Retrospective Study 2019–2021 of Antimicrobial Resistance in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis in Mexicali, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Bacterial Identification and Antimicrobial Susceptibility Testing
2.3. Statistical Analysis
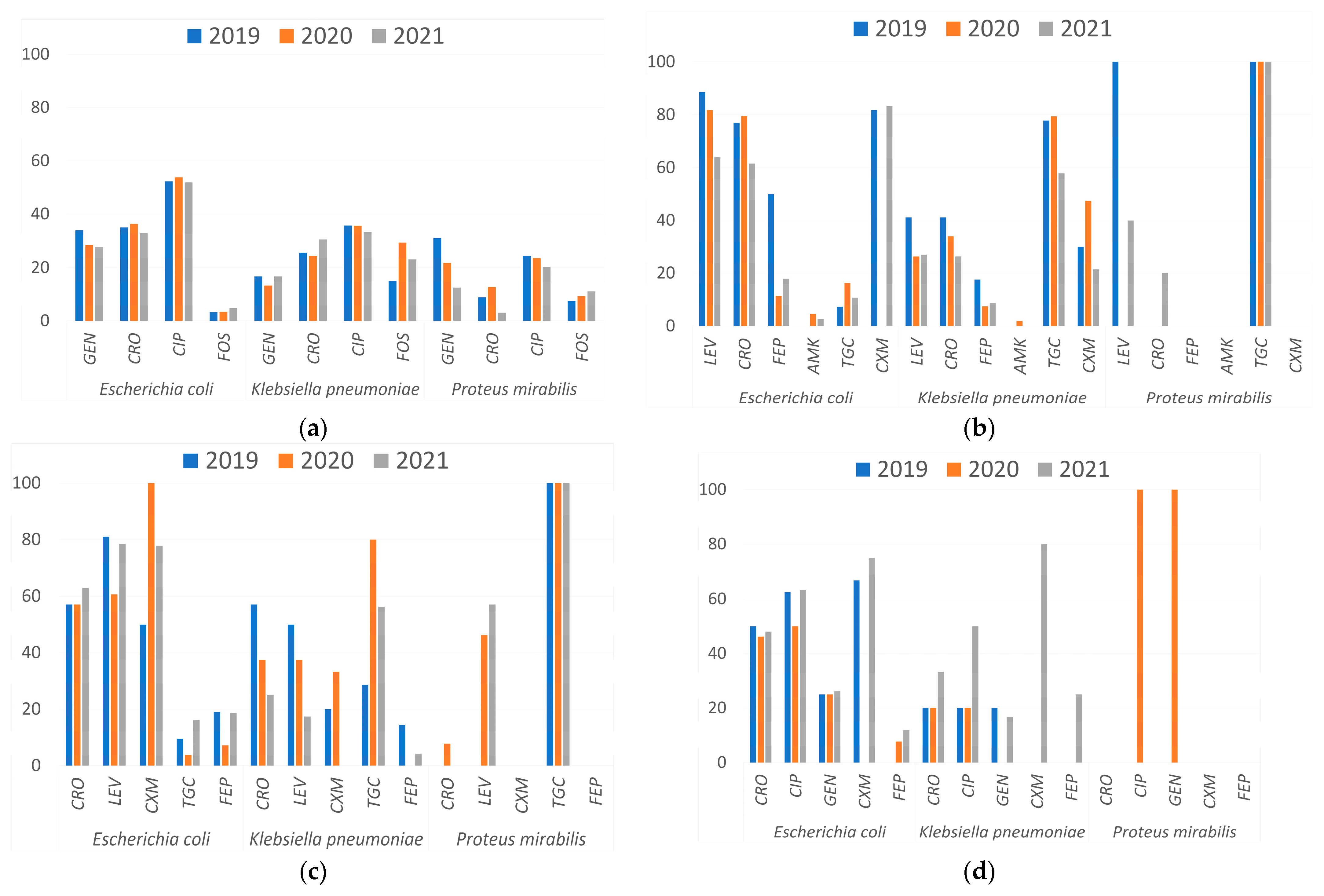
3. Results
3.1. Antimicrobial Resistance Profile by Infection Site
- Urinary Tract Infections (UTIs)
- Lower Respiratory Tract Infections (LRTIs)
- Soft Tissue Infections
- Bloodstream Infections
3.2. Carbapenem Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okeke, I.N.; de Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The Scope of the Antimicrobial Resistance Challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, M.J.; Zarei, J.; Roshani-Asl, P.; Yazdanyar, Z.; Sharif, M.; Rashidi, N. Comprehensive Study of Antimicrobial Susceptibility Pattern and Extended Spectrum Beta-Lactamase (ESBL) Prevalence in Bacteria Isolated from Urine Samples. Sci. Rep. 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Garza-González, E.; Franco-Cendejas, R.; Morfín-Otero, R.; Echaniz-Aviles, G.; Rojas-Larios, F.; Bocanegra-Ibarias, P.; Flores-Treviño, S.; Ponce-De-León, A.; Rodríguez-Noriega, E.; Alavez-Ramírez, N.; et al. The Evolution of Antimicrobial Resistance in Mexico During the Last Decade: Results from the INVIFAR Group. Microb. Drug Resist. 2020, 26, 1372–1382. [Google Scholar] [CrossRef]
- López-Jácome, L.E.; Fernández-Rodríguez, D.; Franco-Cendejas, R.; Camacho-Ortiz, A.; Morfin-Otero, M.D.R.; Rodríguez-Noriega, E.; Ponce-De-León, A.; Ortiz-Brizuela, E.; Rojas-Larios, F.; Velázquez-Acosta, M.D.C.; et al. Increment Antimicrobial Resistance during the COVID-19 Pandemic: Results from the Invifar Network. Microb. Drug Resist. 2022, 28, 338–345. [Google Scholar] [CrossRef]
- Chaaban, T.; Ezzeddine, Z.; Ghssein, G. Antibiotic Misuse during the COVID-19 Pandemic in Lebanon: A Cross-Sectional Study. COVID 2024, 4, 921–929. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Parra, J.; Muiño-Miguez, A.; Bendala-Estrada, A.D.; Ramos-Martínez, A.; Muñez-Rubio, E.; Carracedo, E.F.; Montes, J.T.; Rubio-Rivas, M.; Arnalich-Fernandez, F.; Pérez, J.L.B.; et al. Inappropriate Antibiotic Use in the COVID-19 Era: Factors Associated with Inappropriate Prescribing and Secondary Complications. Analysis of the Registry SEMI-COVID. PLoS ONE 2021, 16, e0251340. [Google Scholar] [CrossRef]
- Palusiak, A. Proteus mirabilis and Klebsiella pneumoniae as Pathogens Capable of Causing Co-Infections and Exhibiting Similarities in Their Virulence Factors. Front. Cell. Infect. Microbiol. 2022, 12, 991657. [Google Scholar] [CrossRef]
- Sokhn, E.S.; Salami, A.; El Roz, A.; Salloum, L.; Bahmad, H.F.; Ghssein, G. Antimicrobial Susceptibilities and Laboratory Profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis Isolates as Agents of Urinary Tract Infection in Lebanon: Paving the Way for Better Diagnostics. Med. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Vance, M.K.; Cretella, D.A.; Ward, L.M.; Vijayvargiya, P.; Garrigos, Z.E.; Wingler, M.J.B. Risk Factors for Bloodstream Infections Due to ESBL-Producing Escherichia coli, Klebsiella Spp., and Proteus mirabilis. Pharmacy 2023, 11, 74. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Uhlemann, A.C.; Peleg, A.Y. Multidrug-Resistant Gram-Negative Bacterial Infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- Torumkuney, D.; De La Torre, C.; Langfeld, K.; Lopez-Turrent, N.P.; Ossaille Beltrame, C. Country Data on AMR in Mexico in the Context of Community-Acquired Respiratory Tract Infections: Links between Antibiotic Susceptibility, Local and International Antibiotic Prescribing Guidelines, Access to Medicine and Clinical Outcome. J. Antimicrob. Chemother. 2022, 77, i43–i50. [Google Scholar] [CrossRef]
- Silva-Sanchez, J.; Barrios, H.; Reyna-Flores, F.; Bello-Diaz, M.; Sanchez-Perez, A.; Rojas, T.; Consortium, B.R.; Garza-Ramos, U. Prevalence and Characterization of Plasmid-Mediated Quinolone Resistance Genes in Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae Isolates in Mexico. Microb. Drug Resist. 2011, 17, 497–505. [Google Scholar] [CrossRef]
- Gallegos-Miranda, V.; Garza-Ramos, U.; Bolado-Martínez, E.; Navarro-Navarro, M.; Félix-Murray, K.R.; Candia-Plata, M.D.C.; Sanchez-Martinez, G.; Dúran-Bedolla, J.; Silva-Sánchez, J. ESBL-Producing Escherichia Coli and Klebsiella Pneumoniae from Health-Care Institutions in Mexico. J. Chemother. 2021, 33, 122–127. [Google Scholar] [CrossRef]
- Silva-Sánchez, J.; Cruz-Trujillo, E.; Barrios, H.; Reyna-Flores, F.; Sánchez-Pérez, A.; Garza-Ramos, U.; Morfin-Otero, R.; Rodríguez-Noriega, E.; Novales, G.M.; Solórzano, F.; et al. Characterization of Plasmid-Mediated Quinolone Resistance (PMQR) Genes in Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae Pediatric Clinical Isolates in Mexico. PLoS ONE 2013, 8, e77968. [Google Scholar] [CrossRef]
- Miranda-Romero, A.L.; Silva-Sanchez, J.; Garza–Ramos, U.; Barrios, H.; Sánchez-Pérez, A.; Reyna-Flores, F. Molecular Characterization of ESBL-Producing Escherichia coli Isolates from Hospital- and Community-Acquired Infections in NW Mexico. Diagn. Microbiol. Infect. Dis. 2017, 87, 49–52. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Mendez-Pfeiffer, P.; Ortíz, B.; Bolado-Martínez, E.; Álvarez-Ainza, M.L.; Enciso-Martínez, Y.; Arenas-Hernández, M.M.P.; Diaz-Murrieta, B.; Barrios-Villa, E.; Valencia, D. Uropathogenic E. coli and Hybrid Pathotypes in Mexican Women with Urinary Tract Infections: A Comprehensive Molecular and Phenotypic Overview. Curr. Issues Mol. Biol. 2024, 46, 5909–5928. [Google Scholar] [CrossRef]
- Reyna-Flores, F.; Barrios, H.; Garza-Ramos, U.; Sánchez-Pérez, A.; Rojas-Moreno, T.; Uribe-Salas, F.J.; Fagundo-Sierra, R.; Silva-Sanchez, J. Molecular Epidemiology of Escherichia coli O25b-ST131 Isolates Causing Community-Acquired UTIs in Mexico. Diagn. Microbiol. Infect. Dis. 2013, 76, 396–398. [Google Scholar] [CrossRef]
- Robles-Torres, J.I.; Ocaña-Munguía, M.A.; Madero Morales, P.A.; Ruiz-Galindo, E.; Garza-González, E.; Gómez-Guerra, L. Antimicrobial Resistance and Extended Spectrum Beta-Lactamases in Urinary Tract Infections: A Serious Problem in Northern Mexico. Rev. Mex. Urología 2020, 80, 1–12. [Google Scholar] [CrossRef]
- Freiwald, A.; Sauer, S. Phylogenetic Classification and Identification of Bacteria by Mass Spectrometry. Nat. Protoc. 2009, 4, 732–742. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. M100 (Ed 32); Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2022. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=518&cHash=2509b0db92646dffba041406dcc9f20c (accessed on 8 January 2024).
- Ballesteros-Monrreal, M.G.; Mendez-Pfeiffer, P.; Barrios-Villa, E.; Arenas-Hernández, M.M.P.; Enciso-Martínez, Y.; Sepúlveda-Moreno, C.O.; Bolado-Martínez, E.; Valencia, D. Uropathogenic Escherichia coli in Mexico, an Overview of Virulence and Resistance Determinants: Systematic Review and Meta-Analysis. Arch. Med. Res. 2023, 54, 247–260. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population Genomics of Klebsiella Pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- CENETEC. Diagnóstico y Tratamiento de La Neumonía Bacteriana Adquirida En La Comunidad En Población Menor a 18 Años. Available online: http://www.cenetec-difusion.com/CMGPC/GPC-SS-120-21/ER.pdf (accessed on 17 September 2024).
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. In An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America; American Thoracic Society Documents: San Francisco, CA, USA, 2019; Volume 200, pp. E45–E67. [Google Scholar] [CrossRef]
- Bontron, S.; Poirel, L.; Kieffer, N.; Savov, E.; Trifonova, A.; Todorova, I.; Kueffer, G.; Nordmann, P. Increased Resistance to Carbapenems in Proteus mirabilis Mediated by Amplification of the blaVIM-1-Carrying and IS26-Associated Class 1 Integron. Microb. Drug Resist. 2019, 25, 663–667. [Google Scholar] [CrossRef]
- Girlich, D.; Bonnin, R.A.; Dortet, L.; Naas, T. Genetics of Acquired Antibiotic Resistance Genes in Proteus Spp. Front. Microbiol. 2020, 11, 500668. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Larios, F.; Martínez-Guerra, B.A.; López-Jácome, L.E.; Bolado-Martínez, E.; Vázquez-Larios, M.D.R.; Velázquez-Acosta, M.D.C.; Romero-Romero, D.; Mireles-Dávalos, C.D.; Quintana-Ponce, S.; Feliciano-Guzmán, J.M.; et al. Active Surveillance of Antimicrobial Resistance and Carbapenemase-Encoding Genes According to Sites of Care and Age Groups in Mexico: Results from the INVIFAR Network. Pathogens 2023, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Valles, R.; Marquez-Salazar, D.A.; Rechy-Iruretagoyena, D.A.; Hernandez-Acevedo, G.N.; Arauz-Cabrera, J.I.; Barrios-Villa, E. Investigation of the Beta-Lactam Resistance Profile in Pseudomonas Aeruginosa Strains in Mexicali: 2019–2021. Rev. Argent. Microbiol. 2024, 56, 368–372. [Google Scholar] [CrossRef]
- Arauz-Cabrera, J.; Marquez-Salazar, D.; Delgadillo-Valles, R.; Caporal-Hernandez, L.; Hernandez-Acevedo, G.N.; Barrios-Villa, E. Genomic Profile of a Multidrug-Resistant Klebsiella Pneumoniae Strain Isolated from a Urine Specimen. Curr. Microbiol. 2024, 81, 276. [Google Scholar] [CrossRef]
- Garza-González, E.; Camacho-Ortiz, A.; Ponce-De-Leon, A.; Ortiz-Brizuela, E.; López-Jácome, L.E.; Colin, C.; Rojas-Larios, F.; Newton-Sánchez, O.A.; Echaniz-Aviles, G.; Carnalla-Barajas, M.N.; et al. Bacterial Incidence and Drug Resistance from Pathogens Recovered from Blood, Cerebrospinal and Pleural Fluids in 2019–2020. Results of the Invifar Network. PeerJ 2023, 11, e14411. [Google Scholar] [CrossRef]
- Tillotson, G.S. Trojan Horse Antibiotics–A Novel Way to Circumvent Gram-Negative Bacterial Resistance? Infect. Dis. 2016, 9, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, Z.; Ghssein, G. Towards New Antibiotics Classes Targeting Bacterial Metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef] [PubMed]
- Fox, V.; Mangioni, D.; Renica, S.; Comelli, A.; Teri, A.; Zatelli, M.; Orena, B.S.; Scuderi, C.; Cavallero, A.; Rossi, M.; et al. Genomic Characterization of Klebsiella pneumoniae Carbapenemase-Producing Klebsiella pneumoniae (KPC-Kp) Strains Circulating in Three University Hospitals in Northern Italy over Three Years. Antimicrob. Resist. Infect. Control. 2024, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Bakr, K.I.; Abdul-Rahman, S.M.; Muhammad Hamasalih, R. Molecular Detection of β-Lactamase Genes in Klebsiella pneumoniae and Escherichia coli Isolated from Different Clinical Sources. Cell. Mol. Biol. 2022, 67, 170–180. [Google Scholar] [CrossRef]
- Ludden, C.; Coll, F.; Gouliouris, T.; Restif, O.; Blane, B.; Blackwell, G.A.; Kumar, N.; Naydenova, P.; Crawley, C.; Brown, N.M.; et al. Defining Nosocomial Transmission of Escherichia Coli and Antimicrobial Resistance Genes: A Genomic Surveillance Study. Lancet Microbe 2021, 2, e472–e480. [Google Scholar] [CrossRef]
| Sample Source | Microorganism | Number of Isolates (%) | ESBL-Producing Isolates | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | Total (%) | |||
| Lower respiratory tract | E. coli | 114 (5.1) | 20 | 34 * | 21 * | 75 (65.8) |
| K. pneumoniae | 164 (37.9) | 6 | 18 | 24 | 48 (29.3) | |
| P. mirabilis ** | 8 (3.9) | 0 | 0 | 0 | 0 (0) | |
| Soft tissue | E. coli | 123 (5.5) | 11 | 15 | 42 | 68 (55.3) |
| K. pneumoniae | 40 (9.2) | 4 | 3 | 7 | 14 (35.0) | |
| P. mirabilis ** | 24 (11.8) | 0 | 0 | 0 | 0 (0) | |
| Sterile body fluids 1 | E. coli | 65 (2.9) | 8 | 5 | 18 | 31 (47.7) |
| K. pneumoniae | 6 (1.4) | 1 | 1 | 0 | 2 (33.3) | |
| P. mirabilis ** | 0 (0) | 0 | 0 | 0 | 0 (0) | |
| Blood | E. coli | 46 (2.1) | 2 | 6 | 9 | 17 (34.0) |
| K. pneumoniae | 17 (3.9) | 1 | 1 | 2 | 4 (23.5) | |
| P. mirabilis ** | 1 (0.005) | 0 | 0 | 0 | 0 (0) | |
| Urinary tract | E. coli | 1803 (80.7) | 198 | 179 | 237 | 614 (34.1) |
| K. pneumoniae | 181 (41.8) | 10 | 11 | 24 | 46 (25.4) | |
| P. mirabilis ** | 164 (80.4) | 0 | 0 | 0 | 0 (0) | |
| Other 2 | E. coli | 83 (3.7) | 11 | 10 | 14 | 35 (42.2) |
| K. pneumoniae | 25 (5.7) | 2 | 2 | 3 | 7 (28.0) | |
| P. mirabilis ** | 7 (3.4) | 0 | 0 | 0 | 0 (0) | |
| Total | E. coli | 2234 | 250 | 249 | 341 | 840 (37.6) |
| K. pneumoniae | 433 | 24 | 36 | 60 | 120 (27.7). | |
| P. mirabilis ** | 204 | 0 | 0 | 0 | 0 (0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Salazar, D.A.; Delgadillo-Valles, R.; Hernández-Acevedo, G.N.; Barrios-Villa, E.; Muñiz-Salazar, R.; López-Valencia, G.; Martínez-Miranda, R.; Arauz-Cabrera, J. Retrospective Study 2019–2021 of Antimicrobial Resistance in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis in Mexicali, Mexico. Microbiol. Res. 2025, 16, 126. https://doi.org/10.3390/microbiolres16060126
Márquez-Salazar DA, Delgadillo-Valles R, Hernández-Acevedo GN, Barrios-Villa E, Muñiz-Salazar R, López-Valencia G, Martínez-Miranda R, Arauz-Cabrera J. Retrospective Study 2019–2021 of Antimicrobial Resistance in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis in Mexicali, Mexico. Microbiology Research. 2025; 16(6):126. https://doi.org/10.3390/microbiolres16060126
Chicago/Turabian StyleMárquez-Salazar, Dolores A., Ricardo Delgadillo-Valles, Gerson N. Hernández-Acevedo, Edwin Barrios-Villa, Raquel Muñiz-Salazar, Gilberto López-Valencia, Rafael Martínez-Miranda, and Jonathan Arauz-Cabrera. 2025. "Retrospective Study 2019–2021 of Antimicrobial Resistance in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis in Mexicali, Mexico" Microbiology Research 16, no. 6: 126. https://doi.org/10.3390/microbiolres16060126
APA StyleMárquez-Salazar, D. A., Delgadillo-Valles, R., Hernández-Acevedo, G. N., Barrios-Villa, E., Muñiz-Salazar, R., López-Valencia, G., Martínez-Miranda, R., & Arauz-Cabrera, J. (2025). Retrospective Study 2019–2021 of Antimicrobial Resistance in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis in Mexicali, Mexico. Microbiology Research, 16(6), 126. https://doi.org/10.3390/microbiolres16060126






