In Vitro Evaluation of the Ovicidal Potential of Proteases from Beauveria bassiana Against Eurytrema pancreaticum Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining and Extracting Extracellular Enzymes
2.2. Enzyme Concentration
2.3. Total Protease Activity
2.4. Total Chitinase Activity
2.5. Determination of Total Protein and Specific Activity
2.6. Enzyme Characterization
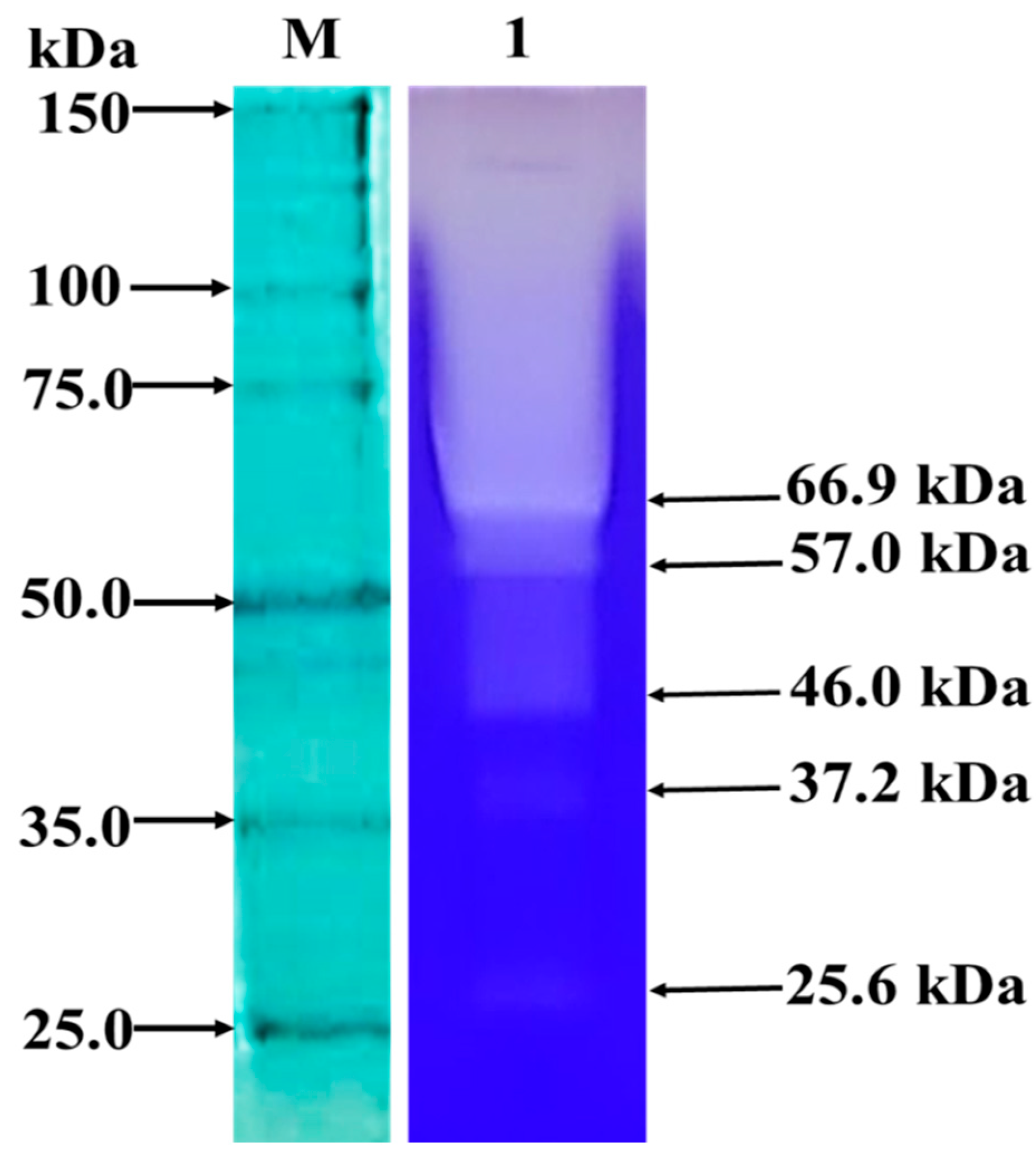
2.6.1. Zymogram
2.6.2. Effects of Inhibitors on Proteolytic Activity
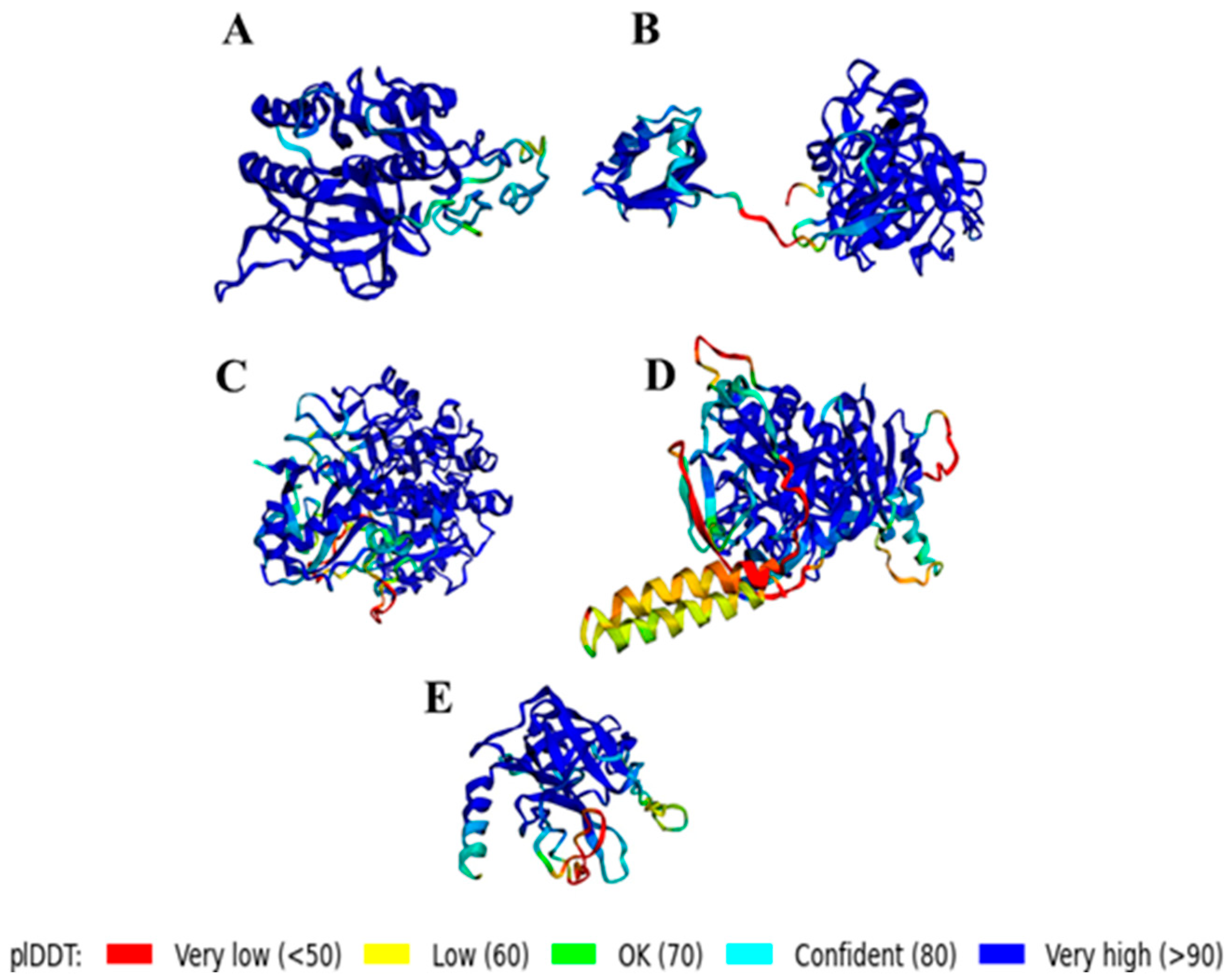
2.6.3. Structural Prediction
2.7. Obtaining Eurytrema Pancreaticum Eggs
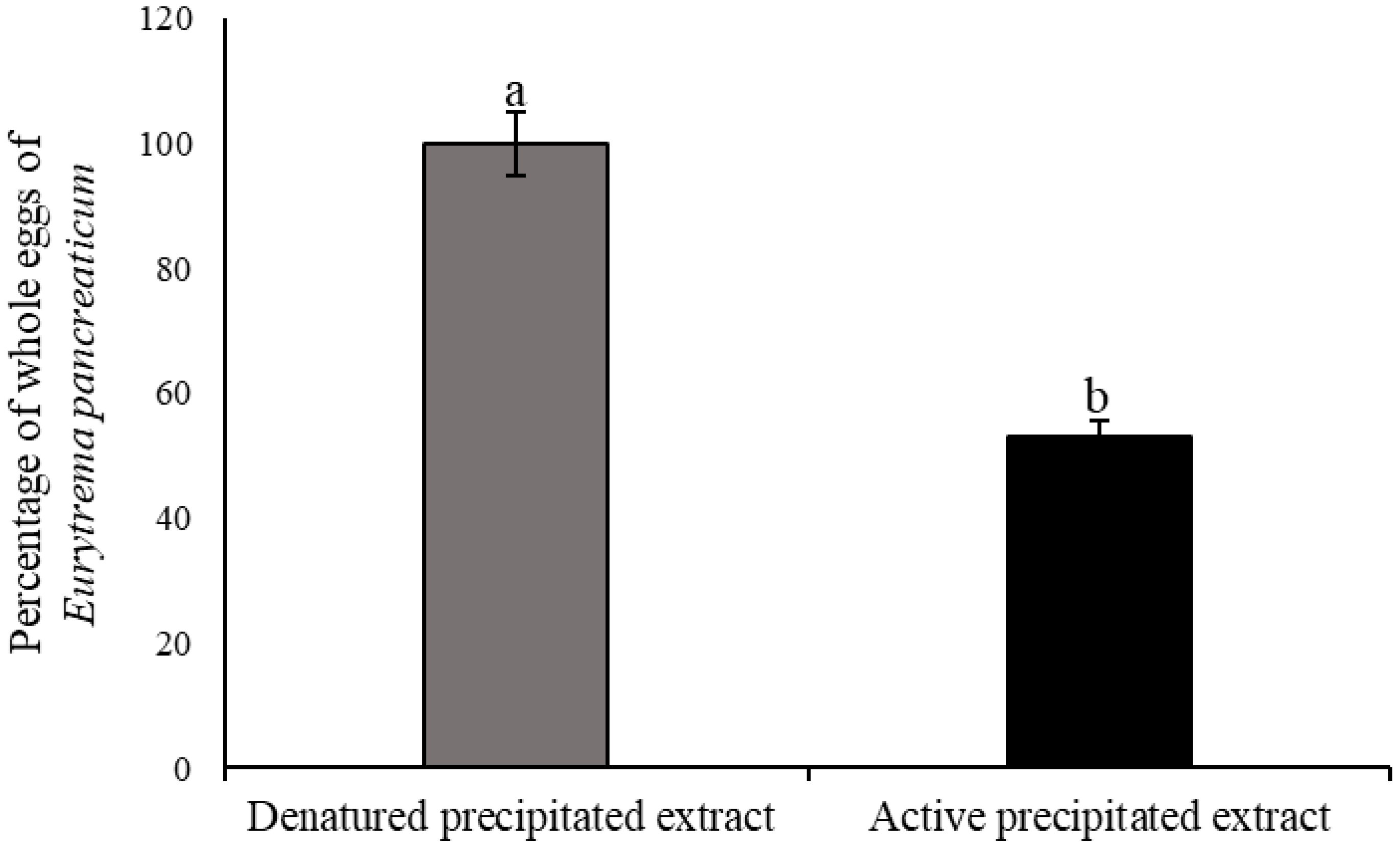
2.8. In Vitro Ovicidal Effect
2.9. Statistical Analysis
3. Results
3.1. Specific Activity of Extracellular Enzymes
3.2. Enzyme Characterization
3.2.1. Zymogram
3.2.2. Effects of Inhibitors on Proteolytic Activity
3.2.3. Structural Prediction
3.3. In Vitro Ovicidal Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandoval, B.A.; Hyster, T.K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Di Cera, E. Serine peptidases: Classification, structure and function. Cell. Mol. Life Sci. 2008, 65, 1220–1236. [Google Scholar] [CrossRef] [PubMed]
- Rekik, H.; Jaouadi, N.Z.; Gargouri, F.; Bejar, W.; Frikha, F.; Jmal, N.; Jaouadi, B. Production, purification and biochemical characterization of a novel detergent-stable serine alkaline protease from Bacillus safensis strain RH12. Int. J. Biol. Macromol. 2019, 121, 1227–1239. [Google Scholar] [CrossRef]
- Naveed, M.; Nadeem, F.; Mehmood, T.; Bilal, M.; Anwar, Z.; Amjad, F. Protease—A versatile and ecofriendly biocatalyst with multi-industrial applications: An updated review. Catal. Lett. 2021, 151, 307–323. [Google Scholar] [CrossRef]
- Dhawan, M.; Joshi, N. Enzymatic comparison and mortality of Beauveria bassiana against cabbage caterpillar Pieris brassicae LINN. Braz. J. Microbiol. 2017, 48, 522–529. [Google Scholar] [CrossRef]
- Bara, G.T.; Laing, M.D. Entomopathogens: Potential to control thrips in avocado, with special reference to Beauveria bassiana. Hortic. Rev. 2020, 47, 325–368. [Google Scholar] [CrossRef]
- dos Reis, C.B.; Sobucki, L.; Mazutti, M.A.; Guedes, J.V. Production of chitinase from Metarhizium anisopliae by solid-state fermentation using sugarcane bagasse as substrate. Ind. Biotechnol. 2018, 14, 230–234. [Google Scholar] [CrossRef]
- Rojas-Osnaya, J.; Rocha-Pino, Z.; Nájera, H.; González-Márquez, H.; Shirai, K. Novel transglycosylation activity of β-N-acetylglucosaminidase of Lecanicillium lecanii produced by submerged culture. Int. J. Biol. Macromol. 2020, 145, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Tong, S.; Yuan, M.; Wang, X.; Wang, J.; Fan, Y. Expression of a Beauveria bassiana chitosanase (BbCSN-1) in Pichia pastoris and enzymatic analysis of the recombinant protein. Protein Expr. Purif. 2020, 166, 105519. [Google Scholar] [CrossRef]
- Grogan, G.J.; Holland, H.L. The biocatalytic reactions of Beauveria spp. J. Mol. Catal. B Enzym. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Pandey, A.; Singh, S.; Pillai, S. Biotechnological potential of Beauveria bassiana as a source of novel biocatalysts and metabolites. Crit. Rev. Biotechnol. 2020, 40, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Rather, B.A.; Kandoo, A.A. Insect pest management by entomopathogenic fungi. J. Entomol. Zool. Stud. 2017, 5, 1185–1190. [Google Scholar]
- Semenova, T.A.; Dunaevsky, Y.E.; Beljakova, G.A.; Belozersky, M.A. Extracellular peptidases of insect-associated fungi and their possible use in biological control programs and as pathogenicity markers. Fungal Biol. 2020, 124, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Sharma, B.; Kaur, S.; Kaur, T.; Khan, S.S.; Kumar, S.; Yadav, A.N. Microbial antagonists: Diversity, formulation and applications for management of pest–pathogens. Egypt. J. Biol. Pest Control. 2023, 33, 105. [Google Scholar] [CrossRef]
- Dhawan, M.; Joshi, N.; Ghuman, S.; Sandhu, S.; Sharma, M. Deciphering the relationships among enzymatic systems and virulence of Beauveria bassiana: A Review. J. Exp. Biol. Agric. Sci. 2020, 8, 730–742. [Google Scholar] [CrossRef]
- Figueroa, L.B.P.; Mamani, R.C.C.; de Souza, D.C.; de Souza Alves, J.C.; de Souza, S.A.; Ferreira, C.B.; Soares, F.E.F. Enzyme production by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae and their application in the control of nematodes (Haemonchus spp. and Meloidogyne incognita) in vitro. J. Nat. Pestic. Res. 2024, 8, 100077. [Google Scholar] [CrossRef]
- de Sousa, D.E.; de Castro, M.B. Pancreatic eurytrematosis in small ruminants: A forgotten disease or an untold history? Vet. Parasitol. 2022, 311, 109794. [Google Scholar] [CrossRef]
- Rachid, M.A.; Aquino, H.M.; Facury-Filho, E.J.; Carvalho, A.U.; Valle, G.R.; Vasconcelos, A.C. Chronic interstitial pancreatitis and chronic wasting disease caused by Eurytrema coelomaticum in Nelore cow. Arq. Bras. Med. Vet. Zootec. 2011, 63, 741–743. [Google Scholar] [CrossRef]
- Rojo-Vázquez, F.A.; Meana, A.; Valcárcel, F.; Martínez-Valladares, M. Update on trematode infections in sheep. Vet. Parasitol. 2012, 189, 15–38. [Google Scholar] [CrossRef]
- de Sousa, D.E.; Barbosa, E.D.; Wilson, T.M.; Machado, M.; Oliveira, W.J.; Duarte, M.A.; de Castro, M.B. Eurytrema coelomaticum natural infection in small ruminants: A neglected condition. Parasitology 2021, 148, 576–583. [Google Scholar] [CrossRef]
- Ilha, M.R.; Loretti, A.P.; Reis, A.C. Wasting and mortality in beef cattle parasitized by Eurytrema coelomaticum in the State of Paraná, southern Brazil. Vet. Parasitol. 2005, 133, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bassani, C.A.; Sangioni, L.A.; Saut, J.P.; Yamamura, M.H.; Headley, S.A. Epidemiology of eurytrematosis (Eurytrema spp. Trematoda: Dicrocoeliidae) in slaughtered beef cattle from the central-west region of the State of Paraná, Brazil. Vet. Parasitol. 2006, 141, 356–361. [Google Scholar] [CrossRef]
- Tandon, V.; Shylla, J.A.; Ghatani, S.; Athokpam, V.D.; Sahu, R. Neglected tropical diseases: Trematodiases—The Indian scenario. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 901–907. [Google Scholar] [CrossRef]
- Toledo, R.; Cortés, A.; Álvarez-Izquierdo, M.; Muñoz-Antoli, C.; Esteban, J.G. New Insights into the Treatment of Foodborne Trematode Infections. In Neglected Tropical Disease: Drug Discovery and Development; Swinney, D., Pollastri, M., Mannhold, R., Buschmann, H., Holenz, J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 289–323. [Google Scholar] [CrossRef]
- Doonan, S. Bulk purification by fractional precipitation. In Protein Purification Protocols: Methods in Molecular Biology; Cutler, P., Ed.; Humana Press: New York, NY, USA, 2004; pp. 117–124. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Soares, F.E.F.; Araujo, J.M.; Geniêr, H.L.A.; Silva, A.R.; Ferreira, S.R. Optimizing protease production from an isolate of the nematophagous fungus Duddingtonia flagrans using response surface methodology and its larvicidal activity on horse cyathostomins. J. helminthol. 2011, 85, 164–170. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. J. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araújo, J.V.; Soares, F.E.D.; Araujo, J.M.; Ferreira, S.R.; Tavela, A.D.; Queiroz, J.H. Proteolytic action of the crude extract of Duddingtonia flagrans on cyathostomins (Nematoda: Cyathostominae) in coprocultures. Rev. Bras. Parasitol. Vet. 2013, 22, 143–146. [Google Scholar] [CrossRef]
- Xiao, Y.Z.; Wu, D.K.; Zhao, S.Y.; Lin, W.M.; Gao, X.Y. Statistical optimization of alkaline protease production from Penicillium citrinum YL-1 under solid-state fermentation. Prep. Biochem. Biotechnol. 2015, 45, 447–462. [Google Scholar] [CrossRef]
- Dennis, W.R.; Stone, W.M.; Swanson, L.E. A new laboratory and field diagnostic test for fluke ova in feces. J. Am. Vet. Med. Assoc. 1954, 124, 47–50. [Google Scholar] [PubMed]
- Ayres, M.; Ayres Júnior, M.; Ayres, D.L.; Santos, A.A. Bioestat—Aplicações Estatísticas nas Áreas das Ciências Bio-Médicas; Ong Mamirauá: Belém, Brazil, 2007. [Google Scholar]
- Mendoza-De Gives, P.; Vazquez-Prats, V.M. Reduction of Haemonchus contortus infective larvae by three nematophagous fungi in sheep faecal cultures. Vet. Parasitol. 1994, 55, 197–203. [Google Scholar] [CrossRef]
- Kassa, A.; Brownbridge, M.; Parker, B.L.; Skinner, M.; Gouli, V.; Gouli, S.; Hata, T. Whey for mass production of Beauveria bassiana and Metarhizium anisopliae. Mycol. Res. 2008, 112, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.A.; Schmaltz, S.; Tres, M.V.; Zabot, G.L.; Kuhn, R.C.; Mazutti, M.A. Process development to obtain a cocktail containing cell-wall degrading enzymes with insecticidal activity from Beauveria bassiana. Biochem. Eng. J. 2020, 156, 107484. [Google Scholar] [CrossRef]
- Bryjak, J.; Rekuć, A. Effective purification of Cerrena unicolor laccase using microfiltration, ultrafiltration and acetone precipitation. Appl. Biochem. Biotechnol. 2010, 160, 2219–2235. [Google Scholar] [CrossRef]
- Baghalabadi, V.; Doucette, A.A. Mass spectrometry profiling of low molecular weight proteins and peptides isolated by acetone precipitation. Anal. Chim. Acta. 2020, 1138, 38–48. [Google Scholar] [CrossRef]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St Leger, R.J.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Monte, J.F.; Moreno, C.J.; Monteiro, J.P.; de Oliveira Rocha, H.A.; Ribeiro, A.R.; Silva, M.S. Use of Zymography in Trypanosomiasis Studies, In Zymography. Methods in Molecular Biology; Wilkesman, J., Kurz, L., Eds.; Humana Press: New York, NY, USA, 2017; pp. 213–220. [Google Scholar] [CrossRef]
- Arias-Aravena, M.; Altimira, F.; Gutiérrez, D.; Ling, J.; Tapia, E. Identification of exoenzymes secreted by entomopathogenic fungus Beauveria pseudobassiana RGM 2184 and their effect on the degradation of cocoons and pupae of quarantine pest Lobesia botrana. J. Fungi. 2022, 8, 1083. [Google Scholar] [CrossRef] [PubMed]
- Vikhe, A.G.; Dale, N.S.; Umbarkar, R.B.; Labade, G.B.; Savant, A.R.; Walunj, A.A. Isolation and Purification of Cuticle Degrading Extra Cellular Proteases from Entomopathogenic Fungal Species of Beauveria bassiana. Int. J. Pure Appl. Biosci. 2016, 4, 130–135. [Google Scholar] [CrossRef]
- Alghanimi, A.A.; Alebadi, S.M.; Al-ethari, A.Y. Partial purification and characterization of protease from local isolate of Beauveria bassiana. Sci. J. Med. Res. 2020, 4, 17–22. [Google Scholar]
- Gao, B.J.; Mou, Y.N.; Tong, S.M.; Ying, S.H.; Feng, M.G. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence 2020, 11, 365–380. [Google Scholar] [CrossRef]
- Urtz, B.E.; Rice, W.C. Purification and characterization of a novel extracellular protease from Beauveria bassiana. Mycol. Res. 2000, 104, 180–186. [Google Scholar] [CrossRef]
- Firouzbakht, H.; Zibaee, A.; Hoda, H.; Sohani, M.M. Purification and characterization of the cuticle-degrading proteases produced by an isolate of Beauveria bassiana using the cuticle of the predatory bug, Andrallus spinidens Fabricius (Hemiptera: Pentatomidae). J. Plant Prot. Res. 2015, 55, 179–186. [Google Scholar] [CrossRef][Green Version]
- Borgi, I.; Dupuy, J.W.; Blibech, I.; Lapaillerie, D.; Lomenech, A.M.; Rebai, A.; Gargouri, A. Hyper-proteolytic mutant of Beauveria bassiana, a new biological control agent against the tomato borer. Agron. Sustain. Dev. 2016, 36, 60. [Google Scholar] [CrossRef][Green Version]
- Shankar, S.; Laxman, R.S. Biophysicochemical characterization of an alkaline protease from Beauveria sp. MTCC 5184 with multiple applications. Appl. Biochem. Biotechnol. 2015, 175, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2017, 46, 624–632. [Google Scholar] [CrossRef]
- Andreis, F.C.; Schrank, A.; Thompson, C.E. Molecular evolution of Pr1 proteases depicts ongoing diversification in Metarhizium spp. Mol. Genet. Genom. 2019, 294, 901–917. [Google Scholar] [CrossRef]
- Sánchez-Pérez, L.D.C.; Barranco-Florido, J.E.; Rodríguez-Navarro, S.; Cervantes-Mayagoitia, J.F.; Ramos-López, M.Á. Enzymes of entomopathogenic fungi, advances and insights. Adv. Enzym. Res. 2014, 2, 65–76. [Google Scholar] [CrossRef]
- Shin, T.Y.; Lee, M.R.; Park, S.E.; Lee, S.J.; Kim, W.J.; Kim, J.S. Pathogenesis-related genes of entomopathogenic fungi. Arch. Insect Biochem. Physiol. 2020, 105, e21747. [Google Scholar] [CrossRef]
- Gilliland, G.L.; Teplyakov, A. Structural calcium (trypsin, subtilisin). In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–14. [Google Scholar] [CrossRef]
- Azrin, N.A.; Ali, M.S.; Rahman, R.N.; Oslan, S.N.; Noor, N.D. Versatility of subtilisin: A review on structure, characteristics, and applications. Biotechnol. Appl. Biochem. 2022, 69, 2599–2616. [Google Scholar] [CrossRef]
- Ramos-Llorca, A.; Decraecker, L.; Cacheux, V.M.; Zeiburlina, I.; De Bruyn, M.; Battut, L.; Augustyns, K. Chemically diverse activity-based probes with unexpected inhibitory mechanisms targeting trypsin-like serine proteases. Front. Chem. 2023, 10, 1089959. [Google Scholar] [CrossRef]
- Dias, B.A.; Neves, P.M.; Furlaneto-Maia, L.; Furlaneto, M.C. Cuticale degrading proteases produced by the entomopathogenic fungus Beauveria bassiana in the presence of coffee berry borer cuticle. Braz. J. Microbiol. 2008, 39, 301–306. [Google Scholar] [CrossRef]
- Pour, S.A.; Zibaee, A.; Rostami, M.G.; Hoda, H.; Shahriari, M. Mortality and immune challenge of a native isolate of Beauveria bassina against the larvae of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Egypt. J. Biol. Pest Control 2021, 31, 37. [Google Scholar] [CrossRef]
- Pinheiro, J.; Franco-Acuña, D.O.; Oliveira-Menezes, A.; Brandolini, S.V.; Adnet, F.A.; Torres, E.L.; Damatta, R.A. Additional study of the morphology of eggs and miracidia of Eurytrema coelomaticum (Trematoda). Helminthologia 2015, 52, 244–251. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic resistance and its mechanism: A review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Charlier, J.; Bartley, D.J.; Sotiraki, S.; Martinez-Valladares, M.; Claerebout, E.; von Samson-Himmelstjerna, G.; Rinaldi, L. Anthelmintic resistance in ruminants: Challenges and solutions. Adv. Parasitol. 2022, 115, 171–227. [Google Scholar] [CrossRef] [PubMed]
- Jubb, K.V. The pancreas. In Pathology of Domestic Animals; Jubb, K.V., Kennedy, P.C., Palmer, N., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 407–424. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa, L.B.P.; Alves, A.d.C.; da Silva, A.T.; de Souza, D.C.; Sales, N.K.L.; Flores, L.V.; Moreira, T.F.; Braga, F.R.; Soares, F.E.d.F. In Vitro Evaluation of the Ovicidal Potential of Proteases from Beauveria bassiana Against Eurytrema pancreaticum Eggs. Microbiol. Res. 2025, 16, 127. https://doi.org/10.3390/microbiolres16060127
Figueroa LBP, Alves AdC, da Silva AT, de Souza DC, Sales NKL, Flores LV, Moreira TF, Braga FR, Soares FEdF. In Vitro Evaluation of the Ovicidal Potential of Proteases from Beauveria bassiana Against Eurytrema pancreaticum Eggs. Microbiology Research. 2025; 16(6):127. https://doi.org/10.3390/microbiolres16060127
Chicago/Turabian StyleFigueroa, Lisseth Bibiana Puentes, Amanda do Carmo Alves, Adriane Toledo da Silva, Debora Castro de Souza, Nivia Kelly Lima Sales, Lorrana Verdi Flores, Tiago Facury Moreira, Fabio Ribeiro Braga, and Filippe Elias de Freitas Soares. 2025. "In Vitro Evaluation of the Ovicidal Potential of Proteases from Beauveria bassiana Against Eurytrema pancreaticum Eggs" Microbiology Research 16, no. 6: 127. https://doi.org/10.3390/microbiolres16060127
APA StyleFigueroa, L. B. P., Alves, A. d. C., da Silva, A. T., de Souza, D. C., Sales, N. K. L., Flores, L. V., Moreira, T. F., Braga, F. R., & Soares, F. E. d. F. (2025). In Vitro Evaluation of the Ovicidal Potential of Proteases from Beauveria bassiana Against Eurytrema pancreaticum Eggs. Microbiology Research, 16(6), 127. https://doi.org/10.3390/microbiolres16060127







