Insights into the Thriving of Bacillus megaterium and Rhodotorula mucilaginosa in Mining Areas: Their Adaptation and Tolerance Under Extreme Levels of Cu and Mn
Abstract
1. Introduction
2. Materials and Methods
2.1. Metallotolerant Microorganisms
2.2. Copper and Manganese Adaptation and Tolerance Study
2.3. Detection of Oxidative Stress by Reactive Oxygen Species (ROS)
2.4. Inhibitory Model
2.5. Correlation Analysis Among Adaptation, Tolerance, and Biosorption Capacity
2.6. Co-Culture Study
2.7. Statistical Analysis
3. Results
3.1. Copper and Manganese Microbial Adaptation and Tolerance
3.2. Detection of Oxidative Stress Under MTC Conditions
3.3. Model Fitting of MACs, MTCs, and MICs
3.4. Correlation Analysis Among Experimental MACs, MTCs, and Biosorption Capacity
3.5. Co-Culture with Copper and Manganese
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAC | Maximum adaptation concentration |
| MTC | Maximum tolerable concentration |
| MIC | Minimum inhibitory concentration |
References
- Macklin, M.G.; Thomas, C.J.; Mudbhatkal, A.; Brewer, P.A.; Hudson-Edwards, K.A.; Lewin, J.; Scussolini, P.; Eilander, D.; Lechner, A.; Owen, J.; et al. Impacts of metal mining on river systems: A global assessment. Science 2023, 381, 1345–1350. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Roane, T.M.; Pepper, I.L.; Gentry, T.J. Chapter 18—Microorganisms and Metal Pollutants. In Environmental Microbiology, 3rd ed.; Pepper, I.L., Gerba, C.P., Gentry, T.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 415–439. [Google Scholar]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Álvarez, A.; Valenzuela-García, J.L.; Meza-Figueroa, D.; O-Villanueva, M.d.l.; Ramírez-Hernández, J.; Almendariz-Tapia, J.; Pérez-Segura, E. Impact of mining activities on sediments in a semi-arid environment: San Pedro River, Sonora, Mexico. Appl. Geochem. 2011, 26, 2101–2112. [Google Scholar] [CrossRef]
- León-García, G.J.; Gómez-Álvarez, A.; Meza-Figueroa, D.M.; Valenzuela-García, J.L.; Encinas-Romero, M.A.; Villalba-Atondo, A.I.; Centeno-García, E.; Encinas-Soto, K.K. Assessment of heavy metal pollution in sediments of the Sonora River basin impacted by mining activities. Environ. Prog. Sustain. Energy 2022, 41, e13796. [Google Scholar] [CrossRef]
- Kiama, C.W.; Njire, M.M.; Kambura, A.K.; Mugweru, J.N.; Matiru, V.N.; Wafula, E.N.; Kagali, R.N.; Kuja, J.O. Prokaryotic diversity and composition within equatorial lakes Olbolosat and Oloiden in Kenya (Africa). Curr. Res. Microb. Sci. 2021, 2, 100066. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Li, D.; Zhou, L. Adsorption of heavy metal tolerance strains to Pb2+ and Cd2+ in wastewater. Environ. Sci. Pollut. Res. 2018, 25, 32156–32162. [Google Scholar] [CrossRef]
- Escamilla-Rodríguez, A.; Carlos-Hernández, S.; Díaz-Jiménez, L. Evidence of Resistance of Heavy Metals from Bacteria Isolated from Natural Waters of a Mining Area in Mexico. Water 2021, 13, 2766. [Google Scholar] [CrossRef]
- Moraleda-Muñoz, A.; Pérez, J.; Extremera Antonio, L.; Muñoz-Dorado, J. Differential Regulation of Six Heavy Metal Efflux Systems in the Response of Myxococcus xanthus to Copper. Appl. Environ. Microbiol. 2010, 76, 6069–6076. [Google Scholar] [CrossRef] [PubMed]
- Havryliuk, O.; Hovorukha, V.; Patrauchan, M.; Youssef, N.H.; Tashyrev, O. Draft whole genome sequence for four highly copper resistant soil isolates Pseudomonas lactis strain UKR1, Pseudomonas panacis strain UKR2, and Pseudomonas veronii strains UKR3 and UKR4. Curr. Res. Microb. Sci. 2020, 1, 44–52. [Google Scholar] [CrossRef]
- Gaur, N.; Flora, G.; Yadav, M.; Tiwari, A. A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 2014, 16, 180–193. [Google Scholar] [CrossRef]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock Biology of Microorganisms, 15th ed.; Pearson: New York, NY, USA, 2018; pp. 399–732. [Google Scholar]
- Dönmez, G.; Aksu, Z. The effect of copper(II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem. 1999, 35, 135–142. [Google Scholar] [CrossRef]
- Shu, W.-S.; Huang, L.-N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2021, 20, 219–235. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Camacho Mateu, J.; Sireci, M.; Muñoz, M.A. Phenotypic-dependent variability and the emergence of tolerance in bacterial populations. PLoS Comput. Biol. 2021, 17, e1009417. [Google Scholar] [CrossRef]
- Martinez Robert, J.; Wang, Y.; Raimondo Melanie, A.; Coombs Jonna, M.; Barkay, T.; Sobecky Patricia, A. Horizontal Gene Transfer of PIB-Type ATPases among Bacteria Isolated from Radionuclide- and Metal-Contaminated Subsurface Soils. Appl. Environ. Microbiol. 2006, 72, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zheng, X.; Zhang, D.; Iqbal, W.; Liu, C.; Yang, B.; Zhao, X.; Lu, X.; Mao, Y. Microbial characterization of heavy metal resistant bacterial strains isolated from an electroplating wastewater treatment plant. Ecotoxicol. Environ. Saf. 2019, 181, 472–480. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. Npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Händel, N.; Schuurmans, J.M.; Brul, S.; ter Kuile Benno, H. Compensation of the Metabolic Costs of Antibiotic Resistance by Physiological Adaptation in Escherichia coli. Antimicrob. Agents Chemother. 2013, 57, 3752–3762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.J.; Andrews, I.W.; Grote, A.T.; Manson, A.L.; Alcantar, M.A.; Earl, A.M.; Collins, J.J. Modulating the evolutionary trajectory of tolerance using antibiotics with different metabolic dependencies. Nat. Commun. 2022, 13, 2525. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G.; Balsberg Påhlsson, A.-M.; Bengtsson, G.; Bååth, E.; Tranvik, L. Heavy-metal ecology of terrestrial plants, microorganisms and invertebrates. Water Air Soil Pollut. 1989, 47, 189–215. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef]
- Voica, D.M.; Bartha, L.; Banciu, H.L.; Oren, A. Heavy metal resistance in halophilic Bacteria and Archaea. FEMS Microbiol. Lett. 2016, 363, fnw146. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Deive, F.J.; Sanromán, M.Á. Chapter 14—Bioreactor Development for the Cultivation of Extremophilic Microorganisms. In Current Developments in Biotechnology and Bioengineering; Larroche, C., Sanromán, M.Á., Du, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 403–432. [Google Scholar]
- Barman, D.; Jha, D.K.; Bhattacharjee, K. Metallotolerant Bacteria: Insights into Bacteria Thriving in Metal-Contaminated Areas. In Microbial Versatility in Varied Environments: Microbes in Sensitive Environments; Singh, R.P., Manchanda, G., Maurya, I.K., Wei, Y., Eds.; Springer: Singapore, 2020; pp. 135–164. [Google Scholar]
- Parades-Aguilar, J.; Calderon, K.; Agustin-Salazar, S.; Cerruti, P.; Ambrogi, V.; Gamez-Meza, N.; Medina-Juarez, L.A. Isolation and identification of metallotolerant bacteria with a potential biotechnological application. Sci. Rep. 2024, 14, 3663. [Google Scholar] [CrossRef]
- Dopson, M.; Ossandon, F.J.; Lövgren, L.; Holmes, D.S. Metal resistance or tolerance? Acidophiles confront high metal loads via both abiotic and biotic mechanisms. Front. Microbiol. 2014, 5, 157. [Google Scholar] [CrossRef]
- Piotrowska-Seget, Z.; Beściak, G.; Bernaś, T.; Kozdrój, J. GFP-tagged multimetal-tolerant bacteria and their detection in the rhizosphere of white mustard. Ann. Microbiol. 2012, 62, 559–567. [Google Scholar] [CrossRef]
- Vera-Bernal, M.; Martínez-Espinosa, R.M. Insights on Cadmium Removal by Bioremediation: The Case of Haloarchaea. Microbiol. Res. 2021, 12, 354–375. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.I.; Caviedes, M.A.; Doukkali, B.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E. Screening beneficial rhizobacteria from Spartina maritima for phytoremediation of metal polluted salt marshes: Comparison of gram-positive and gram-negative strains. Environ. Sci. Pollut. Res. 2016, 23, 19825–19837. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, E.; Peña-Ramos, E.A.; Juneja, V.K.; Martínez-Téllez, M.Á.; González-Ríos, H.; Paredes-Aguilar, M.D.; Valenzuela-Melendres, M.; Aispuro-Hernández, E. Antagonistic Activity of Bacteriocin-like Inhibitory Substances from Enterococcus lactis Isolated from the Surface of Jalapeno Pepper Against Foodborne Pathogens. Microbiol. Res. 2024, 15, 889–899. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Dursun, A.Y.; Uslu, G.; Cuci, Y.; Aksu, Z. Bioaccumulation of copper(II), lead(II) and chromium(VI) by growing Aspergillus niger. Process Biochem. 2003, 38, 1647–1651. [Google Scholar] [CrossRef]
- Chanturia, V.A.; Medyanik, N.L.; Shadrunova, I.V.; Mishurina, O.A. Chemical aspects of manganese removal from mine water at copper–sulfide deposits. J. Min. Sci. 2016, 52, 169–176. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Cervantes, F.J. Microbial recovery of metallic nanoparticles from industrial wastes and their environmental applications. J. Chem. Technol. Biotechnol. 2018, 93, 3091–3112. [Google Scholar] [CrossRef]
- Gálvez-Iriqui, A.C.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Calderón-Santoyo, M.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Synthesis of chitosan biocomposites loaded with pyrrole-2-carboxylic acid and assessment of their antifungal activity against Aspergillus niger. Appl. Microbiol. Biotechnol. 2019, 103, 2985–3000. [Google Scholar] [CrossRef]
- Contreras-Cortés, A.G.; Almendariz-Tapia, F.J.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Rosas-Burgos, E.C.; Rodríguez-Félix, F.; Gómez-Álvarez, A.; Quevedo-López, M.Á.; Plascencia-Jatomea, M. Biosorption of copper by immobilized biomass of Aspergillus australensis. Effect of metal on the viability, cellular components, polyhydroxyalkanoates production, and oxidative stress. Environ. Sci. Pollut. Res. 2020, 27, 28545–28560. [Google Scholar] [CrossRef]
- Mangold, S.; Potrykus, J.; Björn, E.; Lövgren, L.; Dopson, M. Extreme zinc tolerance in acidophilic microorganisms from the bacterial and archaeal domains. Extremophiles 2013, 17, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rusinowski, S.; Szada-Borzyszkowska, A.; Zieleźnik-Rusinowska, P.; Małkowski, E.; Krzyżak, J.; Woźniak, G.; Sitko, K.; Szopiński, M.; McCalmont, J.P.; Kalaji, H.M.; et al. How autochthonous microorganisms influence physiological status of Zea mays L. cultivated on heavy metal contaminated soils? Environ. Sci. Pollut. Res. 2019, 26, 4746–4763. [Google Scholar] [CrossRef]
- Słaba, M.; Gajewska, E.; Bernat, P.; Fornalska, M.; Długoński, J. Adaptive alterations in the fatty acids composition under induced oxidative stress in heavy metal-tolerant filamentous fungus Paecilomyces marquandii cultured in ascorbic acid presence. Environ. Sci. Pollut. Res. 2013, 20, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Garza-Gonzalez, M.T.; Barboza Perez, D.; Vazquez Rodriguez, A.; Garcia-Gutierrez, D.I.; Zarate, X.; Cantú Cardenas, M.E.; Urraca-Botello, L.I.; Lopez-Chuken, U.J.; Trevino-Torres, A.L.; Cerino-Córdoba, F.d.J.; et al. Metal-Induced Production of a Novel Bioadsorbent Exopolysaccharide in a Native Rhodotorula mucilaginosa from the Mexican Northeastern Region. PLoS ONE 2016, 11, e0148430. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Chellam, S.; Yanina, S.; Gassman, P.; Rosso, K.M. Bismuth dimercaptopropanol (BisBAL) inhibits the expression of extracellular polysaccharides and proteins by Brevundimonas diminuta: Implications for membrane microfiltration. Biotechnol. Bioeng. 2008, 99, 634–643. [Google Scholar] [CrossRef]
- Martis, B.S.; Mohan, A.K.; Chiplunkar, S.; Kamath, S.; Goveas, L.C.; Rao, C.V. Bacterium isolated from coffee waste pulp biosorps lead: Investigation of EPS mediated mechanism. Curr. Res. Microb. Sci. 2021, 2, 100029. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Gangadhar, G.; Raghuvanshi, S.; Gupta, S. A comprehensive study on the behavior of a novel bacterial strain Acinetobacter guillouiae for bioremediation of divalent copper. Bioprocess. Biosyst. Eng. 2015, 38, 1749–1760. [Google Scholar] [CrossRef]
- Osman, D.; Martini, M.A.; Foster, A.W.; Chen, J.; Scott, A.J.P.; Morton, R.J.; Steed, J.W.; Lurie-Luke, E.; Huggins, T.G.; Lawrence, A.D.; et al. Bacterial sensors define intracellular free energies for correct enzyme metalation. Nat. Chem. Biol. 2019, 15, 241–249. [Google Scholar] [CrossRef]
- Young, T.R.; Martini, M.A.; Foster, A.W.; Glasfeld, A.; Osman, D.; Morton, R.J.; Deery, E.; Warren, M.J.; Robinson, N.J. Calculating metalation in cells reveals CobW acquires CoII for vitamin B12 biosynthesis while related proteins prefer ZnII. Nat. Commun. 2021, 12, 1195. [Google Scholar] [CrossRef]
- Schlembach, I.; Grünberger, A.; Rosenbaum, M.A.; Regestein, L. Measurement Techniques to Resolve and Control Population Dynamics of Mixed-Culture Processes. Trends Biotechnol. 2021, 39, 1093–1109. [Google Scholar] [CrossRef]
- Clough, S.E.; Young, T.R.; Tarrant, E.; Scott, A.J.P.; Chivers, P.T.; Glasfeld, A.; Robinson, N.J. A metal-trap tests and refines blueprints to engineer cellular protein metalation with different elements. Nat. Commun. 2025, 16, 810. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Foster, K.R. Bacterial species rarely work together. Science 2022, 376, 581–582. [Google Scholar] [CrossRef]
- Foster, A.W.; Clough, S.E.; Aki, Z.; Young, T.R.; Clarke, A.R.; Robinson, N.J. Metalation calculators for E. coli strain JM109 (DE3): Aerobic, anaerobic, and hydrogen peroxide exposed cells cultured in LB media. Metallomics 2022, 14, mfac058. [Google Scholar] [CrossRef]
- Lobanov, A.; Dyckman, S.; Kurkjian, H.; Momeni, B. Spatial structure favors microbial coexistence except when slower mediator diffusion weakens interactions. eLife 2023, 12, e82504. [Google Scholar] [CrossRef]
- García-Béjar, B.; Arévalo-Villena, M.; Guisantes-Batan, E.; Rodríguez-Flores, J.; Briones, A. Study of the bioremediatory capacity of wild yeasts. Sci. Rep. 2020, 10, 11265. [Google Scholar] [CrossRef]
- Helmann, J.D. Metals in Motion: Understanding Labile Metal Pools in Bacteria. Biochemistry 2025, 64, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Puccio, T.; Kunka, K.S.; Zhu, B.; Xu, P.; Kitten, T. Manganese Depletion Leads to Multisystem Changes in the Transcriptome of the Opportunistic Pathogen Streptococcus sanguinis. Front. Microbiol. 2020, 11, 592615. [Google Scholar] [CrossRef]
- Perry, E.K.; Meirelles, L.A.; Newman, D.K. From the soil to the clinic: The impact of microbial secondary metabolites on antibiotic tolerance and resistance. Nat. Rev. Microbiol. 2022, 20, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ares, Á.; Sakai, S.; Sasaki, T.; Shimamura, S.; Mitarai, S.; Nunoura, T. Sequestration and efflux largely account for cadmium and copper resistance in the deep-sea Nitratiruptor sp. SB155-2 (phylum Campylobacterota). Environ. Microbiol. 2022, 24, 6144–6163. [Google Scholar] [CrossRef]
- Chen, M.; Grégoire Daniel, S.; Bain Jeffrey, G.; Blowes David, W.; Hug Laura, A. Legacy copper/nickel mine tailings potentially harbor novel iron/sulfur cycling microorganisms within highly variable communities. Appl. Environ. Microbiol. 2024, 90, e00143-24. [Google Scholar] [CrossRef]



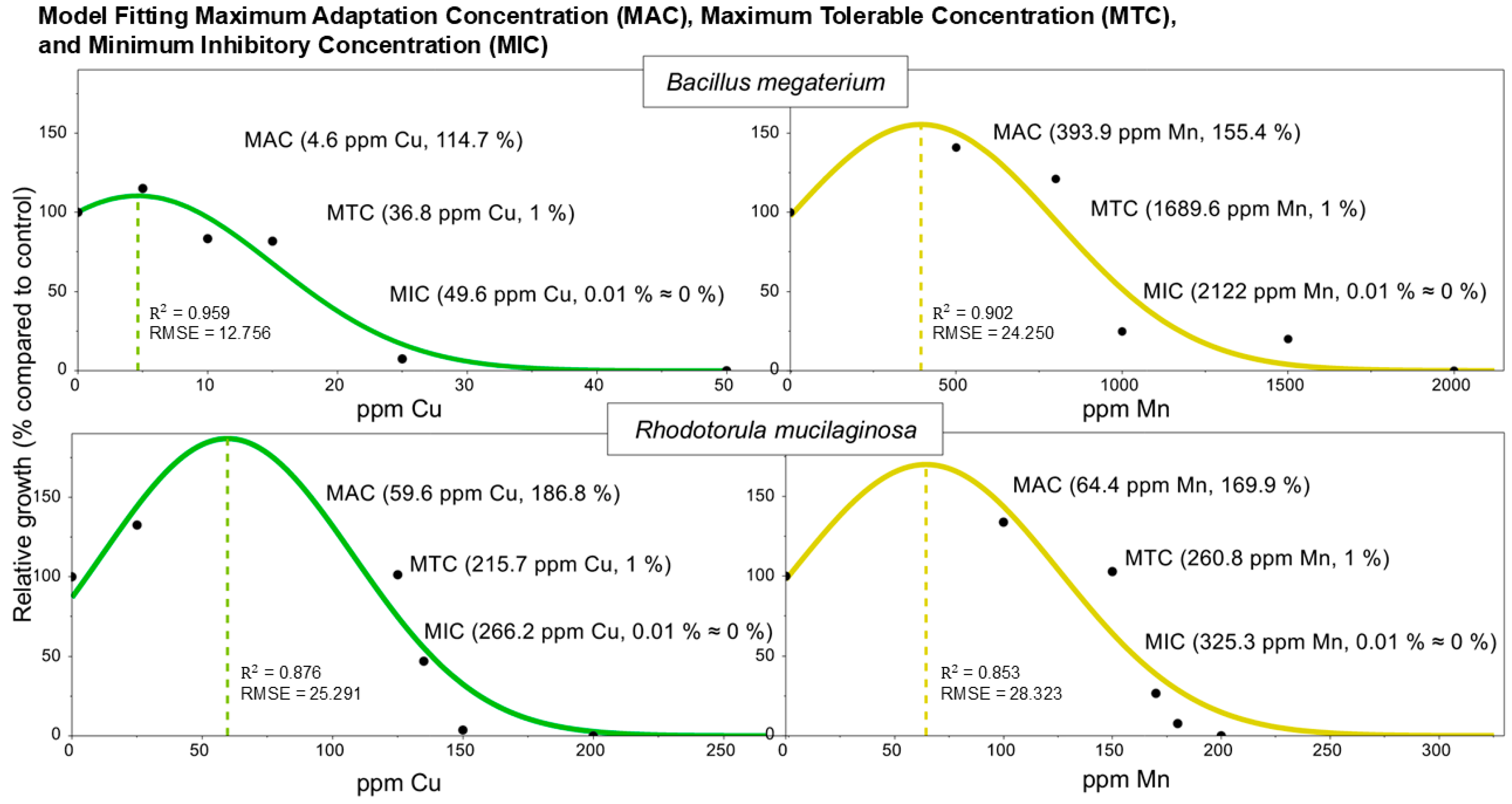
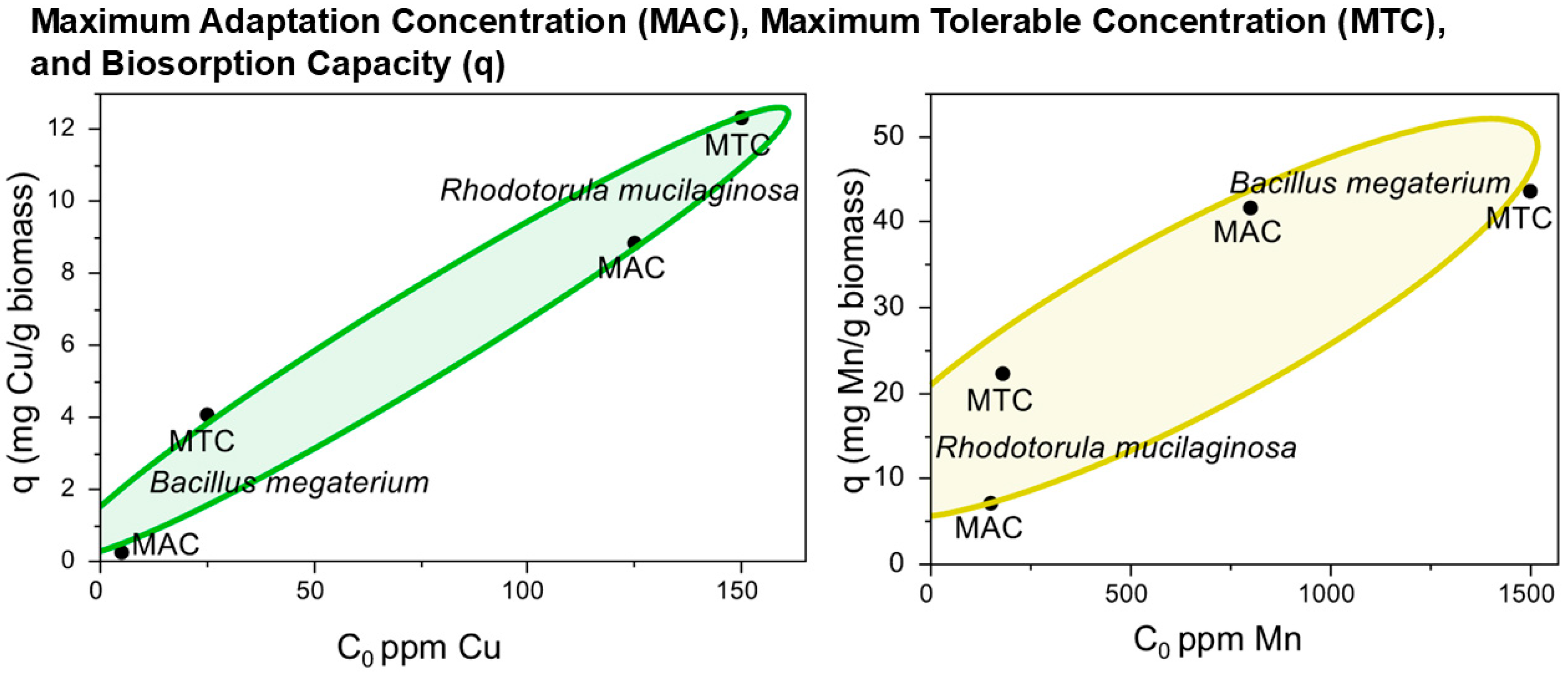

| Microorganism | Heavy Metal | MAC (ppm) | MTC (ppm) | MIC (ppm) | Biosorption Capacity (q) (mg/g Biomass) |
|---|---|---|---|---|---|
| B. megaterium | Cu | 4.6 | 36.8 | 49.6 | 4.1 ± 0.1 |
| Mn | 393.9 | 1689.6 | 2122 | 43.6 ± 7.7 | |
| R. mucilaginosa | Cu | 59.6 | 215.7 | 266.2 | 12.3 ± 1.3 |
| Mn | 64.4 | 260.8 | 325.3 | 22.3 ± 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Villa, A.; Plascencia-Jatomea, M.; Calderón, K.; Arévalo-Niño, K.; López-Avilés, G.; Almendariz-Tapia, F.J. Insights into the Thriving of Bacillus megaterium and Rhodotorula mucilaginosa in Mining Areas: Their Adaptation and Tolerance Under Extreme Levels of Cu and Mn. Microbiol. Res. 2025, 16, 140. https://doi.org/10.3390/microbiolres16070140
Álvarez-Villa A, Plascencia-Jatomea M, Calderón K, Arévalo-Niño K, López-Avilés G, Almendariz-Tapia FJ. Insights into the Thriving of Bacillus megaterium and Rhodotorula mucilaginosa in Mining Areas: Their Adaptation and Tolerance Under Extreme Levels of Cu and Mn. Microbiology Research. 2025; 16(7):140. https://doi.org/10.3390/microbiolres16070140
Chicago/Turabian StyleÁlvarez-Villa, Alfonso, Maribel Plascencia-Jatomea, Kadiya Calderón, Katiushka Arévalo-Niño, Guadalupe López-Avilés, and Francisco Javier Almendariz-Tapia. 2025. "Insights into the Thriving of Bacillus megaterium and Rhodotorula mucilaginosa in Mining Areas: Their Adaptation and Tolerance Under Extreme Levels of Cu and Mn" Microbiology Research 16, no. 7: 140. https://doi.org/10.3390/microbiolres16070140
APA StyleÁlvarez-Villa, A., Plascencia-Jatomea, M., Calderón, K., Arévalo-Niño, K., López-Avilés, G., & Almendariz-Tapia, F. J. (2025). Insights into the Thriving of Bacillus megaterium and Rhodotorula mucilaginosa in Mining Areas: Their Adaptation and Tolerance Under Extreme Levels of Cu and Mn. Microbiology Research, 16(7), 140. https://doi.org/10.3390/microbiolres16070140







