Anti-Influenza A Virus Activity of Rhododendron brachycarpum Extract and Identification of Hyperoside as the Active Constituent
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
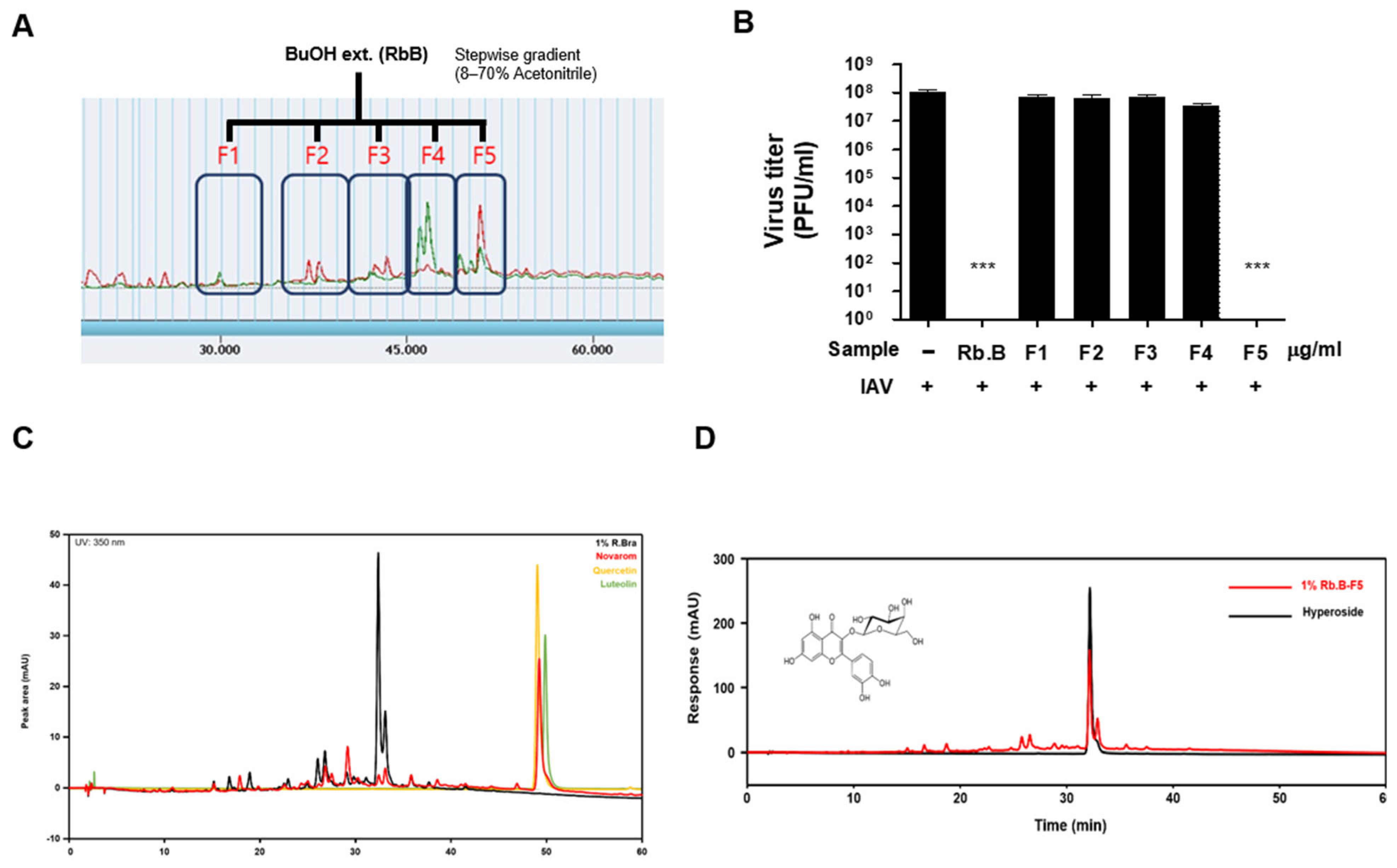
2.2. Fractionation and Active Compound Isolation
2.3. HPLC Analysis
2.4. Virus and Cell Lines
2.5. Cell Infection and Antiviral Assays
2.6. Mechanism-of-Action Analysis
2.7. Binding Site Prediction and in Silico Molecular Docking
2.8. Statistical Analysis
3. Results
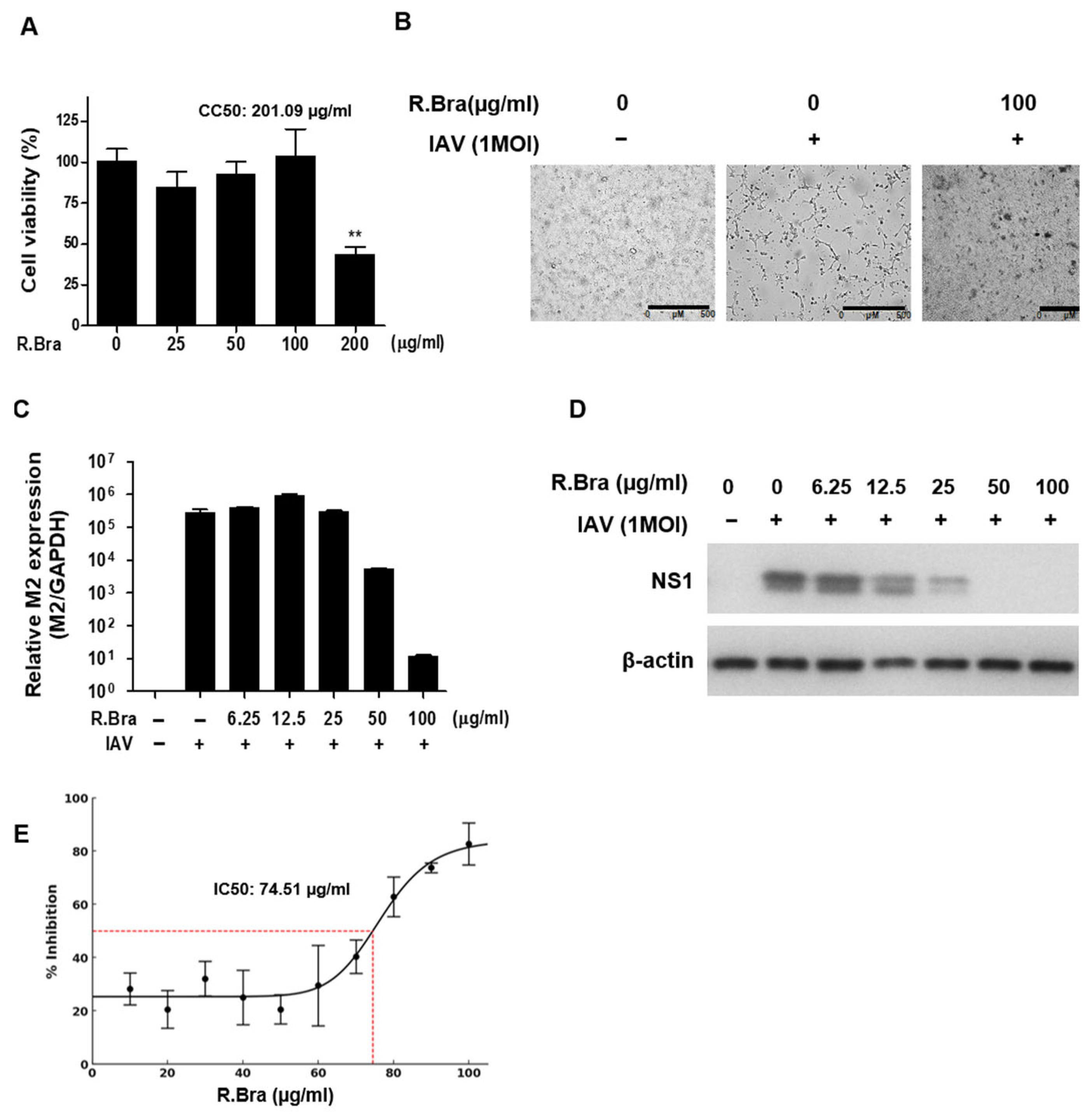
3.1. Cytotoxicity of R. brachycarpum Extract and Antiviral Activity Against IAV
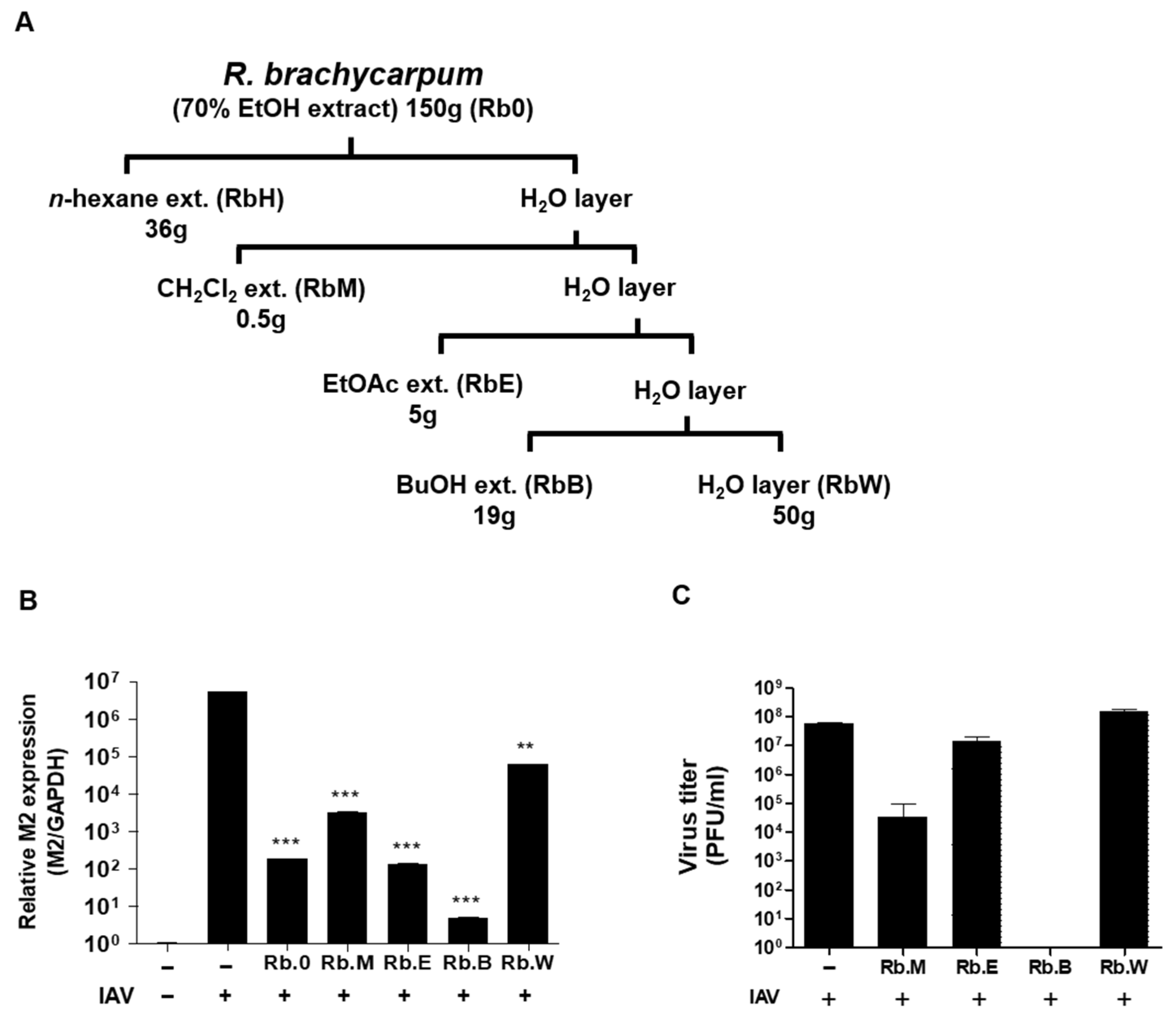
3.2. Comparison of Antiviral Activity of Solvent Fractions
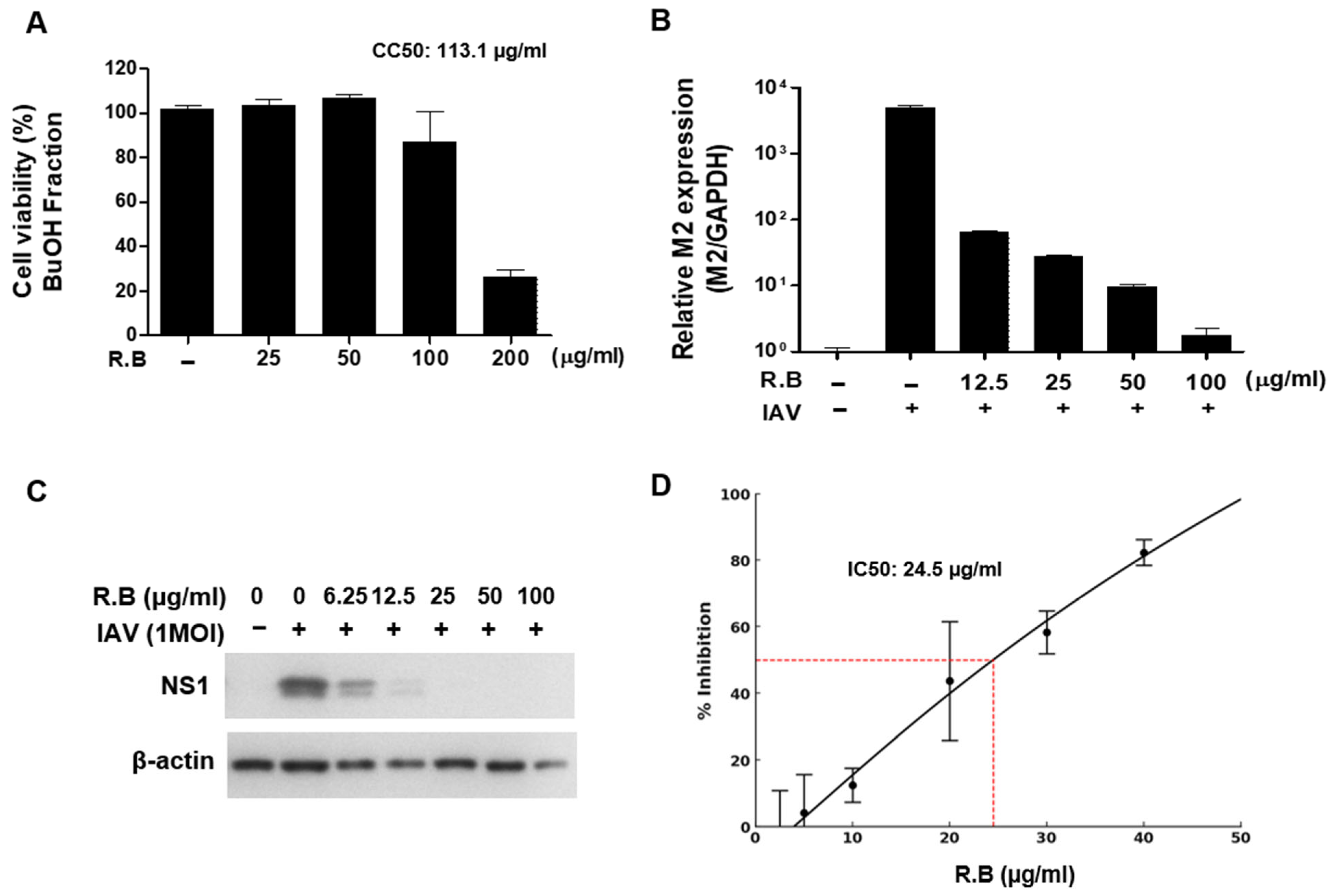
3.3. Antiviral Activity of the Butanol Fraction
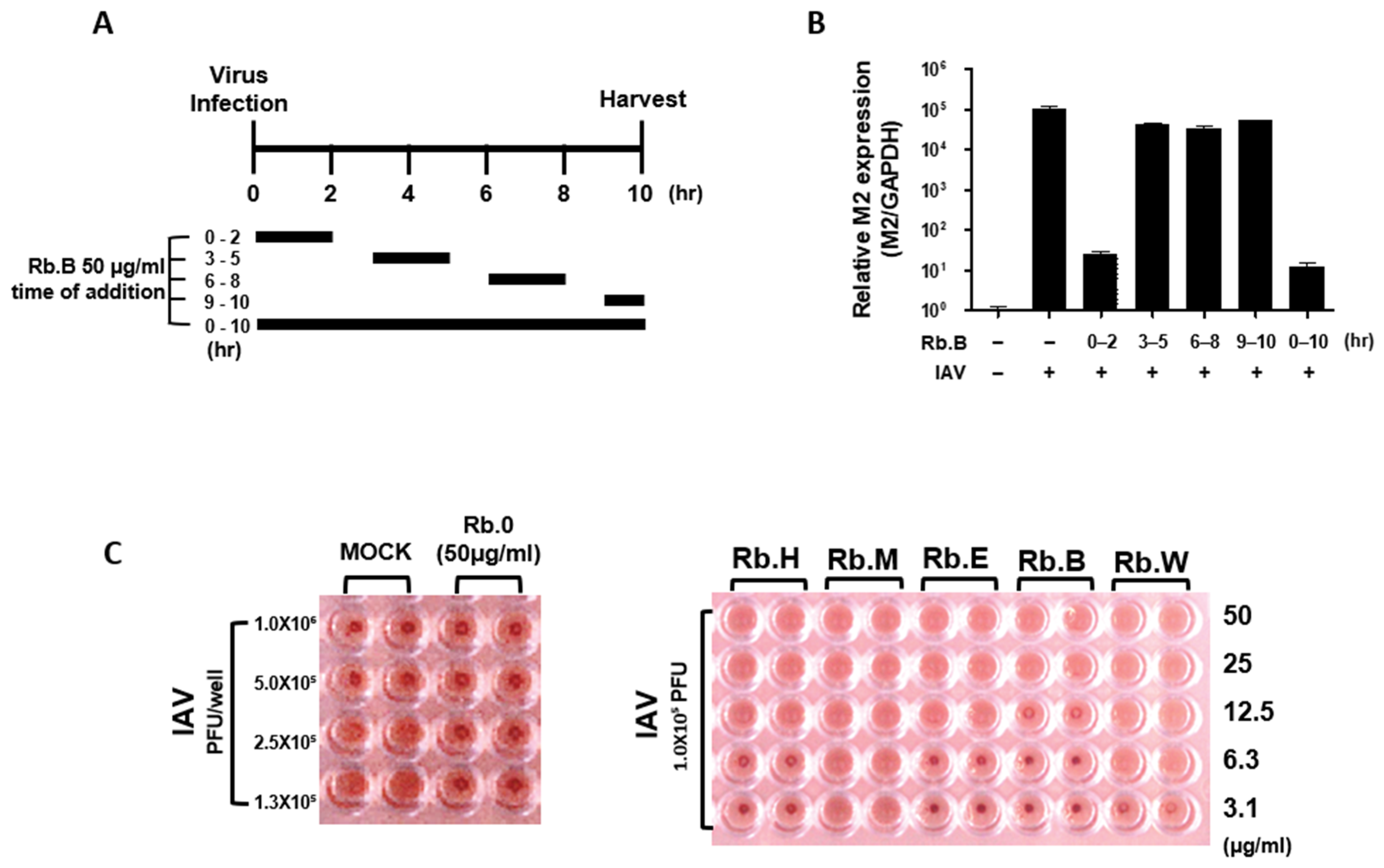
3.4. Antiviral Effect at the Early Stage of the Viral Life Cycle
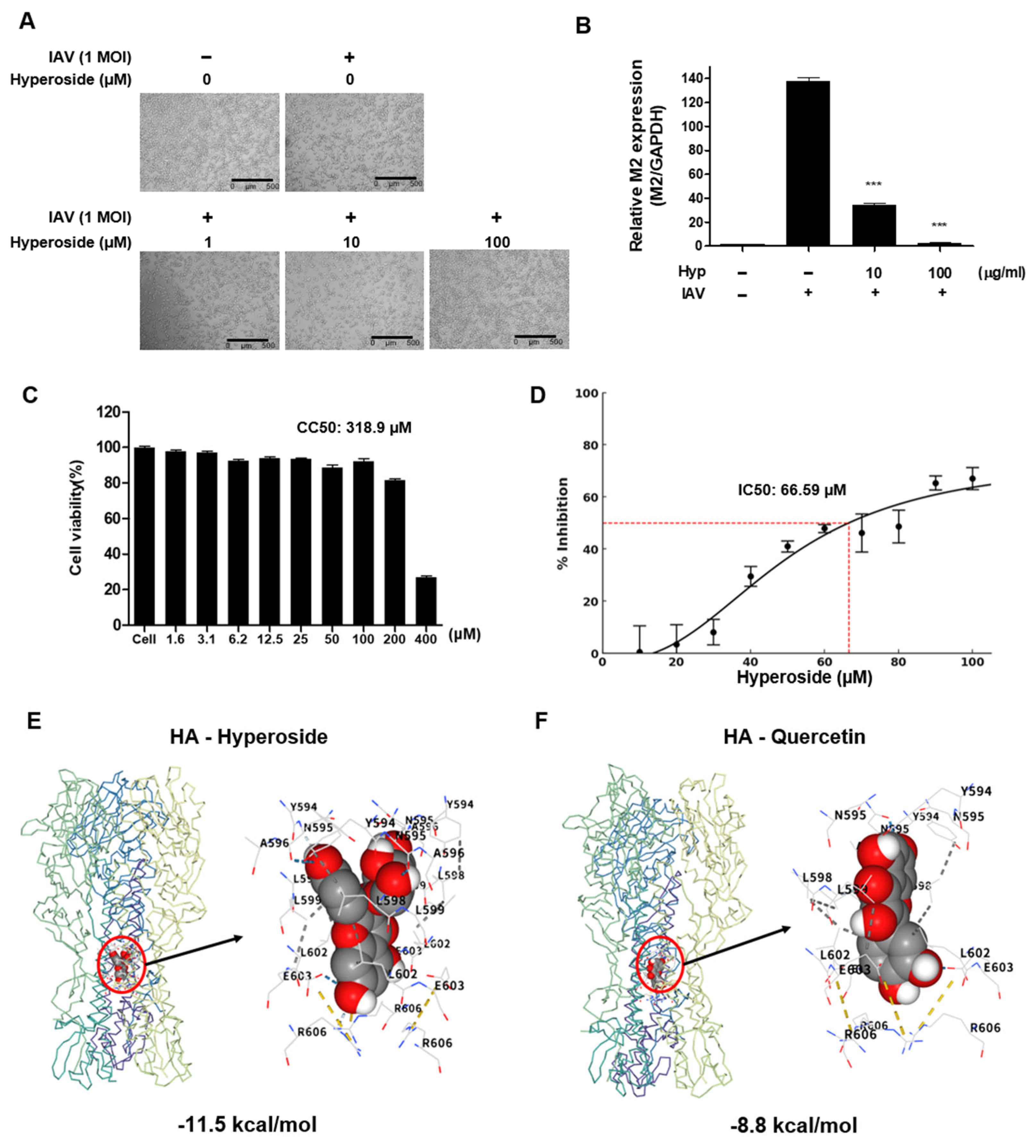
3.5. Identification and Analysis of Hyperoside as the Active Constituent
3.6. Anti-Influenza Activity of Hyperoside
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. S4), D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Jin, Y.; Hu, M.; Zhou, J.; Song, T.; Huang, Z.; Li, B.; Li, K.; Zhou, W.; Dai, H.; et al. Ecological dynamics of influenza A viruses: Cross-species transmission and global migration. Sci. Rep. 2016, 6, 36839. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor, M.; Stevens, J.; Nawrocki, J.; Singh, K. Influenza A virus subtyping: Paradigm shift in influenza diagnosis. J. Clin. Microbiol. 2009, 47, 3055–3056. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Ostbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Wang, M.; Veit, M. Hemagglutinin-esterase-fusion (HEF) protein of influenza C virus. Protein Cell 2016, 7, 28–45. [Google Scholar] [CrossRef]
- Asha, K.; Meseko, C.; Kumar, B. Editorial: Influenza and related viruses: Epidemiology, pathogenesis, and therapeutics. Front. Mol. Biosci. 2022, 9, 1117067. [Google Scholar] [CrossRef]
- Shie, J.J.; Fang, J.M. Development of effective anti-influenza drugs: Congeners and conjugates—A review. J. Biomed. Sci. 2019, 26, 84. [Google Scholar] [CrossRef]
- Duwe, S.C.; Milde, J.; Heider, A.; Wedde, M.; Schweiger, B.; Dürrwald, R. Increase of Synergistic Secondary Antiviral Mutations in the Evolution of A(H1N1)pdm09 Influenza Virus Neuraminidases. Viruses 2024, 16, 1109. [Google Scholar] [CrossRef]
- Li, Y.; Huo, S.; Yin, Z.; Tian, Z.; Huang, F.; Liu, P.; Liu, Y.; Yu, F. Retracted and republished from: “The current state of research on influenza antiviral drug development: Drugs in clinical trial and licensed drugs”. mBio 2024, 15, e00175-24. [Google Scholar] [CrossRef]
- Hay, A.J.; McCauley, J.W. The WHO global influenza surveillance and response system (GISRS)—A future perspective. Influenza Other Respir. Viruses 2018, 12, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Quignon, E.; Ferhadian, D.; Hache, A.; Vivet-Boudou, V.; Isel, C.; Printz-Schweigert, A.; Donchet, A.; Crépin, T.; Marquet, R. Structural Impact of the Interaction of the Influenza A Virus Nucleoprotein with Genomic RNA Segments. Viruses 2024, 16, 421. [Google Scholar] [CrossRef]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Lahlou, M. Screening of natural products for drug discovery. Expert Opin. Drug Discov. 2007, 2, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Oh, J.; Wonhwa, L.; Kwak, S.; Li, W.; Chittiboyina, A.G.; Ferreira, D.; Hamann, M.T.; Lee, S.H.; Bae, J.S.; et al. The first cyclomegastigmane rhododendroside A from Rhododendron brachycarpum alleviates HMGB1-induced sepsis. Biochim. Biophys. Acta 2014, 1840, 2042–2049. [Google Scholar] [CrossRef]
- Hwang, T.; Noh, E.; Jeong, J.H.; Park, S.K.; Shin, D.; Kang, H. Determination of Grayanotoxins from Rhododendron brachycarpum in Dietary Supplements and Homemade Wine by Liquid Chromatography-Quadrupole Time-of-Flight-Mass Spectrometry and Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 1935–1940. [Google Scholar] [CrossRef]
- Zhou, W.; Oh, J.; Li, W.; Kim, D.W.; Yang, M.H.; Jang, J.-H.; Ahn, J.S.; Lee, S.H.; Na, M. Chemical constituents of the Korean endangered species Rhododendron brachycarpum. Biochem. Syst. Ecol. 2014, 56, 231–236. [Google Scholar] [CrossRef]
- Chen, J.Y.; Huang, T.R.; Hsu, S.Y.; Huang, C.C.; Wang, H.S.; Chang, J.S. Effect and mechanism of quercetin or quercetin-containing formulas against COVID-19: From bench to bedside. Phytother. Res. 2024, 38, 2597–2618. [Google Scholar] [CrossRef]
- Ku, S.K.; Zhou, W.; Lee, W.; Han, M.S.; Na, M.; Bae, J.S. Anti-inflammatory effects of hyperoside in human endothelial cells and in mice. Inflammation 2015, 38, 784–799. [Google Scholar] [CrossRef]
- Wu, L.L.; Yang, X.B.; Huang, Z.M.; Liu, H.Z.; Wu, G.X. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol. Sin. 2007, 28, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Rakers, C.; Schwerdtfeger, S.M.; Mortier, J.; Duwe, S.; Wolff, T.; Wolber, G.; Melzig, M.F. Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 4312–4317. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Yoo, H.; Ku, S.-K.; Zhou, W.; Han, M.-S.; Na, M.; Bae, J.-S. Anti-septic effects of phenolic glycosides from Rhododendron brachycarpum in vitro and in vivo. J. Funct. Foods 2015, 16, 448–459. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef]
- Choi, Y.H.; Zhou, W.; Oh, J.; Choe, S.; Kim, D.W.; Lee, S.H.; Na, M. Rhododendric acid A, a new ursane-type PTP1B inhibitor from the endangered plant Rhododendron brachycarpum G. Don. Bioorg. Med. Chem. Lett. 2012, 22, 6116–6119. [Google Scholar] [CrossRef]
- Yang, J.; Kim, M.O.; Kwon, Y.S.; Kim, M.J. Antioxidant Activity, alpha-glucosidase Inhibitory Activity and Chemoprotective Properties of Rhododendron brachycarpum Leaves Extracts. Curr. Pharm. Biotechnol. 2017, 18, 849–854. [Google Scholar] [CrossRef]
- Fang, F.; Bao, S.; Chen, D.; Duan, X.; Zhao, Y.; Ma, Y. Protective effects and mechanism of quercetin from Rhododendron dauricum against cerebral ischemia-reperfusion injury. Eur. J. Pharmacol. 2024, 985, 177126. [Google Scholar] [CrossRef]
- Ngan, C.H.; Hall, D.R.; Zerbe, B.; Grove, L.E.; Kozakov, D.; Vajda, S. FTSite: High accuracy detection of ligand binding sites on unbound protein structures. Bioinformatics 2012, 28, 286–287. [Google Scholar] [CrossRef]
- Kozakov, D.; Grove, L.E.; Hall, D.R.; Bohnuud, T.; Mottarella, S.E.; Luo, L.; Xia, B.; Beglov, D.; Vajda, S. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 2015, 10, 733–755. [Google Scholar] [CrossRef]
- Brenke, R.; Kozakov, D.; Chuang, G.Y.; Beglov, D.; Hall, D.; Landon, M.R.; Mattos, C.; Vajda, S. Fragment-based identification of druggable ‘hot spots’ of proteins using Fourier domain correlation techniques. Bioinformatics 2009, 25, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Jindal, A.; Ghani, U.; Kotelnikov, S.; Egbert, M.; Hashemi, N.; Vajda, S.; Padhorny, D.; Kozakov, D. Elucidation of protein function using computational docking and hotspot analysis by ClusPro and FTMap. Acta Crystallogr. D Struct. Biol. 2022, 78 Pt 6, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Gan, J.; Xiao, Z.X.; Cao, Y. FitDock: Protein-ligand docking by template fitting. Brief. Bioinform. 2022, 23, bbac087. [Google Scholar] [CrossRef]
- Gansukh, E.; Nile, A.; Kim, D.H.; Oh, J.W.; Nile, S.H. New insights into antiviral and cytotoxic potential of quercetin and its derivatives—A biochemical perspective. Food Chem. 2021, 334, 127508. [Google Scholar] [CrossRef]
- Mehrbod, P.; Hudy, D.; Shyntum, D.; Markowski, J.; Łos, M.J.; Ghavami, S. Quercetin as a Natural Therapeutic Candidate for the Treatment of Influenza Virus. Biomolecules 2021, 11, 10. [Google Scholar] [CrossRef]
- Harrington, W.N.; Kackos, C.M.; Webby, R.J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021, 53, 737–749. [Google Scholar] [CrossRef]
- Blut, A. Influenza Virus. Transfus. Med. Hemother. 2009, 36, 32–39. [Google Scholar]
- Li, R.; Han, Q.; Li, X.; Liu, X.; Jiao, W. Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment. Molecules 2024, 29, 2371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.H.; Shin, S.Y.; Choi, H.; Lee, J.H.; Kim, Y.J.; Woo, S.J.; Im, W.R.; Jeon, S.H. Anti-Influenza A Virus Activity of Rhododendron brachycarpum Extract and Identification of Hyperoside as the Active Constituent. Microbiol. Res. 2025, 16, 132. https://doi.org/10.3390/microbiolres16060132
Park YH, Shin SY, Choi H, Lee JH, Kim YJ, Woo SJ, Im WR, Jeon SH. Anti-Influenza A Virus Activity of Rhododendron brachycarpum Extract and Identification of Hyperoside as the Active Constituent. Microbiology Research. 2025; 16(6):132. https://doi.org/10.3390/microbiolres16060132
Chicago/Turabian StylePark, Yung Hun, Soo Yong Shin, Hayeong Choi, Jae Hyeok Lee, You Jin Kim, Seong Ji Woo, Wonkyun Ronny Im, and Sung Ho Jeon. 2025. "Anti-Influenza A Virus Activity of Rhododendron brachycarpum Extract and Identification of Hyperoside as the Active Constituent" Microbiology Research 16, no. 6: 132. https://doi.org/10.3390/microbiolres16060132
APA StylePark, Y. H., Shin, S. Y., Choi, H., Lee, J. H., Kim, Y. J., Woo, S. J., Im, W. R., & Jeon, S. H. (2025). Anti-Influenza A Virus Activity of Rhododendron brachycarpum Extract and Identification of Hyperoside as the Active Constituent. Microbiology Research, 16(6), 132. https://doi.org/10.3390/microbiolres16060132





