG-RAST platform [23], selecting the Silva SSU database [24,25] for the taxonomic study. The calculation of alpha-diversities was conducted based on the Fisher’s alpha, Shannon index, and Simpson index to estimate the average diversity of each sample. Beta-diversities were also calculated to study the rate of species change in the different amplicons. To calculate these diversities, the Past4project application from the University of Oslo was used [26].
2.4. Statistical Methods
GraphPad Prism software v. 8.0.2 was used for statistical analyses. Differences with a p < 0.05 were considered significant. Differences in temperature, pH, age, hand washing and alpha diversity were tested using the unpaired t test after Anderson–Darling and Lilliefors tests to assess normality, whereas the differences in beta diversity were analysed by PERMANOVA [27] using the Past4project application. Comparison of taxonomic composition at different levels was performed by a Mann–Whitney test.
3. Results
3.1. Participant Characteristics and Hand Physiology
The study included 30 participants (16 women and 14 men). No significant differences in alpha diversity (Fisher’s alpha, Shannon, and Simpson indices) were observed between male and female participants (Figure S1). Similarly, beta diversity did not differ significantly by gender (Figure S2). The frequency of handwashing (<10 vs. ≥10 times/day) also showed no significant effect on either alpha or beta diversity (Figures S3 and S4).
The mean age of the participants was 44.1 ± 8.8 years (range: 24–64 years), with no significant difference between the pre-COVID (41.9 ± 9.8 years) and COVID (44.0 ± 9.0 years) periods (Figure S5a).
Interestingly, the average hand temperature was significantly lower in the pre-COVID period (33.6 ± 1.3 °C) compared to those collected during the COVID period (35.1 ± 2.1 °C, Figure S5b). In contrast, no significant differences in skin pH were observed: the mean skin pH was 4.92 ± 0.32 in pre-COVID samples and 4.63 ± 0.46 during the COVID period (Figure S5c).
3.2. Sequencing Results
A total of 30 skin samples were collected from 30 adult volunteers. The rarefaction curve flattening indicates sufficient species richness, as shown in Figure 1. The total number of reads per sample in the pre-COVID and COVID periods ranged from 0.20 to 0.42 M and from 0.16 to 0.39 M, respectively. Additionally, each sample in the pre-COVID and COVID periods had between 0.18 to 0.38 M and 0.15 to 0.35 M high-quality microbial reads, respectively. This represented 88.0–93.4% and 87.3–93.6% of the total reads sequenced, respectively.
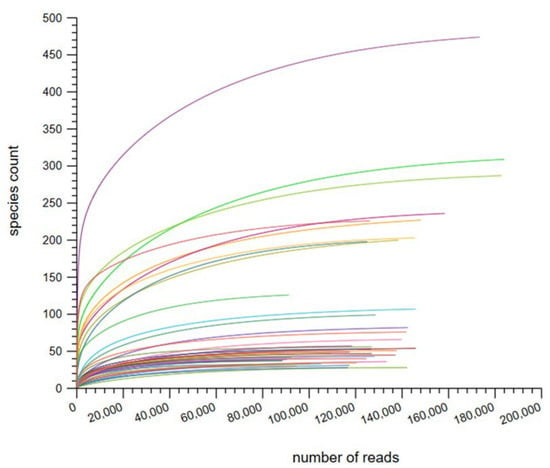
Figure 1.
Rarefaction curves based on species count per sample.
Analysis of high-quality microbial reads, excluding human data, revealed that the microbiological data of the total skin samples in the pre-COVID and COVID periods were predominantly from bacteria (99.7% ± 0.5% and 98.5% ± 5.1%, respectively). Sequences that corresponded to viruses or eukaryotic organisms, as well as those that remained unclassified, were discarded prior to analysis.
3.3. Changes in Microbial Community Diversity in the Pre- and COVID Periods
3.3.1. Alpha Diversity of Microbial Community
Three different indices were used for the study of alpha diversity (Figure 2): (i) the Fisher’s alpha index, which describes the relationship between the number of categories and the number of individuals of those categories; (ii) the Shannon index, which shows the richness and evenness of the categories distribution; and (iii) the Simpson index, which is a measure of relative abundance.
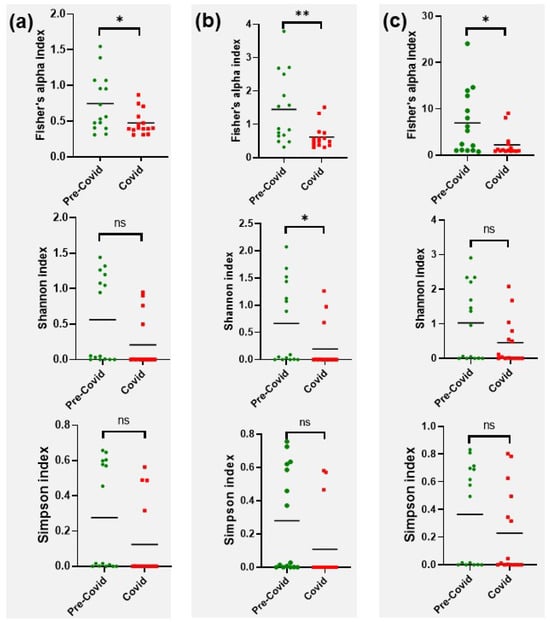
Figure 2.
Comparison of hand microbiome alpha diversity at pre-COVID versus COVID. (a) Phyla. (b) Orders. (c) Genera. *, p<0.05; **, p<0.001; ns, not significant.
Those indices were applied to phylum, order and genus taxa. Fisher’s alpha values were clearly lower in the COVID samples, with a significant reduction at all levels (p = 0.021 for phylum, p = 0.007 for order and p = 0.021 for genus). The Shannon and Simmons indices were also lower in the COVID samples, although significant differences could only be established when the Shannon index was applied to orders (p = 0.045).
3.3.2. Beta Diversity of Microbial Community
The beta diversity (differences between samples) of the microbiomes was determined using principal coordinates analysis (PCoA). The study of this beta diversity showed significant differences between pre-COVID and COVID samples (p = 0.009). We observed similarity and clustering in many of the COVID samples, which translates into similarity in the variability of species. However, the distribution for pre-COVID samples was more heterogeneous, and the samples could not be grouped in the same pattern of similarity. In fact, two different subgroups were observed (Figure 3).
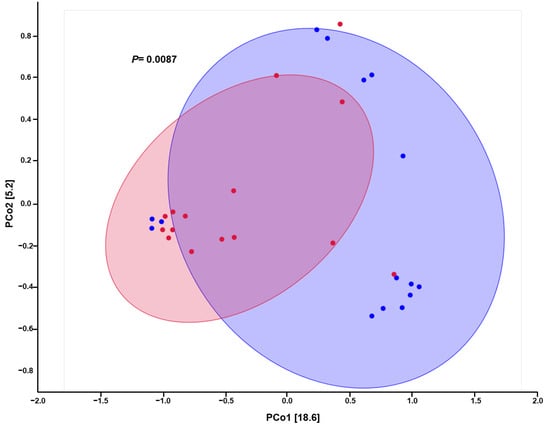
Figure 3.
Principal coordinate analysis (PCoA) plot for comparison of hand microbiome composition in pre-COVID (blue) and COVID (red) periods.
3.4. Microbial Composition Analysis
The bacterial composition of the samples collected from hand surfaces was analysed at different taxa levels. For this, percentage values for taxonomic groups were averaged across all participants within each period (pre-COVID and COVID). The phyla with a relative abundance greater than 0.1% are presented in Figure 4a. Differences in the composition of the microbiota in pre- and COVID groups were observed. The former consisted predominantly of bacteria belonging to phyla Firmicutes (35.7%), Proteobacteria (32.9%) and Actinobacteria (28.5%) and with a minimal representation of Bacteroidetes (2.1%), Fusobacteria (0.2%), Chloroflexi (0.2%), Verrucomicrobia (0.1%) and Cyanobacteria (0.1%). The latter was mainly constituted by Firmicutes (92.6%), while the levels of the other two main phyla decreased significantly (p < 0.01): Proteobacteria (6.8%) and Actinobacteria (0.2%).
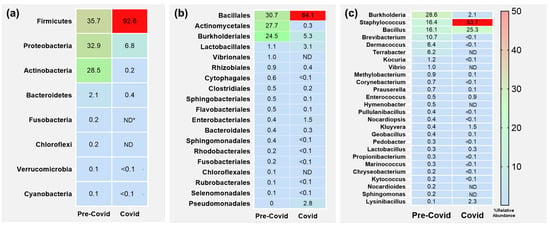
Figure 4.
Heatmap of relative abundance in hand microbiome at different taxa levels. Only taxa with values higher than 0.1% are shown. (a) Phyla. (b) Orders. (c) Genera. * ND, not detected.
We further studied the variability at the order level (Figure 4b). The important alterations observed at the phyla level were also reflected in this case. Thus, the most prevalent orders in pre-COVID samples were Bacillales (30.7%), Actinomycetales (27.7%) and Burkholderiales (24.5%), with minorities including Lactobacillales, Vibrionales and Rhizobiales with levels around 1% or even lower. However, for the COVID samples we found again that members belonging to one single group, in this case Bacillales (84.1%), dramatically increased their levels (p < 0.001). Furthermore, none of the other orders showed levels higher than 6%. The most abundant were Burkholderiales (5.3%), Lactobacillales (3.1%), Pseudomonadales (2.8%) and Enterobacterials (1.5%), the last two increasing compared to pre-COVID levels. Remarkably, the members of the Actinomycetales, the most abundant in the pre-COVID samples after the Bacillales, virtually disappeared.
Finally, we compared the different genera present in pre- and COVID samples (Figure 4c). The most predominant genera in the pre-COVID samples were Burkholderia (28.6%), Staphylococcus (16.4%), Bacillus (16.1%), Brevibacterium (10.7%), Dermacoccus (6.4%) and Terrabacter (6.2%). As expected from the previous data, an important increase in Firmicutes genera was observed in the COVID samples, with a significant increase (p = 0.01) in Staphylococcus (53.7%) standing out above the rest, while the increase for Bacillus was not statistically significant (p = 0.35). On the contrary, significant decreases in Burkholderia (2.1%, p < 0.0001) and Brevibacterium (<0.1%, p = 0.04) were observed.
Our first approach clearly indicates that Firmicutes became predominant in the COVID period. Thus, we carried out a deeper investigation of this group. The study of the majority phylum Firmicutes revealed the absolute predominance of the Bacillales order (Figure 5a), with similar levels in both periods. Then, we analysed the genera within the Bacillales order (Figure 5b), where we detected a significant shift between the two main groups: Staphylococcus and Bacillus. The values of both groups were similar in the pre-COVID period (around 48%), but the staphylococci increased their presence in the COVID period up to 60.4%, subsequently decreasing the Bacillus to 28.4%. Furthermore, Brevibacillus also increased to 8.4% in the COVID period. Finally, we investigated the evolution of the species in the Staphylococcus genus. The four detected species in the pre-COVID period, ordered by prevalence, were S. epidermidis, S. hominis, S. aureus and S. pasteuri (Figure 5c). However, in the COVID period a significant increase in S. pasteuri (p = 0.001) was observed, while S. hominis virtually disappeared.
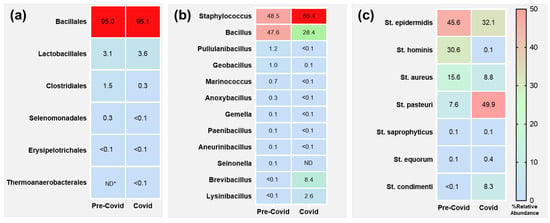
Figure 5.
Heatmap of relative abundance of Firmicutes members in hand microbiome at different taxa levels. Only taxa with values higher than 0.1% are shown. (a) Orders. (b) Bacillales genera. (c) Staphylococcus species. * ND, not detected.
4. Discussion
In this investigation, we analysed the longitudinal changes in the skin microbiome of hands from the pre-COVID era to the COVID period. The results show a decrease in microbial diversity in the COVID period, with a clear concentration in a few bacterial groups. This was somehow expected, as hand hygiene measures were clearly improved in the later period. However, these findings contrast with Vindenes et al. [22], who observed an increase in bacterial diversity when they compared pre-COVID and COVID hand skin microbiomes.
To our knowledge, studies like this, examining the evolution of hand skin microbiome in the medium or long term, including the COVID period, are scarce. The main phyla detected in the pre-COVID samples were Firmicutes, Proteobacteria, Actinobacteria and Bacterioidetes. This agrees with previous studies, where those four groups are always in the top four [11,28]. However, in our study the levels of Proteobacteria are slightly higher than those for Actinobacteria, contrary to previous observations. The fact that in most of those studies the dorsal part of the hand was sampled, whereas in our case only the microbiome from the palm was recovered, may be the probable explanation. Furthermore, when only the palm microbiome is considered, Proteobacteria becomes the predominant phyla [29,30]. The results for the COVID period were more unexpected, showing dramatic changes in phyla distribution. The bacteria present in the hand microbiome concentrated in the Firmicutes phylum, with more than 90% of individuals belonging to this group. Consequently, an important decrease was observed for the rest of the phyla, the Actinobacteria case, which virtually disappeared, being especially significant. Our results contrast with those of Vindenes et al. [21], who reported no significant changes in richness or diversity in hand microbiota between the pre-COVID and COVID periods. However, several methodological and conceptual differences may account for this discrepancy. First, Vindenes et al. assessed short-term, immediate microbial changes before and after a single handwashing or sanitizing event, while our study focuses on medium-term effects, evaluating community-level changes during a period of weeks in which hygiene practices became more frequent and intense. This broader perspective allows us to capture the potential cumulative effects of increased use of alcohol-based sanitizers and disinfectants on the resident microbiota. Second, our study applied a standardized handwashing protocol prior to sampling, minimizing the impact of transient flora and improving the reproducibility of microbiome profiles. In contrast, Vindenes et al. did not control handwashing before disinfection, potentially sequencing both viable and non-viable microbiota, since 16S metagenomics cannot distinguish between live and dead cells. This may partly explain the lack of observed changes in their sanitizer experiment. Interestingly, Vindenes et al. did report significant beta-diversity changes in their handwashing comparison, which supports the idea that repeated hygiene practices can produce measurable shifts in microbial communities. Therefore, the substantial changes observed in our study may reflect the longer-lasting impact of sustained behavioural and environmental changes during the COVID period, rather than the immediate effects of isolated hygiene events.
The distribution of bacteria at the order level also reflected this behaviour. More than 80% of the detected bacteria in the COVID period were assigned to the Bacillales order, belonging to the Firmicutes phylum. A critical decrease in the rest of the orders was observed, with the exception of Lactobacillales (Firmicutes), Enterobacteriales and Pseudomonadales (Proteobacteria). The increase in Pseudomonadales levels was previously described in Vindenes’s study [21]. Furthermore, the levels of Burkholderiales in the pre-COVID period were around 25%, something unexpected that explains the high levels of Proteobacteria. This fact was also present at the genus level: Burkholderia was the most prevalent genus in the pre-COVID period, followed by Staphylococcus, Bacillus, Brevibacterium, Dermacoccus and Terrabacter. However, in the COVID period, bacterial composition shifted to genera belonging to Bacillales, i.e., Staphylococcus and Bacillus. In the pre-COVID literature, we found that, in contrast with our observations, the predominant genera in hand skin microbiomes are Propionibacterium, Streptococcus, Staphylococcus and Corynebacterium [21,31]. Several factors may explain these differences, such as the inter-individual heterogeneity of bacteria, or the age, gender or occupation of the volunteer [17,29,32]. Differences in microbiome composition also vary according to geographical area [33,34]. For instance, Micrococcus was present in studies from Norway [21,31], whereas Moraxella and Burkholderia were detected in the USA [16]. Fewer data for the COVID period are available. Streptococcus, Staphylococcus, Micrococcus and Corynebacterium were common in Norwegian and Chinese investigations [21,31,35]. However, geographical differences were also present: Propionibacterium and Dermacoccus were present in Norway but not in China, whereas the genera Cutibacterium, Gardenella and Moraxella, with an important presence in Chinese microbiomes, were not detected in Norway [21,35].
The reduction in bacterial diversity in the COVID period, focusing on the Firmicutes phylum, was surprising. For this reason, we decided to perform a more detailed analysis of that group. As explained above, the main order was Bacillales (95%), with some presence of Lactobacillales (3.1%) and Clostridiales (1.5%) members. No significant differences were observed in the COVID samples for these orders. Interestingly, the analysis of the Bacillales genera showed changes in the two main genera, Staphylococcus and Bacillus. Their levels were similar in the pre-COVID samples (48.5 and 47.6%, respectively), but Staphylococcus levels clearly increased in the COVID samples (60.4%), with a subsequent decrease in Bacillus (28.4%). Staphylococci are known to survive in dry environments. Therefore, the increasing use of alcohol-based hand sanitiser during the COVID period may be a possible explanation for the observed predominance. However, Bacillus species are also able to survive in such dry environments. Therefore, why did their presence decrease in the COVID period? Bacillus is a ubiquitous genus commonly found in water, soil, and various environmental surfaces [36], and is typically more prevalent in samples collected under normal circumstances. However, the pandemic prompted widespread implementation of enhanced hygiene practices, including frequent handwashing, surface disinfection, and reduced physical contact. These measures likely contributed to a reduction in environmental contamination and human exposure to Bacillus. Consequently, samples collected during the COVID period showed lower levels of Bacillus, reflecting the impact of these comprehensive sanitation efforts. This phenomenon underscores the significant role of environmental and personal hygiene in modulating the presence of microbial communities in our surroundings.
Finally, we focus on the Staphylococcus genus. Our analysis revealed important changes in species composition. S. epidermidis and S. hominis were the main species in the pre-COVID period, followed by S. aureus and S. pasteuri. However, in COVID samples a significant increase in S. pasteuri levels (from 7.6 to 49.9%, p = 0.001) stood out. In fact, this increase contrasts with the virtual disappearance of S. hominis (from 30.6 to 0.1%). S. hominis is a habitual member of human skin microbiome, whereas S. pasteuri is usually found in food products as well as naturally occurring in air and on surfaces, being a rare cause of human disease [37]. However, the reason for these substitutions remains unknown. The selective pressure that Bacillus, with a lower presence in COVID microbiomes, may exert on those species, and the lower concentration of other staphylococci species, could be an explanation.
The microbial imbalance observed during the COVID period may have relevant implications for skin health and immune regulation. A growing body of evidence supports the theory that commensal skin bacteria actively contribute to maintaining the skin’s physical and immune barrier by producing antimicrobial compounds and modulating host responses [38,39,40,41]. For example, Staphylococcus epidermidis has been shown to secrete lipoteichoic acid (LTA), which can inhibit the production of pro-inflammatory cytokines by keratinocytes, thereby reducing skin inflammation [42]. Similarly, Propionibacterium acnes synthesize short-chain fatty acids from sebum components, which not only limit the colonization of pathogens but also influence immune activity [43].
Different skin microbes can stimulate or suppress specific immune pathways, participating in T cell differentiation and shaping antigen presentation [44]. Some species confer protection, while others may promote inflammatory processes or opportunistic colonization. The near disappearance of S. hominis, a known commensal with antimicrobial properties, parallel with the overrepresentation of S. pasteuri, a less-characterized species typically found in food or environmental sources, raises questions about potential dysbiosis. Likewise, the loss of Actinomycetales, a taxon that includes both commensals and opportunistic pathogens, may alter the skin’s ability to regulate microbial interactions and defense [45,46].
In order to further explore potential factors influencing microbial shifts, we considered several physiological and behavioral variables. The mean age of participants did not differ significantly between periods, minimizing the influence of age as a confounding variable. Similarly, neither gender nor frequency of handwashing showed significant associations with microbial diversity, suggesting that the general behavioral context rather than individual hygiene frequency may have played a more prominent role. Interestingly, we observed a significantly higher hand temperature during the COVID period, which may have influenced microbial dynamics by favoring thermotolerant species such as Staphylococcus. In contrast, skin pH values remained relatively stable, indicating that the acid–base balance did not contribute substantially to the observed microbiome alterations.
It is well established that skin microbiota constantly interacts with personal and environmental surfaces, forming a dynamic network of microbial exchange. Disruption of this balance—whether through increased hygiene, environmental changes, or reduced microbial inputs—could reduce ecological resilience and facilitate colonization by external or non-resident species [47]. Although no clinical symptoms were assessed in this study, the shifts observed in community composition suggest that extended periods of intense hygiene may influence the host-microbe equilibrium with potential long-term consequences for skin homeostasis.
These microorganisms of the dermal resident microbiota encounter new habitats beyond the skin, such as surfaces in contact with food, where their ability to adhere, resist environmental stresses, and form biofilms plays a vital role in survival and persistence. This ecological aspect extends the relevance of our findings, linking skin microbiome dynamics with potential impacts on food safety. The formation of biofilms by skin-associated bacteria and their interaction or competition with foodborne pathogens is an active area of research in our laboratory, which aims to further elucidate these complex microbial relationships.
The strengths of the present study include the long follow-up time and the use of standardized sampling methods, ensuring that all the participants equally performed their hand-washing. Most studies focus on the dorsal region of the hand, while our investigation sampled the palm region, which plays a crucial role in bacterial transmission to fomites, foods and/or other people. The diversity among the volunteers, ensuring that no one had skin problems or was taking any treatment, and the equal representation of both sexes are also aspects of interest. The fact that data on microbiomes in the COVID era are scarce and that no study has been performed in our country represents an interesting aspect of our investigation. However, the study also has important limitations that should temper the generalization of the findings. The relatively small sample size limits its statistical power and may affect the robustness of conclusions. Furthermore, all participants were recruited from a single geographical area and specific institutional setting in Majorca, Spain, which may restrict the applicability of the results to broader populations with different environmental, occupational, or cultural contexts. External factors such as regional climate, lifestyle, and age-related variables may also influence hand microbiota composition but were not fully controlled in this study. Therefore, our conclusions should be interpreted with caution and regarded as applicable primarily to populations with similar characteristics and hygiene practices. Further studies with larger, more diverse cohorts are warranted to confirm and extend these findings.
5. Conclusions
Our longitudinal study provides valuable insights into the effects of enhanced hygiene practices during the COVID-19 pandemic on the hand skin microbiome. We observed a marked decrease in microbial diversity in the COVID period, with a notable concentration of bacteria in the Firmicutes phylum, particularly the genus Staphylococcus. These changes contrast with pre-COVID samples, which exhibited a more diverse and evenly distributed microbial community.
The observed shift in microbial composition suggests that the stringent hand hygiene measures implemented during the pandemic, such as frequent hand washing and the use of alcohol-based sanitizers, significantly impacted the skin microbiome. The predominance of Staphylococcus, especially S. pasteuri, during the COVID period, along with the virtual disappearance of S. hominis, may reflect selective pressure favouring bacteria adapted to dry, sanitized environments.
These findings may have important implications for understanding how public health measures can alter microbiomes and potentially affect skin health and resilience. While enhanced hygiene practices are crucial for preventing infectious diseases, their impact on the natural microbial communities that play a role in skin barrier function and immune responses warrants cautious consideration and further investigation.
Future studies with larger, more diverse populations are needed to validate our findings and explore the long-term effects of altered hand hygiene practices on the skin microbiome. Additionally, research focusing on the functional consequences of these microbial shifts, including their effects on microbial interactions and skin health outcomes, is critical. Overall, our study underscores the importance of a balanced approach to hygiene practices that considers both the benefits of pathogen reduction and the preservation of a healthy and diverse skin microbiome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16070144/s1, Figure S1: Comparison of hand microbiome alpha diversity in male versus female. (a) Phyla. (b) Orders. (c) Genera. ns, not significant; Figure S2: Principal coordinate analysis (PCoA) plot for comparison of hand microbiome composition in male (blue) and female (pink); Figure S3: Comparison of hand microbiome alpha diversity for high hand washing (≥10 times per day) versus low hand washing (<10 times per day). (a) Phyla. (b) Orders. (c) Genera. ns, not significant; Figure S4: Principal coordinate analysis (PCoA) plot for comparison of hand microbiome composition in high hand washing (≥10 times per day, green) versus low hand washing (<10 times per day, orange); Figure S5: Comparison of age (a), hand temperature (b) and pH (c) in participants sampled in the pre-COVID period versus those from the COVID period. * p < 0.01; ns, not significant.
Author Contributions
C.T.: Data curation, Methodology, Investigation, Writing—Original draft preparation. A.D.-S.: Conceptualization, Methodology, Writing—review and editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Research of the University of the Balearic Islands (protocol code CER201/14, approved 23 April 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The sequences are available at the MG-RAST server: https://www.mg-rast.org/mgmain.html?mgpage=token&token=YNbDZacyoJzJhkCg7mYf_DEsBmR4LU7cf3ifGtWseF72rePfm9 (accessed on 16 May 2025) should be used for pre-COVID period, and https://www.mg-rast.org/mgmain.html?mgpage=token&token=ImmAzE0dDjqCyyClpMx9VQAFA_o8EGKBEfhwpRKE5_RrxayETg (accessed on 16 May 2025) for the COVID period.
Acknowledgments
The authors thank all the participants who kindly contributed to the present investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Egert, M.; Simmering, R.; Riedel, C.U. The Association of the Skin Microbiota With Health, Immunity, and Disease. Clin. Pharmacol. Ther. 2017, 102, 62–69. [Google Scholar] [CrossRef]
- Swaney, M.H.; Kalan, L.R. Living in your skin: Microbes, molecules, and mechanisms. Infect. Immun. 2021, 89, e00695-20. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Aguwa, C.; Enwereji, N.; Santiago, S.; Hine, A.; Kels, G.G.; McGee, J.; Lu, J. Targeting dysbiosis in psoriasis, atopic dermatitis, and hidradenitis suppurativa: The gut-skin axis and microbiome-directed therapy. Clin. Dermatol. 2023, 41, 640–649. [Google Scholar] [CrossRef]
- Bourkas, A.N.; Lara-Corrales, I. The role of nutrition, food allergies, and gut dysbiosis in immune-mediated in-flammatory skin disease: A narrative review. Curr. Opin. Pediatr. 2023, 35, 452–459. [Google Scholar] [CrossRef]
- Ruiz, G.D.; Barquero, O.D. Cutaneous microbiome, dysbiosis, and its role in atopic dermatitis. Rev. Méd. Sinerg. 2020, 5, 320. [Google Scholar] [CrossRef]
- Connor, V.; German, E.; Pojar, S.; Mitsi, E.; Hales, C.; Nikolaou, E.; Hyder-Wright, A.; Adler, H.; Zaidi, S.; Hill, H.; et al. Hands are vehicles for transmission of Streptococcus pneumoniae in novel controlled human infection study. Eur. Respir. J. 2018, 52, 1800599. [Google Scholar] [CrossRef]
- Padilla-Desgarennes, M.C.; Rosas-Morett, M.T. Microbiota cutánea. Revisión bibliográfica. Rev. Cent. Dermatol. Pascua 2024, 33, 5–11. [Google Scholar] [CrossRef]
- Osorio-Rivera, M.A.; Negrete-Costales, J.H.; Cañar-Rivera, J.L.; Carrillo-Barahona, W.E.; Moreno-Carvajal, J.S. Biofilms: Study of a Bacterial Community. Dominio De Las Cienc. 2021, 7, 1216–1228. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and their role on diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Moskovicz, V.; Gross, A.; Mizrahi, B. Extrinsic Factors Shaping the Skin Microbiome. Microorganisms 2020, 8, 1023. [Google Scholar] [CrossRef]
- Pradhan, D.; Biswasroy, P.; Kumar Naik, P.; Ghosh, G.; Rath, G. A Review of Current Interventions for COVID-19 Prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef]
- WHO. Infection Prevention and Control During Health Care When Coronavirus Disease (COVID-19) is Suspected or Confirmed. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-2021.1 (accessed on 10 July 2024).
- Lotfinejad, N.; Peters, A.; Pittet, D. Hand hygiene and the novel coronavirus pandemic: The role of healthcare workers. J. Hosp. Infect. 2020, 105, 776–777. [Google Scholar] [CrossRef]
- Rocha, L.A.; Ferreira de Almeida e Borges, L.; Gontijo Filho, P.P. Changes in hands microbiota associated with skin damage because of hand hygiene procedures on the health care workers. Am. J. Infect. Control 2009, 37, 155–159. [Google Scholar] [CrossRef]
- Borges LFde Ae Silva, B.L.; Gontijo Filho, P.P. Hand washing: Changes in the skin flora. Am. J. Infect. Control 2007, 35, 417–420. [Google Scholar] [CrossRef]
- Fierer, N.; Hamady, M.; Lauber, C.L.; Knight, R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 17994–17999. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Retuerto, M.; Arters, K.A.; Consolo, M.C.; Patterson, A.; Bajaksouzian, S.; Arbogast, J.W.; Cartner, T.J.; Jacobs, M.R.; et al. Effect of alcohol-based hand rub on hand microbiome and hand skin health in hospitalized adult stem cell transplant patients: A pilot study. J. Am. Acad. Dermatol. 2018, 78, 1218–1221.e5. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. The most important challenges ahead of microbiome pattern in the post era of the COVID-19 pandemic. J. Diabetes Metab. Disord. 2020, 19, 2031–2033. [Google Scholar] [CrossRef]
- Brett Finlay, B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.G.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N.; et al. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef]
- Vindenes, H.K.; Drengenes, C.; Amin, H.; Irgens-Hansen, K.; Svanes, C.; Bertelsen, R.J. Longitudinal analysis of the skin microbiome in association with hand eczema, hand hygiene practices and moisturizer use. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 2118–2129. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Wilke, A.; Bischof, J.; Gerlach, W.; Glass, E.; Harrison, T.; Keegan, K.P.; Paczian, T.; Trimble, W.L.; Bagchi, S.; Grama, A.; et al. The MG-RAST metagenomics database and portal in 2015. Nucleic Acids Res. 2016, 44, D590–D594. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- University of Oslo. Past 4—The Past of the Future n.d. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 11 July 2024).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef]
- Sanmiguel, A.; Grice, E.A. Interactions between host factors and the skin microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Vindenes, H.K.; Drengenes, C.; Amin, H.; Bertelsen, R.J. The impact of alcohol-based hand sanitiser and hand washing with soap and water on bacterial skin microbiota composition. JEADV Clin. Pract. 2023, 2, 764–774. [Google Scholar] [CrossRef]
- Gao, Z.; Tseng, C.H.; Pei, Z.; Blaser, M.J. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl. Acad. Sci. USA 2007, 104, 2927–2932. [Google Scholar] [CrossRef]
- Ying, S.; Zeng, D.N.; Chi, L.; Tan, Y.; Galzote, C.; Cardona, C.; Lax, S.; Gilbert, J.; Quan, Z.-X.; Badger, J.H. The Influence of Age and Gender on Skin-Associated Microbial Communities in Urban and Rural Human Populations. PLoS ONE 2015, 10, e0141842. [Google Scholar] [CrossRef]
- Hospodsky, D.; Pickering, A.J.; Julian, T.R.; Miller, D.; Gorthala, S.; Boehm, A.B.; Peccia, J. Hand bacterial communities vary across two different human populations. Microbiology 2014, 160, 1144–1152. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.Z.; Chen, T.; Min, S.H.; Wang, D.F.; Ding, M.M.; Jiang, G. Effects of wearing personal protective equipment during COVID-19 pandemic on composition and diversity of skin bacteria and fungi of medical workers. J. Eur Acad. Dermatol. Venereol. 2022, 36, 1612–1622. [Google Scholar] [CrossRef]
- Olarte, L.; Bratcher, D.F. Bacillus Species (Including Anthrax). Princ. Pract. Pediatr. Infect. Dis. 2023, 786–789.e3. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Kalainov, D.M.; Mehta, M.P.; Bolon, M.K. An Unusual Case of Staphylococcus pasteuri Osteomyelitis. Infect. Dis. Rep. 2020, 12, 8523. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Gallo, R.L.; Nakatsuji, T. Microbial Symbiosis with the Innate Immune Defense System of the Skin. J. Investig. Dermatol. 2011, 131, 1974–1980. [Google Scholar] [CrossRef]
- Gan, Y.; Zhang, J.; Qi, F.; Hu, Z.; Sweren, E.; Reddy, S.K.; Chen, L.; Feng, X.; Grice, E.A.; Garza, L.A.; et al. Commensal microbe regulation of skin cells in disease. Cell Host Microbe 2024, 32, 1264–1279. [Google Scholar] [CrossRef]
- Liu, Q.; Ranallo, R.; Rios, C.; Grice, E.A.; Moon, K.; Gallo, R.L. Crosstalk between skin microbiota and immune system in health and disease. Nat. Immunol. 2023, 24, 895–898. [Google Scholar] [CrossRef]
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.R.; Hooper, L.V.; Schmidt, R.R.; Von Au-lock, S.; et al. Commensal bacteria regulate toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef]
- Sanford, J.A.; O’Neill, A.M.; Zouboulis, C.C.; Gallo, R.L. Short-Chain Fatty Acids from Cutibacterium acnes Activate Both a Canonical and Epigenetic Inflammatory Response in Human Sebocytes. J. Immunol. 2019, 202, 1767–1776. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef]
- Castrillón-Rivera, L.E.; Palma-Ramos, A.; Castañeda-Sánchez, J.I. Actinomycetes: Pathogenicity Mechanisms. Dermatol. Rev. Mex. 2020, 64, 556–570. Available online: https://dermatologiarevistamexicana.org.mx/article/actinomicetos-mecanismos-de-patogenicidad/ (accessed on 20 April 2025).
- Brüggemann, H.; Salar-Vidal, L.; Gollnick, H.; Lood, R. A Janus-Faced Bacterium: Host-Beneficial and -Detrimental Roles of Cutibacterium acnes. Front. Microbiol. 2021, 12, 673845. [Google Scholar] [CrossRef]
- Patiño, L.A.; Andrés Morales, C.; Federico Lleras Acosta, D. Microbiota de la piel: El ecosistema cutáneo. Rev. Asoc. Colomb. Dermatol. Cir. Dermatol. 2013, 21, 147–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).