Zika Virus in Malaria-Endemic Populations: A Climate Change-Driven Syndemic in the Sudan Savannah, Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design
2.3. Sample Collection
2.4. Serological Assay
2.5. Data Analysis
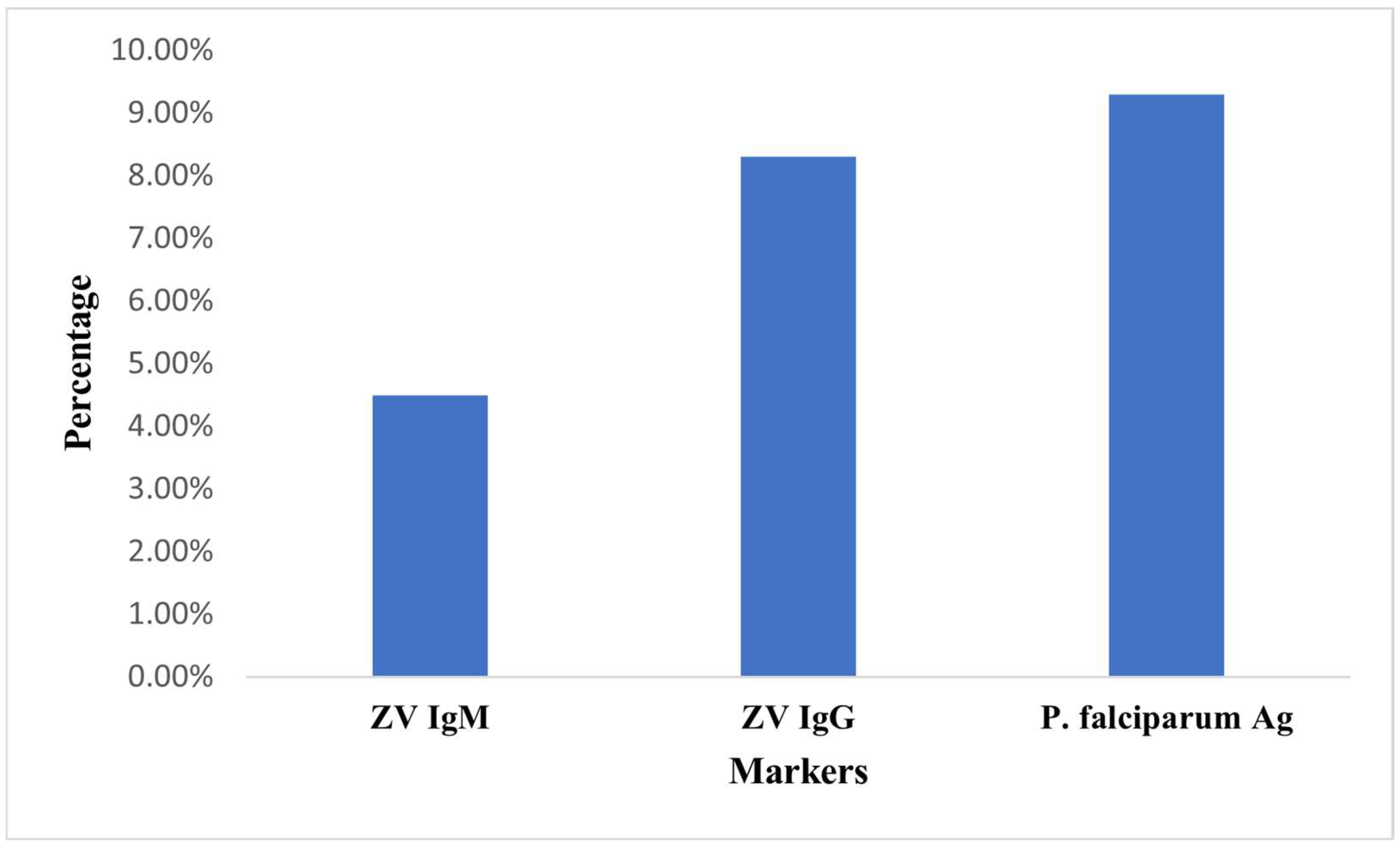
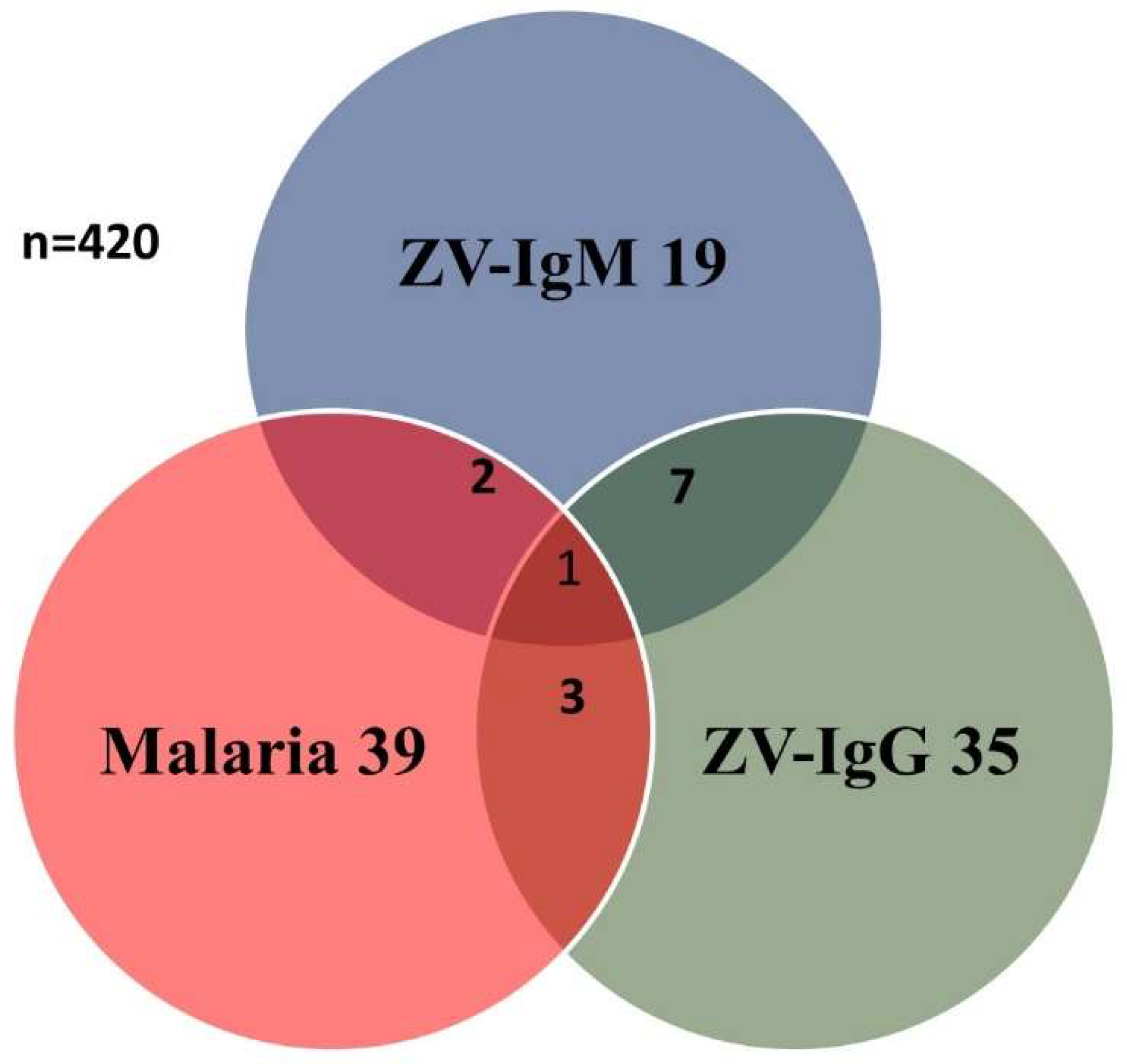
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LGA | Local Government Area |
| ZV | Zika Virus |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| ELISA | Enzyme Linked Immunosorbent Assay |
| RNA | Ribonucleic Acid |
| ORF | Open Reading Frame |
| C | Capsid |
| M | Membrane |
| E | Envelope |
| NS | Non-structural |
| ZVF | Zika Virus Fever |
| GBS | Guillain-Barre Syndrome |
| ADEM | Acute Disseminated Encephalomyelitis |
| EIP | Extrinsic Incubation Period |
| SD | Senatorial District |
| RDT | Rapid Diagnostic Test |
| Ag | Antigen |
| CI | Confidence Interval |
| OR | Odds Ratio |
| ITNs | Insecticide Treated Nets |
| χ2 | Chi Square |
References
- Kindhauser, K.M.; Allen, T.; Frank, V.; Santhana, S.R.; Dye, C. Zika: The origin and spread of a mosquito-borne virus. Bull. World Health Organ. 2016, 94, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Zika Virus Seroprevalence, French Polynesia, 2014–2015. Emerg. Infect Dis. 2017, 23, 669–672. [Google Scholar] [CrossRef]
- Noisumdaeng, P.; Dangsagul, D.; Sangsiriwut, K.; Prasertsopon, J.; Changsom, D.; Yoksan, S.; Ajawatanawong, P.; Buathong, R.; Puthavathana, P. Molecular characterization and geographical distribution of Zika virus worldwide from 1947 to 2022. Int. J. Infect. Dis. 2023, 133, 5–10. [Google Scholar] [CrossRef]
- Faye, O.; Faye, O.; Diallo, D.; Diallo, M.; Weidmann, M.; Sall, A.A. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol. J. 2013, 10, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; Signor Ldel, C. Phylogeny of Zika Virus in Western Hemisphere, 2015. Emerg. Infect Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Centres for Disease Control and Prevention. Clinical Signs and Symptoms of Zika Virus Disease. Available online: https://www.cdc.gov/zika/hcp/clinical-signs/index.html#:~:text=Characteristic%20clinical%20findings%20associated%20with,several%20days%20to%20a%20week (accessed on 15 May 2025).
- Hamel, R.; Liégeois, F.; Wichit, S.; Pompon, J.; Diop, F.; Talignani, L.; Missé, D. Zika virus: Epidemiology, clinical features and host-virus interactions. Microbes. Infect. 2016, 18, 441–449. [Google Scholar] [CrossRef]
- World Health Organization. Zika Virus Technical Report: Interim Risk Assessment WHO European Region. 2016. Available online: https://www.who.int/europe/publications/zika-virus-technical-report.-interim-risk-assessment-for-who-european-region (accessed on 23 May 2025).
- Rojas, D.P.; Dean, N.E.; Yang, Y.; Kenah, E.; Quintero, J.; Tomasi, S.; Ramirez, E.L.; Kelly, Y.; Castro, C.; Carrasquilla, G.; et al. The Epidemiology and Transmissibility of Zika Virus in Girardot and San Andres Island, Colombia, September 2015 to January 2016. Euro Surveill. 2016, 21, 30283. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Zika Virus: Symptoms & Diagnosis. 2024. Available online: https://www.cdc.gov (accessed on 26 March 2025).
- Brito Ferreira, M.L.; Antunes de Brito, C.A.; Moreira, Á.J.P.; de Morais Machado, M.Í.; Henriques-Souza, A.; Cordeiro, M.T.; de Azevedo Marques, E.T.; Pena, L.J. Guillain-Barré Syndrome, Acute Disseminated Encephalomyelitis and Encephalitis Associated with Zika Virus Infection in Brazil: Detection of Viral RNA and Isolation of Virus during Late Infection. Am. J. Trop. Med. Hyg. 2017, 97, 1405–1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization (WHO). Zika Virus Fact Sheet. 2024. Available online: https://www.who.int (accessed on 26 March 2025).
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection-After the Pandemic. NEJM. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Ramos da Silva, S.; Gao, S. Zika Virus: An Update on Epidemiology, Pathology, Molecular Biology, and Animal Model. J. Med. Virol. 2016, 88, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, Y.; Zhang, W. Zika virus outbreak: ‘A perfect storm’. Emerg. Microbes Infect. 2016, 5, e21. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, A.P.R.; Gibson, G.; Ramos, A.N., Jr.; Bloch, K.V.; Sousa, G.dS.; de Silva, T.L.N.; Braga, U.J.; Castro, C.M.; Werneck, L.G. Sociodemographic and environmental factors associated with dengue, Zika, and chikungunya among adolescents from two Brazilian capitals. PLoS Negl. Trop. Dis. 2023, 17, e0011197. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.; Liu, M.; Liu, J. Global burden and incidence trends of zika virus infection among women aged 15–49 years from 2011 to 2021: A systematic analysis. J. Infect. Public Health 2024, 17, 102557. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Berto, A.; Sinigaglia, A.; Franchin, E.; Lavezzo, E.; Brugnaro, P.; Palu, G. Isolation of Infectious Zika Virus from Saliva and Prolonged Viral RNA Shedding in a Traveller Returning from the Dominican Republic to Italy. Euro Surveill. 2016, 21, 30159. [Google Scholar] [CrossRef] [PubMed]
- Ioos, S.; Mallet, H.P.; Leparc Goffart, I.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika Virus Epidemiology and Recent Epidemics. Med. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef]
- Fagbami, A.H. Zika virus infections in Nigeria: Virological and Seroepidemiological Investigations in Oyo State. J. Hyg. 1978, 83, 213–219. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Malaria Report 2024. Lancet Microbe 2024, 6, 101073. Available online: https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(25)00001-1/fulltext (accessed on 26 March 2025).
- Pattanshetty, S.; Dsouza, V.S.; Shekharappa, A.; Yagantigari, M.; Raj, R.; Inamdar, A.; Alsamara, I.; Rajvanshi, H.; Brand, H. A Scoping Review on Malaria Prevention and Control Intervention in Fragile and Conflict-Affected States (FCAS): A Need for Renewed Focus to Enhance International Cooperation. J. Epidemiol. Glob. Health 2024, 14, 4–12. [Google Scholar] [CrossRef]
- ELmojtaba, I.M.; Al-Maqrashi, K.; Al-Musalhi, F.; Al-Salti, N. Optimal control and cost effectiveness analysis of a Zika–Malaria co-infection model. Partial. Differ. Equ. Appl. Math. 2024, 11, 100754. [Google Scholar] [CrossRef]
- Mac, P.A.; Kroeger, A.; Daehne, T.; Anyaike, C.; Velayudhan, R.; Panning, M. Zika, Flavivirus and Malaria Antibody Cocirculation in Nigeria. Trop. Med. Infect. Dis. 2023, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.M.; Ahmed, A.; Jackson, S.Y.; Oderinde, B.S. Cryptic Zika virus infections unmasked from suspected malaria cases in Northeastern Nigeria. PLoS ONE 2023, 18, e0292350. [Google Scholar] [CrossRef] [PubMed]
- McKinney, K.L.; Wu, H.M.; Tan, K.R.; Gutman, J.R. Malaria in the pregnant traveler. J. Travel Med. 2020, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, A.O.; Afinowi, A.O.; Tauheed, M.A.; Danazumi, U.A.; Dibba, B.S.L.; Balogun, B.J.; Flore, G.; Saidu, U.; Ibrahim, B.; Balogun, O.O.; et al. Multisectoral Perspectives on Global Warming and Vector-borne Diseases: A Focus on Southern Europe. Curr. Trop. Med. Rep. 2023, 10, 47–70. [Google Scholar] [CrossRef]
- Delrieu, M.; Martinet, J.-P.; O’Connor, O.; Viennet, E.; Menkes, C.; Burtet-Sarramegna, V.D.; Frentiu, F.; Dupont-Rouzeyrol, M. Temperature and transmission of chikungunya, dengue, and Zika viruses: A systematic review of experimental studies on Aedes aegypti and Aedes albopictus. Curr. Res. Parasitol. Vector-Borne Dis. 2023, 4, 100139. [Google Scholar] [CrossRef]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef]
- Stopard, I.J.; Sanou, A.; Suh, E.; Cator, L.J.; Thomas, M.B.; Guelbéogo, W.M.; Sagnon, N.; Lambert, B.; Churcher, T.S. Modelling the effects of diurnal temperature variation on malaria infection dynamics in mosquitoes. Commun. Biol. 2025, 8, 581. [Google Scholar] [CrossRef]
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef]
- Rabiu, I.; Musa, H.A.; Isaiah, Z.; Hussaini, M.; Umar, M.M.; Mustapha, S.; Abdullahi, J.I.; Shehu, A.; Sani, M.A. Dengue outbreaks in northern Nigeria: Evaluating the recommended Takeda vaccine and future prevention strategies. World J. Virol. 2024, 13, 95555. [Google Scholar] [CrossRef]
- Adesola, R.O.; Ajibade, F.A.; Idris, I.; Scott, G.Y.; Agaie, M.I. Addressing the Dengue fever challenges in Nigeria: A narrative review and recommendations for control. Infez. Med. 2024, 32, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Onoja, B.A.; Oguzie, J.U.; George, U.E.; Asoh, K.E.; Ajayi, P.; Omofaye, T.F.; Igeleke, I.O.; Eromon, P.; Harouna, S.; Parker, E.; et al. Whole Genome Sequencing Unravels Cryptic Circulation of Divergent Dengue Virus Lineages in the Rainforest Region of Nigeria. Emerg. Microbes Infect. 2024, 13, 2307511. [Google Scholar] [CrossRef] [PubMed]
- Kaduna State City Population–Population Statistics, Charts, Maps and Location. Available online: https://citypopulation.de/en/nigeria/admin/NGA019__kaduna/ (accessed on 23 May 2025).
- Yayock, H.C.; Ndams, I.S.; Kogi, E.; Ahmed, A.B.; Vagime, C.G. Distribution of Mosquito Species in Kaduna Metropolis, Kaduna State, Northern Nigeria. Nig. J. Ent. 2015, 31, 137–145. Available online: https://www.esnjournal.com.ng/download/Vol_31/Vol_31_X23.pdf (accessed on 23 May 2025). [CrossRef]
- Centres for Disease Control and Prevention. Zika Virus Symptoms from Zika Virus. 2019. Available online: https://www.cdc.gov/zika/signs-symptoms/?CDC_AAref_Val=https://www.cdc.gov/zika/symptoms/index.html (accessed on 23 May 2025).
- Mathé, P.; Egah, D.Z.; Müller, J.A.; Shehu, N.Y.; Obishakin, E.T.; Shwe, D.D.; Pam, B.C.; Okolo, M.O.; Yilgwan, C.; Gomerep, S.S.; et al. Low Zika Virus seroprevalence among pregnant women in North Central Nigeria. J. Clin. Virol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Babaniyi, A.O.; Mwaba, P.; Songolo, P.; Mazaba-Liwewe, L.M.; Mweene-Ndumba, I.; Masaninga, F.; Rudatsikira, E.; Siziya, S. Seroprevalence of Zika virus infection specific IgG in Western and North-Western Provinces of Zambia. Int. J. Public Health 2015, 4, 110–114. [Google Scholar]
- Otu, A.A.; Udoh, A.U.; Ita, I.O.; Hicks, P.J.; Ukpeh, I.; Walle, J. Prevalence of Zika and malaria in patients with fever in secondary healthcare facilities in south-eastern Nigeria. Trop. Doct. 2020, 50, 22–30. [Google Scholar] [CrossRef]
- Anejo-Okopi, J.; Gotom, D.Y.; Chiehiura, N.A.; Okojokwu, J.O.; Amanyi, D.O.; Egbere, J.O.; Adetunji, J.; Ujah, O.I.; Audu, O. The seroprevalence of zika virus infection among HIV positive and HIV negative pregnant women in Jos, Nigeria. Hosts Viruses 2020, 7, 129–136. [Google Scholar] [CrossRef]
- Ekesiobi, A.O.; Anene, C.C.; Nwigwe, H.C.; Emmy-Egbe, I.O.; Igbodika, M.C. Seasonal Distribution and Abundance of Yellow Fever Mosquito Vector, Aedes Aegypti, (Diptera: Culicidae) in Aguatu L.G.A. Anambra State, Nigeria. Chukwuemeka Odumegwu Ojukwu University. Int. Multidiscip. Res. J. 2014, 1, 25–32. [Google Scholar]
- Owusu-Asenso, C.M.; Mingle, J.A.A.; Weetman, D.; Afrane, Y.A. Spatiotemporal distribution and insecticide resistance status of Aedes aegypti in Ghana. Parasites Vectors 2022, 15, 61. [Google Scholar] [CrossRef]
- National Malaria Elimination Programme, Federal Ministry of Health: Malaria Indicator Survey 2015. Abuja 2016. Available online: https://nmcp.gov.ng (accessed on 17 May 2025).
- Bajoga, U.A.; Balarabe, H.S.; Olufemi, A.A.; Dalhat, M.M.; Sule, I.B.; Ibrahim, M.S.; Ajumobi, O.O. Trend of malaria cases in Kaduna State using routine surveillance data, 2011–2015. Pan Afr. Med. J. 2019, 32, 8. [Google Scholar] [CrossRef]
- Lozier, M.; Adams, L.; Febo, M.F.; Torres-Aponte, J.; Bello-Pagan, M.; Ryff, K.R.; Rivera-Garcia, B. Incidence of Zika Virus Disease by Age and Sex—Puerto Rico. Wkly. Rep. 2016, 65, 1219–1223. [Google Scholar] [CrossRef]
- Dos Santos, T.; Rodriguez, A.; Almiron, M.; Sanhueza, A.; Ramon, P.; de Oliveira, W.K.; Espinal, M.A. Zika Virus and the Guillain-Barré Syndrome—Case Series from Seven Countries. N. Engl. J. Med. 2016, 3, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- MacNamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Griffin, J.T.; Ferguson, N.M.; Ghani, A.C. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat. Commun. 2014, 5, 3136. [Google Scholar] [CrossRef]
- Okunlola, O.A.; Oyeyemi, O.T. Spatio-temporal analysis of association between incidence of malaria and environmental predictors of malaria transmission in Nigeria. Sci. Rep. 2019, 9, 17500. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Quijano, F.A.; Martínez-Vega, R.A.; Rodriguez-Morales, A.J.; Rojas-Calero, R.A.; Luna-González, M.L.; Díaz-Quijano, R.G. Association between the level of education and knowledge, attitudes and practices regarding dengue in the Caribbean region of Colombia. BMC Public Health 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Nery, N.J.; Aguilar Ticona, J.P.; Gambrah, C.; Doss-Gollin, S.; Aromolaran, A.; Rastely-Júnior, V.; Costa, F. Social determinants associated with Zika virus infection in pregnant women. PLoS Negl. Trop. Dis. 2021, 15, e0009612. [Google Scholar] [CrossRef]
- Maharajan, M.K.; Rajiah, K.; Belotindos, J.A.S.; Basa, M.S. Social Determinants Predicting the Knowledge, Attitudes, and Practices of Women Toward Zika Virus Infection. Front. Public Health 2020, 8, 170. [Google Scholar] [CrossRef]
- Bearcroft, W.G. Zika virus infection experimentally induced in a human volunteer. Trans. R. Soc. Trop. Med. Hyg. 1956, 50, 442–448. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Hayes, E.B. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Wimalasiri-Yapa, B.M.C.R.; Yapa, H.E.; Huang, X.; Hafner, L.M.; Kenna, T.J.; Frentiu, F.D. Zika Virus and Arthritis/Arthralgia: A Systematic Review and Meta-Analysis. Viruses 2020, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria World Report. 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 12 May 2025).
- Oyefolu, A.O.B.; Shaibu, O.J.; Anjorin, A.A.; Akinyemi, O.K. A Review on The State of Zika Virus in Nigeria. J. Res. Rev. Sci. 2019, 6, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.E.; Cha, G.W.; Cho, J.E.; Lee, E.J.; Jee, Y.; Lee, W.J. Viral and serological kinetics in Zika virus-infected patients in South Korea. Virol. J. 2017, 14, 70. [Google Scholar] [CrossRef]
| Marital Status | No. of Samples | ZV IgM | ZV IgG | Malaria | |||
|---|---|---|---|---|---|---|---|
| No + ve (%) | p Value/χ2 | No + ve (%) | p Value/χ2 | No + ve (%) | p Value/χ2 | ||
| Married | 271 | 13 (4.8) | 0.879/0.247 | 30 (11.1) | 0.023/7.535 | 17 (6.3) | 0.011/9.023 |
| Single | 146 | 6 (4.1) | 5 (3.4) | 22 (15.1) | |||
| Divorced | 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Total | 420 | 19 (8.9) | 35 (14.5) | 39 (21.4) | |||
| Age (Years) | No. of Samples | ZV IgM | ZV IgG | Malaria | |||
|---|---|---|---|---|---|---|---|
| No + ve (%) | p Value/χ2 | No + ve (%) | p Value/χ2 | No + ve (%) | p Value/χ2 | ||
| 1–10 | 49 | 1 (2.0) | 0.834/2.110 | 1 (2.0) | 0.004/17.418 | 9 (18.4) | 0.008/15.656 |
| 11–20 | 69 | 3 (4.3) | 3 (4.3) | 10 (14.5) | |||
| 21–30 | 114 | 7 (6.1) | 10 (8.8) | 12 (10.5) | |||
| 31–40 | 73 | 4 (5.5) | 4 (5.5) | 4 (5.5) | |||
| 41–50 | 45 | 1 (2.2) | 3 (6.7) | 4 (8.9) | |||
| >50 | 70 | 3 (4.3) | 14 (20.0) | 0 (0.0) | |||
| Total | 420 | 19 (24.4) | 34 (47.3) | 39 (57.8) | |||
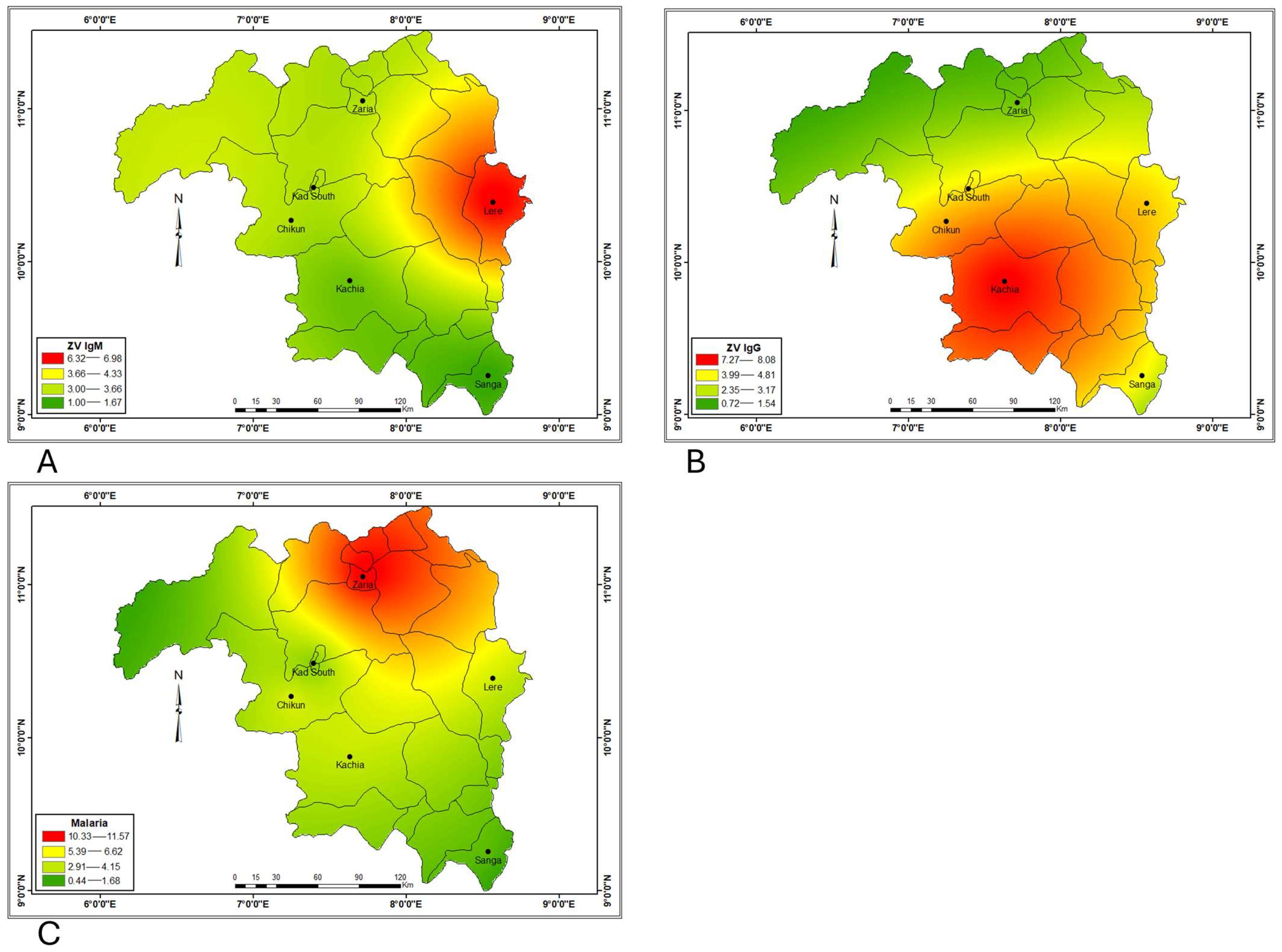
| Variables | No of Samples | Zaria +ve (%) | Chikun + ve (%) | Lere + ve (%) | Sanga + ve (%) | Kachia + ve (%) | K/South + ve (%) | p Value/χ2 |
|---|---|---|---|---|---|---|---|---|
| ZV IgM | 70 | 3 (4.3) | 3 (4.3) | 7 (10.0) | 1 (1.4) | 2 (2.9) | 3 (4.3) | 0.220/ 6.891 |
| ZV IgG | 70 | 2 (2.9) | 6 (8.6) | 6 (8.6) | 6 (8.6) | 10 (14.3) | 5 (7.1) | 0.292/ 6.140 |
| Malaria | 70 | 13 (18.6) | 7 (10.0) | 6 (8.6) | 4 (5.7) | 6 (8.6) | 3 (4.3) | 0.0639/ 10.430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atai, R.B.; Aminu, M.; Ella, E.E.; Kia, G.S.N.; Obishakin, E.T.; Luka, H.G.; Joel, G.S.; Onoja, A.B. Zika Virus in Malaria-Endemic Populations: A Climate Change-Driven Syndemic in the Sudan Savannah, Nigeria. Microbiol. Res. 2025, 16, 109. https://doi.org/10.3390/microbiolres16060109
Atai RB, Aminu M, Ella EE, Kia GSN, Obishakin ET, Luka HG, Joel GS, Onoja AB. Zika Virus in Malaria-Endemic Populations: A Climate Change-Driven Syndemic in the Sudan Savannah, Nigeria. Microbiology Research. 2025; 16(6):109. https://doi.org/10.3390/microbiolres16060109
Chicago/Turabian StyleAtai, Rebecca B., Maryam Aminu, Elijah E. Ella, Grace S. N. Kia, Emmanuel T. Obishakin, Helen G. Luka, Ganih S. Joel, and Anyebe B. Onoja. 2025. "Zika Virus in Malaria-Endemic Populations: A Climate Change-Driven Syndemic in the Sudan Savannah, Nigeria" Microbiology Research 16, no. 6: 109. https://doi.org/10.3390/microbiolres16060109
APA StyleAtai, R. B., Aminu, M., Ella, E. E., Kia, G. S. N., Obishakin, E. T., Luka, H. G., Joel, G. S., & Onoja, A. B. (2025). Zika Virus in Malaria-Endemic Populations: A Climate Change-Driven Syndemic in the Sudan Savannah, Nigeria. Microbiology Research, 16(6), 109. https://doi.org/10.3390/microbiolres16060109






