Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control
Abstract
1. Introduction
2. Silver Nanoparticles in Antimicrobial Coatings: Effect of Size, Shape, and Surface Chemistry
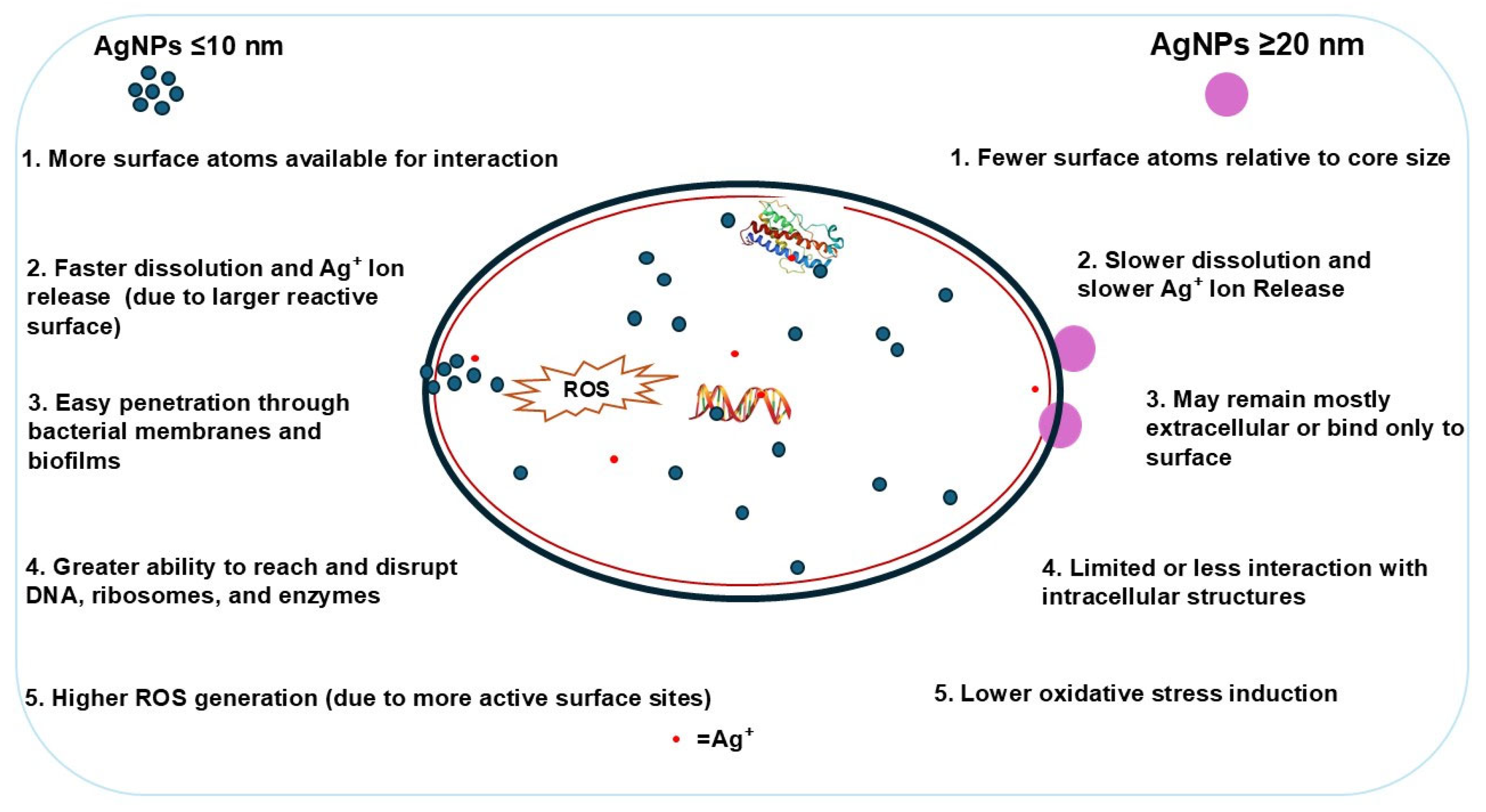
2.1. Effect of Size on Silver Nanoparticles (AgNPs) Antimicrobial Activity
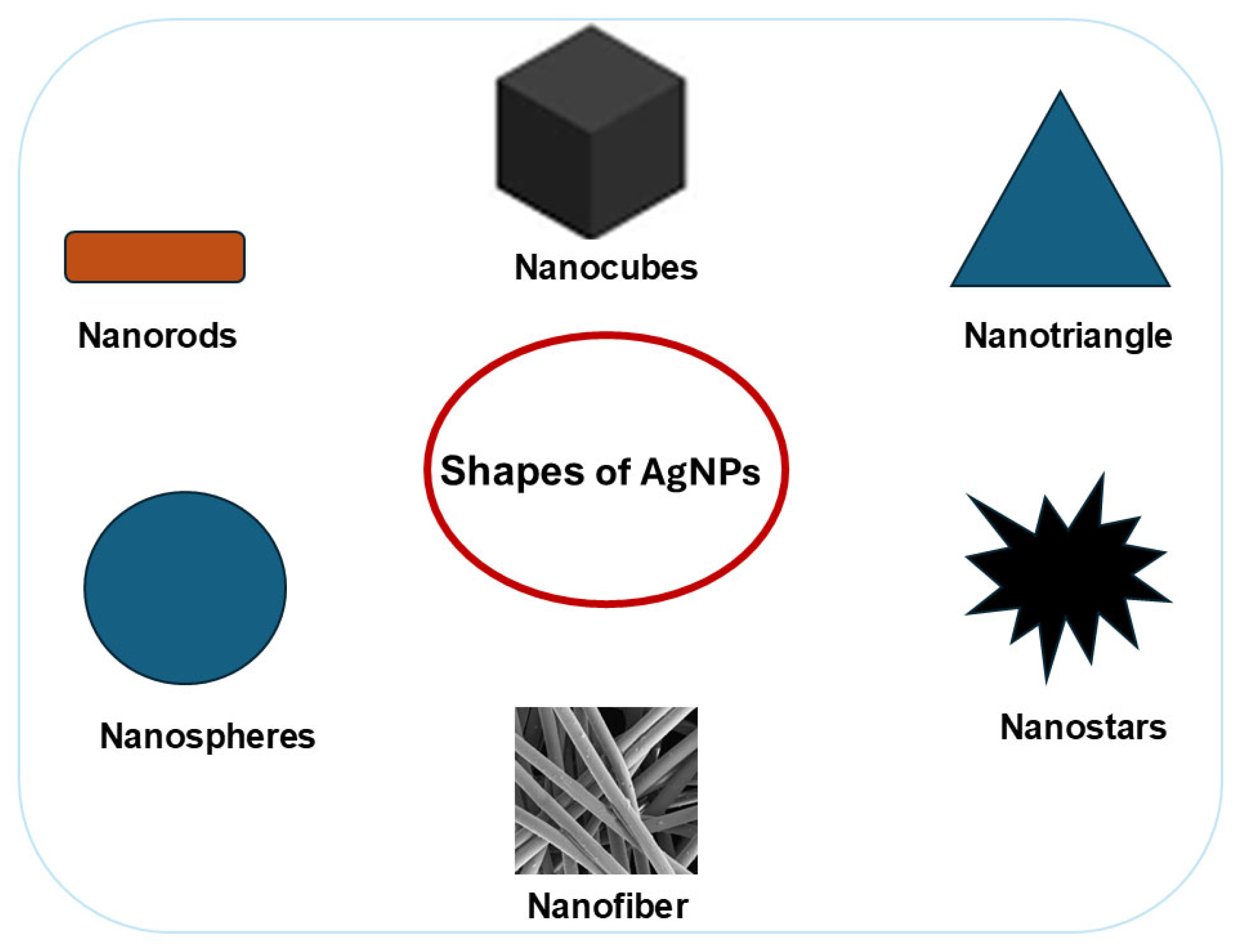
2.2. Effect of Shape on Silver Nanoparticles (AgNPs) Antimicrobial Activity
2.3. Effect of Surface Chemistry and Functionalization on Silver Nanoparticles (AgNPs) Antimicrobial Activity
3. Mechanisms of Antimicrobial Action of Silver Nanoparticles (AgNPs)
3.1. Cell Membrane Disruption and Increased Permeability Nanoparticles
3.2. Penetration and Intracellular Damage
3.3. Silver Nanoparticles (AgNPs) Interaction with Microbial Deoxyribonucleic Acid (DNA), Proteins, and Enzymes
3.3.1. Interaction with Deoxyribonucleic Acid
3.3.2. Interaction with Proteins and Enzymes
3.4. Biofilm Inhibition and Disruption
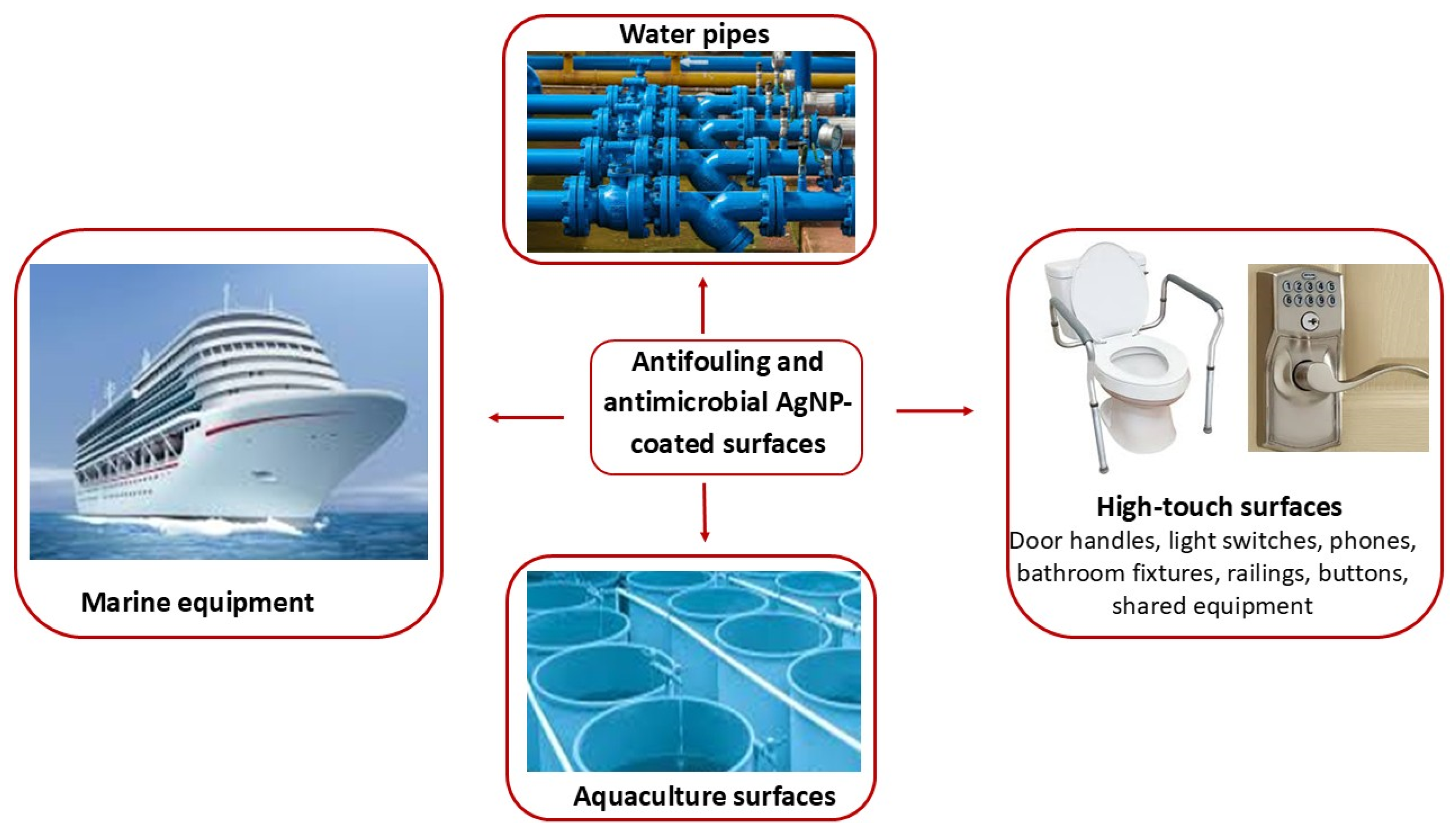
4. Applications of Silver Nanoparticles (AgNPs) Coatings in Antimicrobial Protection
4.1. Silver Nanoparticles (AgNPs) Coating for Medical and Healthcare Applications
| Material | Preparation | Effect | Ref |
|---|---|---|---|
| Poly(3-hydroxybutyrate) (PHB) combined with poly(ethylene glycol) (PEG) and esterified sodium alginate (ALG-e), enhanced with AgNPs for wound dressing | ALG was esterified to form ALG-e, then combined with PHB and PEG in chloroform to create polymeric gels. These gels were loaded with AgNPs and cast into composite films. | The composite film showed good antimicrobial activity in vitro against S. aureus and E.coli. | [119] |
| Polyglactin suture | A dip-coating method was employed, where sutures were first immersed in a polyethyleneimine solution and then dipped into an AgNP solution. | AgNP-coated polyglactin sutures exhibited activity against E. coli, S. aureus, and P. aeruginosa. | [120] |
| Silk sutures | Silk sutures were coated with AgNPs using a dip coating method | Highest antibacterial effectiveness against S. aureus, E. coli, and P. aeruginosa. | [121] |
| Silk sutures were coated with AgNPs using a dip coating method | Significant inhibitory activity against S. aureus. | [122] | |
| The suture sample was immersed in a silver solution, where in situ photoreduction facilitated the synthesis of silver particles on its surface. | AgNP-coated silk suture showed significant antimicrobial activity against S.mutans and S.aureus, persisting for 7 days. | [123] | |
| Surgical fabric and surgical blade | AgNPs were applied to surgical fabric using a layer-by-layer method, with fabric swatches alternately dipped in poly(diallyldimethylammonium chloride) and AgNPs solutions. Additionally, stainless steel surgical blades were coated with AgNPs via electrochemical deposition. | Antibacterial activity against P. aeruginosa, E. coli, A. baumannii, K. pneumoniae, S. aureus, and E. faecalis and antibiofilm efficacy against P. aeruginosa. | [110] |
| Medical protective masks | AgNPs and polyvinylidene fluoride (PVDF) solutions were mixed and electrospun into nanofibrous membranes. | AgNPs/PVDF membranes have antibacterial efficiency of 99.98% and 99.86% against E. coli and S. aureus. | [124] |
| In situ synthesis was carried out by dissolving silver nitrate in a solution containing chitosan, polyvinyl alcohol (PVA), and poly-ε-caprolactone, followed by electrospinning to produce nanofibers. | AgNP-activated nanofibers provided potent, long-lasting antiviral protection against betacoronavirus. | [107] | |
| AgNP-coated polypropylene surgical masks | AgNPs were deposited on the surface of the spun-bond polypropylene microfiber layer. | Antibacterial activity against S. aureus and E. coli and antiviral effectiveness against SARS-CoV-2. | [125] |
| Medical cotton gauze | Amino-modified cotton gauze was immersed in an AgNPs solution, leading to the formation of AgNP-loaded gauze. | Strong bactericidal activity and strong resistance to the adhesion of E. coli, S. aureus, and MRSA. | [126] |
| Extracorporeal biomaterials (hemodialysis blood tubing) | The dialysis tubing surface was coated with polyethylene glycol, with AgNPs immobilized on the coating. | Antimicrobial activity against E. coli, P. aeruginosa, K. pneumonia, S. aureus, and C. albicans. | [127] |
| Hospital room surface coating | The transparent acid siloxane-based sol-gel was combined with AgNPs and 4,5-dichloro-2-octyl-4-isothiazolin-3-one (DCOIT). The resulting coatings were then sprayed onto the test surfaces. | The coating consistently reduced bacterial load on frequently touched surfaces in a real-world clinical setting for 90 days. | [108] |
| Plasma-polymerized hexamethyldisiloxane (ppHMDSO) protective film on 3D printed polylactic acid substrates | AgNPs coatings were deposited on 3D printed polylactic acid substrates by DC magnetron sputtering. | Overcoating AgNPs with ppHMDSO results in a material with antibacterial properties against P. aeruginosa and antiviral activity against human rhinovirus species A/type 2. | [128] |
| Dental alginate | Dental alginate powder mixed with AgNPs suspension to create a paste. | AgNPs alginate exhibited broad-spectrum activity against the three tested microorganisms: S. aureus, E. coli, and Candida albicans. | [129] |
| AgNPs mixed with alginate powder. | Incorporating AgNPs enhanced dental alginate’s antimicrobial activity against C. albicans, Streptococcus mutans, E. coli, S. aureus, and Micrococcus luteus. | [130] | |
| Denture resins | AgNPs were blended with heat-curing acrylic resin powder polymethyl methacrylate (PMMA) and then mixed with a resin monomer to create a paste. | The PMMA composite disks were active against C. albicans and S. mutans. | [35] |
4.2. Silver Nanoparticle (AgNP)-Coated Textiles Applications
| Material | Preparation | Effect | Ref |
|---|---|---|---|
| Cotton fabric | Cotton fabric soaked in AgNPs solution. | AgNP-treated fabrics exerted antimicrobial activity against P. aeruginosa. | [137] |
| The fabric was immersed in the AgNPs solution with Tween 80, dried at 80 °C, and cured at 130 °C to ensure nanoparticle attachment. | AgNP-coated fabric exhibited superior inhibition against Acinetobacter baumannii, Klebsiella pneumoniae, P. aeruginosa, S. aureus, E. faecalis, and C. albicans. | [117] | |
| Polyamide (PA) fabric | AgNP-treated fabrics were produced via a hybrid plasma reactor using surface activation and AgNP deposition. The PA-elastane fabric was plasma-activated to enhance adhesion before AgNPs were nebulized into the plasma region. | AgNP-treated fabrics exhibited significant antimicrobial activity against S. aureus and K. pneumoniae. | [138] |
| In situ incorporation of AgNPs onto PA fabrics during the dyeing process. | Significant inhibition against both S. aureus and E. coli. | [139] | |
| Three-layer mask with water-repellent mulberry silk, oil-repellent eri silk, and a cotton middle layer treated with AgNPs. | AgNPs coated on the cotton middle layer of a silk face covering. | Silk face coverings treated with AgNPs inhibited S. aureus and E. coli by over 99.9%, maintaining effectiveness even after 20 wash cycles. | [140] |
| Polyimide nanofibers (PINFs), AgNPs, carbon nanotubes (CNTs), and waterborne polyurethane (WPU) nanofabric. | AgNPs are embedded in PINFs using electrospinning and in situ reduction, followed by thermal imidization to form Ag@PINFs. A conductive CNT layer was then added via suction filtration of a CNT/WPU mixture and sandwiched between two Ag@PINF layers. | Inactivation of E. coli and S. aureus. | [114] |
| Polyester Fabrics | The polyester surface was chemically modified with hydrazide groups. Silver ions were then adsorbed and reduced using either glucose to form AgNP-loaded polyester or dopamine to simultaneously create AgNPs and a polydopamine (PDA) coating | AgNP-loaded polyester hydrazide exhibited a bactericidal effect against E. coli after 24 h and a bacteriostatic effect against S. aureus. In contrast, the PDA-coated AgNP-loaded polyester hydrazide composite demonstrated a bactericidal effect against both pathogens. | [141] |
| Polyester fabrics were functionalized via spray deposition with AgNPs dispersed in ethanol. Formulations incorporating chitosan or hexamethyldisiloxane enhanced AgNP adhesion and minimized release during washing. | Adding chitosan or HMDSO layers affected the antimicrobial performance and stability of AgNPs on polyester textiles. Chitosan enhanced adhesion and showed a synergistic effect, particularly against E. coli, while HMDSO contributed to a more controlled release of AgNPs. | [142] | |
| Polyester and cotton fabrics | Polyester and cotton fabrics were silver-plated using electroless plating. Fabrics were pre-treated with tin(II) chloride and hydrochloric acid for uniform metal deposition, then immersed in silver nitrate for 30 s. Daylight facilitated silver nucleation, creating catalytic sites for plating. | AgNP-coated polyester reduced E. coli by 95% in 30 min and 100% in 120 min, while cotton achieved a 78% reduction. Against SARS-CoV-2, cotton achieved a 100% reduction in 30 min and polyester in 60 min. Both fabrics rapidly inhibited Feline Calicivirus, reaching 100% in 30 min. | [9] |
| Cotton, nylon and cotton/nylon fabrics | The antibacterial fabrics were prepared by coating them with chitosan, using triethyl orthoformate as a crosslinker to enhance durable attachment. For silver nanoparticle immobilization, the chitosan-coated fabrics were immersed in an AgNP solution for 24 h at 0 °C, allowing effective nanoparticle binding to the modified fabric surface | AgNP-loaded fabrics demonstrated significant and durable antibacterial activity against both E. coli and S. aureus in laboratory tests and showed strong bacterial control in a real-life scenario, even after repeated washing cycles. | [135] |
| Viscose fabric | The viscose fabric undergoes pre-treatment with a polysiloxane matrix to improve AgNP absorption. Functionalization is achieved through either in situ synthesis or pre-functionalization of AgNPs within the poly(N-isopropylacrylamide)/chitosan hydrogel, leading to the development of an e-textile. | Modified viscose textiles exhibited excellent antibacterial activity, achieving over 90% growth reduction against both Gram-negative E. coli and S. aureus. | [132] |
4.3. Silver Nanoparticles (AgNPs) Coating for Antifouling and Environmental Protection Applications
| Material | Preparation | Effect | Ref |
|---|---|---|---|
| Polymeric membrane ion-selective electrodes | Immersing the electrode in AgNO3 solution, followed by immersion in a NaBH4 solution to reduce the silver ions into AgNPs. | Surface modification demonstrated long-term antifouling and anti-biofouling effectiveness against P. aeruginosa in bacterial suspension. | [147] |
| Microfiltration membrane | A membrane for anti-biofouling microfiltration was prepared using non-solvent-induced phase separation followed by mild alkali treatment for in situ AgNP formation. Polyacrylonitrile and silver nitrate were dissolved in N-methyl-2-pyrrolidone, cast onto a patterned mold, and immersed in isopropyl alcohol for phase separation. | Confocal laser scanning microscopy confirmed that the bacteria that adhered to the membrane surfaces were non-viable. A prolonged anti-biofouling effect was observed. | [148] |
| Ag/ZrO2-SiO2 composite ceramic membranes | Ag/ZrO2-SiO2 composite ceramic membranes were synthesized via the sol-gel method by incorporating AgNO3 into the ZrO2-SiO2 matrix, leading to AgNP formation within the membrane. The process involved preparing SiO2 and ZrO2 sols, combining them to obtain Agx/ZrO2-SiO2 sols, followed by dip-coating α-Al2O3 substrates, drying, and calcination. | Antibacterial efficacy against E. coli and S. aureus was observed. | [17] |
| AgNP-enhanced nanofiber membrane made of polyvinyl alcohol (PVA) and thermoplastic polyurethane (TPU) | AgNPs were incorporated into the prepared PVA and TPU solutions. The resulting Ag-polymer solutions were then electrospun into nanofibrous membranes. | TPU-AgNPs and PVA-AgNPs showed significant antiviral activity against HIV-1 and SARS-CoV-2 and broad antibacterial activity. | [149] |
| Urushiol-based benzoxazine coating | Urushiol-based benzoxazine monomers (UOB) are synthesized via a Mannich reaction using urushiol, n-octylamine, and paraformaldehyde. AgNPs are in situ generated by reducing silver ions with the phenolic groups in UOB. The UOB/AgNPs composites are cast onto glass slides, cured, and thermoset, resulting in dark-brown coatings. | Antimicrobial activity towards E. coli, S. aureus, Vibrio alginolyticus, and Bacillus sp. Strong inhibition of the settlement and attachment of marine microalgae. | [28] |
| Water-based acrylic paint | In situ synthesis of AgNPs for water-based acrylic emulsion or polymeric dispersion paint involves stirring a polymer solution, adding silver nitrate, and refluxing under argon. Sodium acrylate is then introduced, resulting in polymer-coated AgNPs. These coatings are applied to wood via brush coating and to stainless and mild steel through dip coating. | The water-based acrylic paint doped with AgNPs exhibited strong antimicrobial activity and remarkable resistance against E. coli, B. cereus, A. baumannii, Klebsiella pneumoniae, Aspergillus niger, A. terreus and Rhizopus arrhizus. | [150] |
| Silicone–epoxy resin (hydrophobic tetramethyldis-iloxane eugenol–epoxy resin, HMME-EP) modified by poly(N-isopropylacrylamide)–thiol (PNIPAM-SH) | Silicone–epoxy resin (HMME-EP) and isophoronediamine (IPDA) were prepolymerized under stirring, then combined with reduced graphene oxide (rGO)-AgNPs and PNIPAM-SH dispersed in anhydrous ethanol. After stirring and vacuum degassing, the mixture was bar-coated onto a steel substrate and cured at room temperature, forming an anti-fouling coating. | After 24 h, rGO/AgNP-based coating demonstrated a 100% elimination rate against E. coli, Vibrio parahaemolyticus, and S. aureus, along with effective anti-algae activity against Phaeodactylum tricornutum. | [151] |
| Commercial white painting | Natural zeolite (NZ) was combined with AgNPs to form the NZ-AgNPs composite, which was mixed with commercial white paint (CWP) and agitated to produce CWP-NZ-AgNPs paint. A 35-mm test specimen was dip-coated twice by immersing it for 5 s, allowing for proper drying between applications. | CWP-NZ-AgNPs exhibited significant antibacterial activity, producing a 41 mm inhibition zone against E. coli. | [152] |
| Polyurethane foams | Polyurethane foams were incubated in a 1:1 mixture of Amba turmeric extract and AgNO3, enabling the in situ synthesis of AgNPs on their surfaces. The foams were subsequently heated in a water bath to enhance nanoparticle formation and adhesion. | High-density foams demonstrated bactericidal activity, achieving a 1.2 log reduction in E. coli within 30 min of treatment. | [153] |
| An open-cell polyurethane foam sample is immersed in a silver nitrate solution containing dopamine hydrochloride under continuous stirring for 24 h. This process facilitates the simultaneous deposition of polydopamine and in situ synthesis of AgNPs on the foam surface. | Exhibited strong antimicrobial activity against C. albicans, E. coli, S. aureus, and Legionella pneumophila. | [29] | |
| Polydopamine/chitosan hydrogels-functionalized polyurethane foams | Chitosan (CT) hydrogels are grafted onto polyurethane foam (PUF) to form CT/PUF, which is then coated with polydopamine (PDA) by immersion in a dopamine solution. AgNPs are in situ immobilized by soaking PDA/CT/PUF in silver nitrate for 24 h, followed by reduction with sodium borohydride. | PDA/CT/PUF@AgNPs exhibited excellent reusability over five cycles, consistently achieving effective disinfection of E. coli. It successfully purified the water sample, meeting microbial safety standards for drinking water. | [146] |
4.4. Silver Nanoparticles (AgNPs) Coating for Food Packaging Applications
| Material | Preparation | Effect | Ref |
|---|---|---|---|
| Cellulose acetate (CA)-based films intended for food packaging | CA films were fabricated using the solution casting method. CA was dissolved in glacial acetic acid, and silver nitrate was added. The mixture was then heated, forming a gel that facilitated the controlled reduction in silver ions. | The films exhibited antimicrobial activity against E. coli, S. aureus, C. albicans, and A. niger. | [159] |
| Sugarcane bagasse-derived carboxymethyl cellulose, cassava (CMC) starch (CV), and chitosan (CT) bioplastic film | A bioplastic film was produced using the film casting method. The cassava starch solution was first gelatinized, then CT and CMC solutions were heated to 65 °C. Subsequently, the vanillin solution was incorporated, and AgNPs were introduced into the mixture before casting to a film. | The film demonstrated significant antibacterial activity against S. aureus and E. coli. | [160] |
| Polylactic acid (PLA) films doped with carob pod (CP) powder | CP and AgNPs were integrated into the PLA matrix to produce eco-composite films. The process involved CP pretreatment with citric acid, PLA dissolution, ultrasonication, extrusion, and film formation using injection molding or hot pressing. | Adding AgNPs enhanced antibacterial activity against both S. aureus and E. coli. | [161] |
| Sodium alginate (SA), persimmon polysaccharide (PP) AgNPs film | The SA-PP-AgNPs film was produced using a casting method, where PP, SA, and PPE-AgNPs were combined, followed by the addition of glycerol, thorough stirring, ultrasonication, and casting. | The film showed excellent antibacterial activities against E. coli, S. aureus, Saccharomyces cerevisiae and Aspergillus niger. | [162] |
| Pectin-derived biopackaging film | Films were prepared using the evaporation casting method. Pectin from grapefruit was dissolved in water, mixed with sorbitol, CaCl2, and acetic acid for demethylation, then combined with AgNPs before casting. | Pectin/AgNPs films demonstrated effective antibacterial activity against both E. coli and B. subtilis. | [163] |
| Bacterial cellulose (BC) for food packaging | A bacterial cellulose membrane was immersed in an AgNO3 solution, allowing for the incorporation of AgNPs to form the BC-AgNP membrane. This membrane was then immersed in a montmorillonite (MMT) solution, forming the BC-Ag-MMT membrane. | BC-Ag-MMT membranes showed inhibitory effects against Salmonella paratyphi, E. coli, S. aureus, Bacillus subtilis, and Salmonella enterica. | [118] |
| Bacterial cellulose (BC) was oxidized and reacted with a silver-ammonia solution to form BC/AgNPs. These were dispersed in ethanol and ammonium hydroxide, followed by TEOS addition to create BC/AgNPs/SiO2 composites. The composites were emulsified with octadecenyl succinic anhydride (ODSA) to form pickering emulsions and applied to paper and fruits for preservation. | The coating exhibits strong antimicrobial activity against E. coli, Salmonella typhimurium, S. aureus, and Listeria monocytogenes. | [164] | |
| Paper-based packaging | AgNPs are incorporated into the starch solution and then applied to paper-based packaging. The coating is evenly distributed using the bar coating method, and the treated paper is subsequently left to air dry. | Strong and concentration-dependent activity was observed against E. coli. | [165] |
| Paper | Carbamate starch, calcium lignosulfonate, and cellulose nanofibrils were mixed with water to form a coating solution, to which AgNPs were added and dispersed ultrasonically. The resulting coatings were then applied to paper using a rod coating method. | The coated paper exhibited inhibition zones against E. coli and S. aureus. | [101] |
| Polypropylene film | Dried nisin and montmorillonite K10 were dispersed in distilled water and then combined with AgNPs. The mixtures were thoroughly homogenized and coated onto the plasma-treated polypropylene (PP) films, followed by air drying to produce stable antimicrobial coatings. | Incorporating AgNPs significantly enhanced its antimicrobial effectiveness against E. coli and S. aureus. | [166] |
| Biopolymers like cellulose nanocrystals, alginate, chitin nanocrystals, or chitosan were combined with AgNO3 for in situ AgNP reduction. The polypropylene (PP) film was plasma-treated, then subjected to a layer-by-layer deposition using polyelectrolytes (poly(allylamine hydrochloride) (PAH) and/or poly(sodium 4-styrenesulfonate) (PSS)) and biopolymer/AgNP hybrid suspensions to form a uniform, antimicrobial coating. | - | [167] |
5. Biocompatibility Considerations in Silver Nanoparticle (AgNPs)-Based Coatings
5.1. Role of Silver Nanoparticles (AgNPs) Physicochemical Properties in Determining Their Biocompatibility
5.2. Role of Silver Nanoparticles (AgNPs) Surface Chemistry and Functionalization in Determining Their Biocompatibility
5.3. Controlled Silver Ion Release for Enhanced Biocompatibility
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashid, S.A.; Noh, N.A.M.; Zarkasi, K.Z.; Salikin, N.H.; Jalil, M.T.M.; Zakaria, N.A.; Tong, W.Y. Pollution by Microorganisms and Its Impact on the Food Safety. In Controlling Environmental Pollution; Yaser, A.Z., Samah, M.A.A., Ariffin, F., Haghi, A.K., Eds.; Springer: Singapore, 2025; pp. 47–72. [Google Scholar]
- Belay, M.M.; Ambelu, A.; Mekonen, S.; Karbana, G.; Yemane, B. Investigating Microbial Contamination of Indoor Air, Environmental Surfaces, and Medical Equipment in a Southwestern Ethiopia Hospital. Environ. Health Insights 2024, 18, 11786302241266052. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Launches First Ever Global Report on Infection Prevention and Control. 2022. Available online: https://www.who.int/news/item/06-05-2022-who-launches-first-ever-global-report-on-infection-prevention-and-control (accessed on 10 April 2025).
- Kerketta, S.; Rout, U.K.; Kshatri, J.S.; Kerketta, A.S.; Paikaray, A.K.; Dash, A.; Pradhan, R.; Padhi, A.; Patra, T.; Behera, A.K.; et al. An Acute Diarrheal Disease Outbreak in Urban Setting of Odisha, India. BMC Infect. Dis. 2025, 25, 283. [Google Scholar] [CrossRef] [PubMed]
- Kalu, C.M.; Mudau, K.L.; Masindi, V.; Ijoma, G.N.; Tekere, M. Occurrences and Implications of Pathogenic and Antibiotic-Resistant Bacteria in Different Stages of Drinking Water Treatment Plants and Distribution Systems. Heliyon 2024, 10, e26380. [Google Scholar] [CrossRef]
- WHO Report. World Health Organization Food Safety. Available online: https://www.who.int/health-topics/food-safety#tab=tab_1 (accessed on 18 May 2025).
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking Microbial Contamination to Food Spoilage and Food Waste: The Role of Smart Packaging, Spoilage Risk Assessments, and Date Labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, B.; Zhou, S.; Shi, C.; Liang, Y.; Hu, L. Development and Application of Intelligent Coating Technology: A Review. Coatings 2024, 14, 597. [Google Scholar] [CrossRef]
- Ferrari, I.V.; Castellino, M.; Pisani, A.; Giuntoli, G.; Cavallo, A.; Al Kayal, T.; Mazzetti, P.; Rosellini, A.; Sidoti, M.; Cataldo, A.; et al. Electroless Silver Plating on Fabrics for Antimicrobial Coating: Comparison between Cotton and Polyester. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241277383. [Google Scholar] [CrossRef]
- Polívková, M.; Hubáček, T.; Staszek, M.; Švorčík, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef]
- Uddin Rabbi, M.B.; Haque, S.; Bedoura, S. Advancements in Synthesis, Immobilization, Characterization, and Multifaceted Applications of Silver Nanoparticles: A Comprehensive Review. Heliyon 2024, 10, e40931. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Qari, H.A. Therapeutic Applications of Biogenic Silver Nanomaterial Synthesized from the Paper Flower of Bougainvillea glabra (Miami, Pink). Nanomaterials 2023, 13, 615. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Amara, H.; Alam, F.; El, S.; Butt, H. 3D-Printed and In-Situ Prepared Hydrogel Anti-Bacterial Wound Patch with Silver Nanoparticle Embedded Matrix. Heliyon 2025, 11, e42186. [Google Scholar] [CrossRef] [PubMed]
- Harun-ur-rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A. Bin Recent Advances of Silver Nanoparticle-Based Polymer Nanocomposites for Biomedical Applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; de Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Yang, J.; Zhang, R.; Li, H.; Guo, Y. Enhanced Antibacterial Activity, Dye Rejection and Anti-Fouling Performance of ZrO2-SiO2 Composite Ceramic Membranes Embedded by Silver Nanoparticles. J. Sol-Gel Sci. Technol. 2025, 114, 413–429. [Google Scholar] [CrossRef]
- Fu, H.; Bing, W.; Liu, X. Intelligent Antibacterial Surface with Controllable Sterilization and Bacteria-Releasing Ability. Prog. Org. Coat. 2024, 187, 108105. [Google Scholar] [CrossRef]
- Jangid, H.; Singh, S.; Kashyap, P.; Singh, A.; Kumar, G. Advancing Biomedical Applications: An in-Depth Analysis of Silver Nanoparticles in Antimicrobial, Anticancer, and Wound Healing Roles. Front. Pharmacol. 2024, 15, 1438227. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Elshahat Abdallah, S.; Elmessery, W.M.; Elfallawi, F.E.; Shoueir, K.R. Utilizing Agricultural Biowaste for Food Safety: Integrating Naturally Synthesized Silver Nanoparticles as Antibacterial Coating. Inorg. Chem. Commun. 2024, 163, 112337. [Google Scholar] [CrossRef]
- Nematollahi, S.; Maghsoudian, S.; Motasadizadeh, H.; Nouri, Z.; Azad, K.; Fatahi, Y.; Samadi, N.; Mahmoudieh, M.; Shaabani, A.; Dinarvand, R. Polyhexamethylene Biguanidine Coated Silver Nanoparticles Embedded into Chitosan Thiourea/PVA Nanofibers as Wound Healing Mats: In Vitro and In Vivo Studies. Carbohydr. Polym. 2025, 347, 122704. [Google Scholar] [CrossRef]
- de Souza, T.B.; Rosa, A.S.; Constantino-Teles, P.; Ferreira, V.N.S.; Archanjo, B.S.; Soares, C.A.G.; Picciani, P.H.S.; Allão Cassaro, R.A.; Miranda, M.D.; Poneti, G. Silver Nanoparticles-Functionalized Textile against SARS-CoV-2: Antiviral Activity of the Capping Oleylamine Molecule. ACS Appl. Mater. Interfaces 2025, 17, 5710–5718. [Google Scholar] [CrossRef]
- Ilickas, M.; Guobienė, A.; Gedvilas, K.; Merkis, M.; Abakevičienė, B. UV-Mediated Photochemical Synthesis and Investigation of the Antiviral Properties of Silver Nanoparticle-Polyvinyl Butyral Nanocomposite Coatings as a Novel Antiviral Material with High Stability and Activity. Appl. Mater. Today 2024, 38, 102203. [Google Scholar] [CrossRef]
- Tooklang, P.; Audtarat, S.; Chaisen, K.; Thepsiri, J.; Chingsungnoen, A.; Jittabut, P.; Dasri, T. Functionalization of Silver Nanoparticles Coating Cotton Fabrics Through Hydrothermal Synthesis for Improved Antimicrobial Properties. Nano Express 2024, 5, 025009. [Google Scholar] [CrossRef]
- Chittratan, P.; Detsri, E.; Chalitangkoon, J.; Mathaweesansurn, A.; Monvisade, P. Antimicrobial Nanolayer Films of Chloroxylenol–Carboxyethylchitosan–Modified Silver Nanoparticles for Enhanced Surgical Suture Performance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 133957. [Google Scholar] [CrossRef]
- Zaniolo, K.M.; Biaggio, S.R.; Cirelli, J.A.; Cominotte, M.A.; Bocchi, N.; Rocha-Filho, R.C.; de Souza, C.W.O. Production and Characterization of Bioactive and Antimicrobial Titanium Oxide Surfaces with Silver Nanoparticles and a Poly(Lactic Acid) Microfiber Coating. J. Braz. Chem. Soc. 2023, 35, e-20230134. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Jian, R.; Bai, W.; Zheng, G.; Xie, Z.; Lin, Q.; Lin, F.; Xu, Y. In Situ Reduction of Silver Nanoparticles/Urushiol-Based Polybenzoxazine Composite Coatings with Enhanced Antimicrobial and Antifouling Performances. Polymers 2024, 16, 1167. [Google Scholar] [CrossRef]
- Larrieu, M.; Mouniee, D.; Agusti, G.; Blaha, D.; Edouard, D. Antimicrobial Foam-Filter Based on Commercial Support Coated with Polydopamine and Silver Nanoparticles for Water Treatment. Environ. Technol. Innov. 2024, 33, 103468. [Google Scholar] [CrossRef]
- Gouda, M.; Khalaf, M.M.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Fabrication of Silver Nanoparticles Loaded Acacia Gum/Chitosan Nanogel to Coat the Pipe Surface for Sustainable Inhibiting Microbial Adhesion and Biofilm Growth in Water Distribution Systems. Int. J. Biol. Macromol. 2024, 262, 130085. [Google Scholar] [CrossRef]
- Khan, M.; Saeed, M.A.; Ullah, S.; Repon, M.R.; Pranta, A.D.; Yunusov, N.; Hossain, M.M. Development of Self-Cleaning and Antibacterial Properties on Cotton Fabric Using Silver Nanoparticles and PFOTS. SPE Polym. 2024, 5, 568–575. [Google Scholar] [CrossRef]
- Dumas, L.; de Souza, M.C.; Bonafe, E.G.; Martins, A.F.; Monteiro, J.P. Optimized Incorporation of Silver Nanoparticles onto Cotton Fabric Using K-Carrageenan Coatings for Enhanced Antimicrobial Properties. ACS Appl. Bio Mater. 2024, 7, 6908–6918. [Google Scholar] [CrossRef]
- Jin, X.; Peng, N.; Cui, A.; Liu, Y.; Peng, X.; Huang, L.; Ed-Dra, A.; He, F.; Li, Y.; Yang, S.; et al. Sodium Dodecyl Sulfate-Coated Silver Nanoparticles Accelerate Antimicrobial Potentials by Targeting Amphiphilic Membranes. mLife 2024, 3, 551–564. [Google Scholar] [CrossRef]
- Pradhan, L.; Sah, P.; Nayak, M.; Upadhyay, A.; Pragya, P.; Tripathi, S.; Singh, G.; Mounika, B.; Paik, P.; Mukherjee, S. Biosynthesized Silver Nanoparticles Prevent Bacterial Infection in Chicken Egg Model and Mitigate Biofilm Formation on Medical Catheters. J. Biol. Inorg. Chem. 2024, 29, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Abar, E.S.; Vandghanooni, S.; Memar, M.Y.; Eskandani, M.; Torab, A. Enhancing Antifungal and Antibacterial Properties of Denture Resins with Nystatin-Coated Silver Nanoparticles. Sci. Rep. 2024, 14, 23770. [Google Scholar] [CrossRef] [PubMed]
- Aouay, M.; Aguado, R.J.; Bayés, G.; Fiol, N.; Putaux, J.L.; Boufi, S.; Delgado-Aguilar, M. In-Situ Synthesis and Binding of Silver Nanoparticles to Dialdehyde and Carboxylated Cellulose Nanofibrils, and Active Packaging Therewith. Cellulose 2024, 31, 5687–5706. [Google Scholar] [CrossRef]
- Ahire, J.H.; Wang, Q.; Rowley, G.; Chambrier, I.; Crack, J.C.; Bao, Y.; Chao, Y. Polyurethane Infused with Heparin Capped Silver Nanoparticles Dressing for Wound Healing Application: Synthesis, Characterization and Antimicrobial Studies. Int. J. Biol. Macromol. 2024, 282, 136557. [Google Scholar] [CrossRef]
- Iurilli, M.; Porrelli, D.; Turco, G.; Lagatolla, C.; Piloni, A.C.; Medagli, B.; Nicolin, V.; Papa, G. Electrospun Collagen-Coated Nanofiber Membranes Functionalized with Silver Nanoparticles for Advanced Wound Healing Applications. Membranes 2025, 15, 39. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, P.; Li, R.; Han, M.; Shen, Y. Natural Light-Induced Self-Healing Antibacterial Superhydrophobic Coatings Based on ODA/Ag-PDA@HMS-ODA. Appl. Surf. Sci. 2025, 684, 161803. [Google Scholar] [CrossRef]
- Kılıç, H.; Ceylan, D. Multi-Responsive Shape Memory and Self-Healing Hydrogels with Gold and Silver Nanoparticles. J. Mater. Chem. B 2025, 13, 336–353. [Google Scholar] [CrossRef]
- Bai, G.; Niu, C.; Liang, X.; Li, L.; Feng, Y.; Wei, Z.; Chen, K.; Bohinc, K.; Guo, X. Engineering Robust Silver-Decorated Calcium Peroxide Nano-Antibacterial Platforms for Chemodynamic Enhanced Sterilization. J. Colloid Interface Sci. 2025, 680, 684–695. [Google Scholar] [CrossRef]
- Arghand, N.; Reiisi, S.; Karimi, B.; Khorasgani, E.M.; Heidari, R. Biosynthesis of Nanocomposite Alginate-Chitosan Loaded with Silver Nanoparticles Coated with Eugenol/Quercetin to Enhance Wound Healing. Bionanoscience 2024, 14, 5149–5166. [Google Scholar] [CrossRef]
- Viana, M.M.; Souza, T.R.; Bueno-Silva, B.; Gonçalves, F.; Braga, R.R.; Nascimento, F.D.; Pereira, R.M.; Batista, B.L.; Seabra, A.B.; Rodrigues, M.C. Antimicrobial and Optical Properties of a New Biogenic Silica-Coated Silver Nanoparticles Incorporated into Experimental Resin. J. Clin. Exp. Dent. 2024, 16, 151–158. [Google Scholar] [CrossRef]
- Gomaa, M.H.; Abdallah, S.S.; Ghayad, I.M.; El-Sayed, K.; Hamid, Z.A. Instant and Synergistic Antibacterial Coating of Silver Loaded With Silica Nanoparticles for Different Applications. Surf. Interface Anal. 2025, 57, 137–147. [Google Scholar] [CrossRef]
- Nuti, S.; Fernández-Lodeiro, A.; Galhano, J.; Oliveira, E.; Duarte, M.P.; Capelo-Martínez, J.L.; Lodeiro, C.; Fernández-Lodeiro, J. Tailoring Mesoporous Silica-Coated Silver Nanoparticles and Polyurethane-Doped Films for Enhanced Antimicrobial Applications. Nanomaterials 2024, 14, 462. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Shenoy, B.M.; Verma, M.; Nayak, S.; Hegde, G.; John, N.S. Self-Cleaning Formulations of Mixed Metal Oxide-Silver Micro-Nano Structures with Spiky Coronae as Antimicrobial Coatings for Fabrics and Surfaces. Mater. Adv. 2024, 5, 4293–4310. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Cao, S.; Bao, Y.; Wang, L.; He, Z.E.; Li, J.; Zhou, Y.; Lv, M. Engineering Bacterial Biofilm Development and Structure via Regulation of Silver Nanoparticle Density in Graphene Oxide Composite Coating. J. Am. Chem. Soc. Au 2024, 4, 855–864. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Khan, B.; Nawaz, M.; Hussain, R.; Price, G.J.; Warsi, M.F.; Waseem, M. Enhanced Antibacterial Activity of Size-Controlled Silver and Polyethylene Glycol Functionalized Silver Nanoparticles. Chem. Pap. 2021, 75, 743–752. [Google Scholar] [CrossRef]
- Sorinolu, A.J.; Godakhindi, V.; Siano, P.; Vivero-Escoto, J.L.; Munir, M. Influence of Silver Ion Release on the Inactivation of Antibiotic Resistant Bacteria Using Light-Activated Silver Nanoparticles. Mater. Adv. 2022, 3, 9090–9102. [Google Scholar] [CrossRef]
- Rosário, F.; Creylman, J.; Verheyen, G.; Van Miert, S.; Santos, C.; Hoet, P.; Oliveira, H. Impact of Particle Size on Toxicity, Tissue Distribution and Excretion Kinetics of Subchronic Intratracheal Instilled Silver Nanoparticles in Mice. Toxics 2022, 10, 260. [Google Scholar] [CrossRef]
- Wang, L.; Periyasami, G.; Aldalbahi, A.; Fogliano, V. The Antimicrobial Activity of Silver Nanoparticles Biocomposite Films Depends on the Silver Ions Release Behaviour. Food Chem. 2021, 359, 129859. [Google Scholar] [CrossRef]
- Salmani-Zarchi, H.; Mousavi-Sagharchi, S.M.A.; Sepahdoost, N.; Ranjbar-Jamalabadi, M.; Gross, J.D.; Jooya, H.; Samadi, A. Antimicrobial Feature of Nanoparticles in the Antibiotic Resistance Era: From Mechanism to Application. Adv. Biomed. Res. 2024, 13, 113. [Google Scholar] [CrossRef]
- Kladko, D.V.; Falchevskaya, A.S.; Serov, N.S.; Prilepskii, A.Y. Nanomaterial Shape Influence on Cell Behavior. Int. J. Mol. Sci. 2021, 22, 5266. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Chen, X.; Mourdikoudis, S.; Fan, S.; Li, H.; Gómez-graña, S.; Ren, S.; Zheng, G. Disentangling the “Tip-Effects” Enhanced Antibacterial Mechanism of Ag Nanoparticles. Dalt. Trans. 2024, 53, 12281–12290. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Mukherji, S. Enhanced Antibacterial Activity of Decahedral Silver Nanoparticles. J. Nanopart. Res. 2021, 23, 36. [Google Scholar] [CrossRef]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J. Aggregation and Dissolution of Silver Nanoparticles in Natural Surface Water. Environ. Sci. Technol. 2012, 46, 5378–5386. [Google Scholar] [CrossRef]
- Velgosova, O.; Mačák, L.; Múdra, E.; Vojtko, M.; Lisnichuk, M. Preparation, Structure, and Properties of PVA–AgNPs Nanocomposites. Polymers 2023, 15, 379. [Google Scholar] [CrossRef]
- Ortiz-Magdaleno, M.; Sánchez-Vargas, L.; Gardea-Contreras, D.; Campos-Ibarra, V.; Pozos-Guillén, A.; Márquez-Preciado, R. Antibiofilm Properties of Silver Nanoparticles Incorporated into Polymethyl Methacrylate Used for Dental Applications. Biomed. Mater. Eng. 2023, 34, 357–373. [Google Scholar] [CrossRef]
- Petousis, M.; Nasikas, N.K.; Papadakis, V.; Valsamos, I.; Gkagkanatsiou, K.; Mountakis, N.; Argyros, A.; Dimitriou, E.; Michailidis, N.; Vidakis, N. Printability and Performance Metrics of New-Generation Multifunctional PMMA/Antibacterial Blend Nanocomposites in MEX Additive Manufacturing. Polymers 2025, 17, 410. [Google Scholar] [CrossRef]
- Salh, I.B.A.E.A.; El-Salam, E.B.A.; Ezzat, A.A.; Aboshama, M.; Elhagali, A.F. Antimicrobial Effect of Three Different Nanoparticles-Modified 3D-Printed Denture Resin: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2025, 15, 42–49. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications–A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and Antibacterial Activity of Chitosan-Silver Nanoparticles for Application in Preservation of Minced Meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Mendez-pfeiffer, P.; Ballesteros-monrreal, M.G.; Juarez, J.; Gastelum-cabrera, M.; Martinez-flores, P.; Taboada, P.; Valencia, D. Chitosan-Coated Silver Nanoparticles Inhibit Adherence and Biofilm Formation of Uropathogenic. ACS Infect. Dis. 2024, 10, 1126–1136. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Smitha, C.; Abbubakkar, B.M.; Govindhan, P.; Krishnan, N.A. Materials Today: Proceedings Synthesis and Characterization of TiO2/ZnO—Ag Nanocomposite for Photocatalytic Degradation of Dyes and Anti-Microbial Activity. Mater. Today Proc. 2021, 45, 3357–3364. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vu, V.T.; Nguyen, T.H.; Nguyen, T.A.; Tran, V.K. Antibacterial Activity of TiO2—And ZnO-Decorated with Silver Nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef]
- Chaudhary, R.; Singh, N.B.; Nagpal, G.; Saah, F.K. Antibacterial Activity of Reduced Graphene-Silver Oxide Nanocomposite against Gram-Negative Bacteria. Microbe 2024, 5, 100221. [Google Scholar] [CrossRef]
- Lange, A.; Kutwin, M.; Zawadzka, K.; Ostrowska, A.; Strojny-cieślak, B.; Nasiłowska, B.; Bombalska, A.; Jaworski, S. Impaired Biofilm Development on Graphene Oxide-Metal Nanoparticle Composites. Nanotechnol. Sci. Appl. 2024, 7, 303–320. [Google Scholar] [CrossRef]
- Singh, P.; Ali, S.W.; Kale, R.D. Antimicrobial Nanomaterials as Advanced Coatings for Self- Sanitizing of Textile Clothing and Personal Protective Equipment. ACS Omega 2023, 8, 8159–8171. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Dube, E. Antibacterial Activity of Engineered Nanoparticles against Fish Pathogens. Aquac. Rep. 2024, 37, 102240. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Oh, S.G.; Mun, J.Y.; Han, S.S. Effects of Silver Nanoparticles on the Fluidity of Bilayer in Phospholipid Liposome. Colloids Surf. B Biointerfaces 2005, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sun, J.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, M.; Pei, J.; He, R.; Chen, W. Disruption of Cell Membranes and Redox Homeostasis as an Antibacterial Mechanism of Dielectric Barrier Discharge Plasma against Fusarium oxysporum. Int. J. Mol. Sci. 2024, 25, 7875. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial Activity of Silver Nanoparticles of Different Particle Size against Vibrio natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Khina, A.G.; Krutyakov, Y.A. Similarities and Differences in the Mechanism of Antibacterial Action of Silver Ions and Nanoparticles. Appl. Biochem. Microbiol. 2021, 57, 683–693. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Basu, S.; Jana, S.; Pande, S.; Pal, T. Interaction of DNA Bases with Silver Nanoparticles: Assembly Quantified Through SPRS and SERS. J. Colloid Interface Sci. 2008, 321, 288–293. [Google Scholar] [CrossRef]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Exploring the Ct-DNA and Plasmid DNA Binding Affinity of the Biogenic Synthesized Chloroxine-Conjugated Silver Nanoflowers: Spectroscopic and Gel Electrophoresis Methods. Process Biochem. 2022, 121, 360–370. [Google Scholar] [CrossRef]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial Effects of Silver Nanoparticles on Gram-Negative Bacteria: Influence on the Growth and Biofilms Formation, Mechanisms of Action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef]
- Jiang, H.S.; Zhang, Y.; Lu, Z.W.; Lebrun, R.; Gontero, B.; Li, W. Interaction between Silver Nanoparticles and Two Dehydrogenases: Role of Thiol Groups. Small 2019, 15, 1900860. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Matpan Bekler, F.; Tunç, A.; Güven, K. The Effects of Silver Nanoparticles (AgNPs) on Thermophilic Bacteria: Antibacterial, Morphological, Physiological and Biochemical Investigations. Microorganisms 2024, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Barbir, R.; Capjak, I.; Crnković, T.; Debeljak, Z.; Jurašin, D.D.; Ćurlin, M.; Šinko, G.; Weitner, T.; Včcek, I.V. Chemico-Biological Interactions Interaction of Silver Nanoparticles with Plasma Transport Proteins: A Systematic Study on Impacts of Particle Size, Shape and Surface Functionalization. Chem. Biol. Interact. 2021, 335, 109364. [Google Scholar] [CrossRef]
- Girma, A. Alternative Mechanisms of Action of Metallic Nanoparticles to Mitigate the Global Spread of Antibiotic-Resistant Bacteria. Cell Surf. 2023, 10, 100112. [Google Scholar] [CrossRef]
- Marutyan, S.; Karapetyan, H.; Khachatryan, L.; Muradyan, A.; Marutyan, S.; Poladyan, A.; Trchounian, K. The Antimicrobial Effects of Silver Nanoparticles Obtained through the Royal Jelly on the Yeasts Candida Guilliermondii NP-4. Sci. Rep. 2024, 14, 19163. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J.; May, R.V.; Re, V.; Recei, M.; July, V. Interaction of Silver (I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Zhang, N.; Shen, B.; Jiang, L.; Zhang, L.; Zhang, Z. Deinococcus wulumuqiensis R12 Synthesized Silver Nanoparticles with Peroxidase-like Activity for Synergistic Antibacterial Application. Biotechnol. J. 2024, 19, 2300584. [Google Scholar] [CrossRef]
- Khari, M.; Tuong, T.; Truong, V.; Chen, C.; Lai, J.; Jessie, S. Journal of the Taiwan Institute of Chemical Engineers Exploring Antibacterial Effectiveness: A Comparative Analysis of Green and Chemical Synthesis of Silver Nanoparticles against Staphylococcus Aureus. J. Taiwan Inst. Chem. Eng. 2024, 165, 105750. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative Stress Generation of Silver Nanoparticles in Three Bacterial Genera and Its Relationship with the Antimicrobial Activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Bihari, A.; Rekha, R.; Kumar, N.; Duttaroy, A.K. Biomedicine & Pharmacotherapy Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Chang, R.L.; Stanley, J.A.; Robinson, M.C.; Sher, J.W.; Li, Z.; Chan, Y.A.; Omdahl, A.R.; Wattiez, R.; Godzik, A.; Matallana-surget, S. Protein Structure, Amino Acid Composition and Sequence Determine Proteome Vulnerability to Oxidation-Induced Damage. EMBO J. 2020, 39, e104523. [Google Scholar] [CrossRef]
- González-fernández, S.; Blanco-agudín, N.; Rodríguez, D.; Fernández-vega, I.; Merayo-lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. [Google Scholar] [CrossRef]
- Haris, Z.; Ahmad, I. Green Synthesis of Silver Nanoparticles Using Moringa Oleifera and Its Efficacy against Gram-Negative Bacteria Targeting Quorum Sensing and Biofilms. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 156–167. [Google Scholar] [CrossRef]
- Aflakian, F.; Hashemitabar, G. Biosynthesized Silver Nanoparticles at Subinhibitory Concentrations as Inhibitors of Quorum Sensing, Pathogenicity, and Biofilm Formation in Pseudomonas Aeruginosa PAO1. Heliyon 2025, 11, e42899. [Google Scholar] [CrossRef]
- Rather, M.A.; Mandal, M. Attenuation of Biofilm and Quorum Sensing Regulated Virulence Factors of an Opportunistic Pathogen Pseudomonas aeruginosa by Phytofabricated Silver Nanoparticles. Microb. Pathog. 2023, 185, 106433. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, S.; Meng, F.; Xu, Z.; Fang, Q.; Gu, Z.; Zhang, C.; Li, P.; Kong, F. Eco-Friendly Food Packaging Based on Paper Coated with a Bio-Based Antibacterial Coating Composed of Carbamate Starch, Calcium Lignosulfonate, Cellulose Nanofibrils, and Silver Nanoparticles. Int. J. Biol. Macromol. 2024, 254, 127659. [Google Scholar] [CrossRef]
- Colin, C.; Akpo, E.; Perrin, A.; Cornu, D.; Cambedouzou, J. Encapsulation in Alginates Hydrogels and Controlled Release: An Overview. Molecules 2024, 29, 2515. [Google Scholar] [CrossRef]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical Synthesis of Chitosan/Silver Nanoparticles Multilayer Hydrogel Coating with PH-Dependent Controlled Release Capability and Antibacterial Property. Colloids Surf. B Biointerfaces 2021, 202, 111711. [Google Scholar] [CrossRef]
- Cassa, M.A.; Gentile, P.; Girón-hernández, J.; Ciardelli, G.; Carmagnola, I. Smart Self-Defensive Coatings with Bacteria- Triggered Antimicrobial Response for Medical Devices. Biomater. Sci. 2024, 12, 5433–5449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ao, J.; Ding, L.; Chen, Y.; Xu, J.; Sun, Z.; Li, Y.; Wu, L.; Liu, X.; Jiang, S. Flexible, Robust and Reusable Ag Nanoparticle-Coated Polyamide SERS Substrates for Rapid Identification of Toxic Substances on Curved Surfaces. Microchem. J. 2025, 209, 112899. [Google Scholar] [CrossRef]
- Tandel, T.; Govekar, A.; Deogaonkar-Baride, S. Development of Excellent Antimicrobial and Wash Durable Fabric with Biosynthesized Silver Nanoparticles Using Soluble Starch, Maize Starch and Rice Extract. J. Appl. Polym. Sci. 2025, 0, e56926. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Souza, P.R.; Jacinto, G.S.; Braga, T.L.; Ricken, Y.S.; Souza, G.K.; Caetano, W.; Radovanovic, E.; Arns, C.W.; Rai, M.; et al. Silver Nanoparticles In Situ Synthesized and Incorporated in Uniaxial and Core–Shell Electrospun Nanofibers to Inhibit Coronavirus. Pharmaceutics 2024, 16, 268. [Google Scholar] [CrossRef]
- Weber, J.; Henssler, L.; Zeman, F.; Pfeifer, C.; Alt, V.; Nerlich, M.; Huber, M.; Herbst, T.; Koller, M.; Schneider-brachert, W.; et al. Nanosilver/DCOIT-Containing Surface Coating Effectively and Constantly Reduces Microbial Load in Emergency Room Surfaces. J. Hosp. Infect. 2023, 135, 90–97. [Google Scholar] [CrossRef]
- De Simone, S.; Gallo, A.L.; Paladini, F.; Sannino, A.; Pollini, M. Development of Silver Nano-Coatings on Silk Sutures as a Novel Approach against Surgical Infections. J. Mater. Sci. Mater. Med. 2014, 25, 2205–2214. [Google Scholar] [CrossRef]
- Tahir, I.; Jabeen, S.; Mahmood, N.; Ahmad, H. Antimicrobial Coating of Biologically Synthesized Silver Nanoparticles on Surgical Fabric and Surgical Blade to Prevent Nosocomial Infections. Heliyon 2024, 10, e35968. [Google Scholar] [CrossRef]
- Kylián, O.; Shelemin, A.; Solař, P.; Pleskunov, P.; Nikitin, D.; Kuzminova, A.; Štefaníková, R.; Kúš, P.; Cieslar, M.; Hanuš, J.; et al. Magnetron Sputtering of Polymeric Targets: From Thin Films to Heterogeneous Metal/Plasma Polymer Nanoparticles. Materials 2019, 12, 2366. [Google Scholar] [CrossRef]
- Tharwat, M.; Algahtani, F.D.; Othman, M.S.; Ahmad, K.; Maysara, S.; Al-najjar, M.A.A.; El-morsy, M.A.; Menazea, A. Laser Deposited Ultra-Thin Silver Nanoparticles on CMC-PVA Blend Film as Sheet for Wound Dressings. Mater. Chem. Phys. 2024, 318, 129246. [Google Scholar] [CrossRef]
- Sharma, G.K.; James, N.R. Electrospinning: The Technique and Applications. In Recent Developments in Nanofibers Research; Khan, M., Chelladurai, S.J.S., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Wang, C.; Feng, L.; Xu, S.; Zhao, Y.; Yao, L.; Ge, J. Nano Energy Fabrication of Reusable Bioprotective Nanofabric with Passive Nano-Ag/Joule Thermal Disinfection Properties for Real-Time Wireless Fine Biomotion and Gesture Sensing. Nano Energy 2024, 124, 109525. [Google Scholar] [CrossRef]
- Ariga, K. Layer-by-Layer Nanoarchitectonics: A Method for Everything in Layered Structures. Materials 2025, 18, 654. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Gouda, M.; Khalaf, M.M.; Abou, M.F.; Abdelaziz, M.A.; El-lateef, H.M.A. Functionalization of Cotton Fabric Using the Biogenic Synthesized Silver Nanoparticles for Enhanced Dye Reduction and Antimicrobial Efficiency: Response Surface Methodology. Int. J. Biol. Macromol. 2025, 307, 141853. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Y.; Yu, L.; Song, G.; Ge, J.; Du, R. Characterization of Nanosilver Antibacterial Bacterial Cellulose Composite Membranes Coated with Montmorillonite and Their Potential Application in Food Packaging. Int. J. Biol. Macromol. 2025, 289, 138685. [Google Scholar] [CrossRef]
- Pandey, V.; Gupta, A.; Choudhary, I.S.; Imran, M.; Mudavath, S.L.; Kar, A.G.; Nandan, R. Impact of Dual-coated Silver Nanoparticle and Antibiotic Sutures on Wound Healing in Inflammatory Mouse Models. J. Indian Assoc. Pediatr. Surg. 2024, 29, 612. [Google Scholar] [CrossRef]
- Ramani, S.; Senthil, S.; Rajaram, V.; Kumari, B.N. Comparison of Mechanical, Antibacterial and Morphological Properties of Silk Sutures Coated with Silver Nanoparticles and Aloe Vera Herbal Extract: An In-Vitro Study. J. Clin. Diagn. Res. 2023, 17, 18–22. [Google Scholar] [CrossRef]
- Baygar, T.; Ugur, A.; Karaca, I.R.; Kilinc, Y.; Gultekin, S.E.; Sarac, N. Fabrication of a Biocompatible Nanoantimicrobial Suture for Rapid Wound Healing after Surgery. ACS Omega 2024, 9, 22573–22580. [Google Scholar] [CrossRef]
- Mathew, S.; Kumar, K.V.; Prabhu, A.; Shastry, R.P.; Rajesh, K.S. Braided Silk Sutures Coated with Photoreduced Silver Nanoparticles for Eradicating Staphylococcus aureus and Streptococcus mutans Infections. J. Microbiol. Methods 2024, 220, 106923. [Google Scholar] [CrossRef]
- Kang, L.; Huang, S.; Qin, X.; Gao, X.; Li, Y.; Zhang, X. Ag Nanoparticles-Decorated PVDF Nanofiber/Net Membranes with Enhanced Filtration and Antibacterial Efficiency for Personal Protective Equipment. ACS Appl. Nano Mater. 2024, 7, 9252–9261. [Google Scholar] [CrossRef]
- da Silva, M.R.P.; Matos, R.S.; Monteiro, M.D.S.; Santos, S.B.; Filho, H.D.F.; Andrade, G.R.S.; Salerno, M.; Almeida, L.E. Exploiting the Physicochemical and Antimicrobial Properties of PHB/PEG and PHB/PEG/ALG-e Blends Loaded with Ag Nanoparticles. Materials 2022, 15, 7544. [Google Scholar] [CrossRef]
- Xiang, J.; Zhu, R.; Lang, S.; Yan, H.; Liu, G.; Peng, B. Mussel-Inspired Immobilization of Zwitterionic Silver Nanoparticles toward Antibacterial Cotton Gauze for Promoting Wound Healing. Chem. Eng. J. 2021, 409, 128291. [Google Scholar] [CrossRef]
- Ferrari, I.V.; Giuntoli, G.; Pisani, A.; Cavallo, A.; Mazzetti, P.; Fonnesu, R.; Rosellini, A.; Pistello, M.; Al, T.; Cataldo, A.; et al. One-Step Silver Coating of Polypropylene Surgical Mask with Antibacterial and Antiviral Properties. Heliyon 2024, 10, e23196. [Google Scholar] [CrossRef] [PubMed]
- Karademir, F.; Ayhan, F. Antimicrobial Surface Functionality of PEG Coated and AgNPs Immobilized Extracorporeal Biomaterials. Biointerface Res. Appl. Chem. 2022, 12, 1039–1052. [Google Scholar]
- Machková, A.; Vaňková, E.; Obrová, K.; Fürhacker, P.; Košutová, T.; Lion, T.; Hanuš, J.; Scholtz, V. Silver Nanoparticles with Hexamethyldisiloxane Coating on 3D Printed Substrates Are Non-Cytotoxic and Effective against Respiratory Pathogens. Front. Microbiol. 2023, 19, 1217617. [Google Scholar] [CrossRef]
- Bendary, I.M.; Omar, A.A.; Goda, R.M.; Ali, A.A.; Lotfy, K.A.; Shohayeb, M.M. Evaluation of Two Different Self-Disinfection Alginate Impression Material. BDJ Open 2024, 10, 84. [Google Scholar] [CrossRef]
- Singer, L.; Karacic, S.; Szekat, C.; Bierbaum, G.; Bourauel, C. Biological Properties of Experimental Dental Alginate Modified for Self-Disinfection Using Green Nanotechnology. Clin. Oral Investig. 2023, 27, 6677–6688. [Google Scholar] [CrossRef]
- Deng, P.; Shi, Z.; Fang, F.; Xu, Y.; Zhou, L.A.; Liu, Y.; Jin, M.; Chen, T.; Wang, Y.; Cao, Y.; et al. Wireless Matrix Metalloproteinase-9 Sensing by Smart Wound Dressing with Controlled Antibacterial Nanoparticles Release toward Chronic Wound Management. Biosens. Bioelectron. 2025, 268, 116860. [Google Scholar] [CrossRef]
- Glažar, D.; Štular, D.; Jerman, I.; Simončič, B.; Tomšič, B. Embedment of Biosynthesised Silver Nanoparticles in PolyNIPAAm/Chitosan Hydrogel for Development of Proactive Smart Textiles. Nanomaterials 2025, 15, 10. [Google Scholar] [CrossRef]
- Liu, T.; Xie, F.; Geng, L.; He, R.; Sun, M.; Ni, T.; Xu, P.; Liu, T.; Xie, F.; Geng, L.; et al. Micro-Electro Nanofibrous Dressings Based on PVDF- AgNPs as Wound Healing Materials to Promote Healing in Active Areas Micro-Electro Nanofibrous Dressings Based on PVDF-AgNPs as Wound Healing Materials to Promote Healing in Active Areas. Int. J. Nanomed. ISSN 2025, 20, 771–789. [Google Scholar] [CrossRef]
- Javed, A.; Tariq, A.; Farhan, M.; Khan, A.; Mirza, R.; Usman, M.; Nadir, A.; Khan, A. Multifunctional Tilapia Skin Based Smart Dressing of Silver Capped Allopurinol Nanoparticles for Treatment of Infectious Burn Wounds. J. Drug Deliv. Sci. Technol. 2025, 107, 106804. [Google Scholar] [CrossRef]
- Mehmood, S.; Akhtar, N.; Arshad, M.; Azhar, U.; Ullah, S.; Waris, T.S.; Jabbar, F.; Hasan, A.; Iqbal, F.; Chaudhry, A.A.; et al. A Novel Methodology for Stabilization of Silver Nanoparticles on Cotton, Nylon and Cotton/Nylon Fabrics Using Chitosan and Triethyl Orthoformate for Enhanced and Elongated Antibacterial Performance. Int. J. Biol. Macromol. 2024, 267, 129256. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.; Parajuli, P.; Hossain, M.T.; Chaudhari, H.; Abidi, N. Antimicrobials for Protective Clothing. In Antimicrobial Textiles from Natural Resources; Mondal, M., Ibrahim, H., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 349–376. [Google Scholar]
- Veeraraghavan, P.V.; Periadurai, N.D.; Karunakaran, T.; Hussain, S.; Surapaneni, K.M.; Jiao, X. Green Synthesis of Silver Nanoparticles from Aqueous Extract of Scutellaria Barbata and Coating on the Cotton Fabric for Antimicrobial Applications and Wound Healing Activity in Fibroblast Cells (L929). Saudi J. Biol. Sci. 2021, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Francelino, I.G.; Tavares, V.K.F.; Leite, L.D.P.; da Silva, D.M.; de Souza Miranda, F.; Koga-Ito, C.Y.; Filho, G.P. Silver Nanoparticle Incorporation on Polyamide 6,6 Fabrics by Hybrid Corona—Dielectric Barrier Discharge for Antimicrobial Applications. J. Nanopart. Res. 2025, 27, 53. [Google Scholar] [CrossRef]
- Sadeghi-kiakhani, M.; Hashemi, E.; Norouzi, M.; Tehrani-Bagha, A.R. Bio-Synthesis of Silver Nanoparticles Using Rhus coriaria L. Seed Extract on Polyamide: Characterization, Antibacterial Efficacy, and Colorimetric Analysis. Inorg. Chem. Commun. 2025, 174, 113942. [Google Scholar] [CrossRef]
- Phromphen, P.; Phoophat, P.; Sukatta, U.; Rugthaworn, P.; Rungruangkitkrai, N.; Tuntariyanond, P.; Chartvivatpornchai, N.; Sichola, P.; Boonyarit, J.; Apipatpapha, T.; et al. Enhancement of Antibacterial Silk Face Covering with the Biosynthesis of Silver Nanoparticles from Garcinia mangostana Linn. Peel and Andrographis paniculata Extract and a Bacterial Cellulose Filter. Coatings 2024, 14, 379. [Google Scholar] [CrossRef]
- Allehyani, E.S.; Almulaiky, Y.Q.; Al-Harbi, S.A.; El-Shishtawy, R.M. In Situ Coating of Polydopamine-AgNPs on Polyester Fabrics Producing Antibacterial and Antioxidant Properties. Polymers 2022, 14, 3794. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. Stabilization of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138. [Google Scholar] [CrossRef]
- Zorraquín-Peña, I.; Cueva, C.; Bartolomé, B.; Moreno-Arribas, M.V. Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations. Microorganisms 2020, 8, 132. [Google Scholar] [CrossRef]
- Júnior, H.L.O.; Neves, R.M.; Monticeli, F.M.; Agnol, L.D. Smart Fabric Textiles: Recent Advances and Challenges. Textiles 2022, 2, 582–605. [Google Scholar] [CrossRef]
- Li, X.; Su, X.; Deng, P. PH—Responsive Superhydrophobic Fabric Based on AgNP/Copolymer Composites for Controllable Oil—Water Separation. Cellulose 2025, 32, 1755–1770. [Google Scholar] [CrossRef]
- Wu, S.; Luo, H.; Li, S.; Zheng, Z.; Long, Q.; Wei, C. Polydopamine/Chitosan Hydrogels-Functionalized Polyurethane Foams in Situ Decorated with Silver Nanoparticles for Water Disinfection. J. Environ. Manag. 2024, 366, 121858. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Liao, Z.; Liu, Y.; Liang, R. In Situ and Rechargeable Generation of Silver Nanoparticles on Polymeric Membrane Ion-Selective Electrodes for Marine Biofouling Mitigation. Sens. Actuators B. Chem. 2025, 427, 137170. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, M.G.; Yoo, C.H.; Lee, M.-S.; Park, H.; Hyeong, J.; Seong, M.S.; Na, J.-G.; Lee, J.-H.; Lee, J.S. A New Anti-Biofouling Microfiltration: Combining Sharkskin-Inspired Topology with in-Situ Silver Nanoparticles. Sep. Purif. Technol. 2025, 355, 129636. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Hagar, M.; Al-mutabagani, L.A.; Abozaid, G.M.; Abdallah, S.M.; Shehata, N.; Ahmed, H.; Hassanin, A.H. Hybrid Nanofibrous Membranes as a Promising Functional Layer for Personal Protection Equipment: Manufacturing and Antiviral/Antibacterial Assessments. Polymers 2021, 13, 1776. [Google Scholar] [CrossRef]
- Shahzadi, P.; Majeed, M.A.; Ibrahim, S.; Asif, S.; Kalsoom, R.; Hussain, I. Polymeric Coating Doped with Nanomaterials for Functional Impact on Different Substrates. Sci. Rep. 2024, 14, 578. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Liu, S.; Li, J.; Pu, J.; Hao, Y.; Ying, G.; Xue, Q.; Lu, G. An Integrated Anti-Fouling and Anti-Corrosion Coating Enabled by RGO/AgNPs and Amphiphilic Networks. Engineering 2024, 42, 223–234. [Google Scholar] [CrossRef]
- Carla, A.; Marjorie, A.; Oliveira, D.; Bergamasco, L.; Anne, G.; Magalh, V.; Di, A.; Neves, C.; Almeida, F. De Antibacterial Activity of Functionalized Natural Zeolites (NZ-AgNPs) and Its Application in Bacteriological Water Treatment and Commercial Paints. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101001. [Google Scholar]
- Ali, H.; Anwar, S.; Aziz, U.; Nurjis, F. Antimicrobial Efficacy of Polyurethane Foams Impregnated with Amba Turmeric Mediated Silver Nanoparticles. Mater. Chem. Phys. 2025, 334, 130454. [Google Scholar] [CrossRef]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef]
- Rajaramon, S.; David, H.; Sajeevan, A.; Shanmugam, K.; Sriramulu, H.; Dandela, R.; Solomon, A.P. Multi-Functional Approach in the Design of Smart Surfaces to Mitigate Bacterial Infections: A Review. Front. Cell. Infect. Microbiol. 2023, 13, 1139026. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Jouyban, A.; Soleymani, J.; Rahimi, M. Rational Design of Smart Nano-Platforms Based on Antifouling-Nanomaterials toward Multifunctional Bioanalysis. Adv. Colloid Interface Sci. 2022, 302, 102637. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-H.; Liao, Y.-Q.; Wang, Y.-Y.; Zhu, J.; Liu, P.; Ding, R.; Guo, D.-M.; Wang, F.; Song, F.; Wang, Y.-Z. Conductive Superhydrophobic Smart Coatings Based on Spherical Silver Nanoparticles and Waterborne Polyurethane for Flexible and Wearable Electronics. ACS Appl. Mater. Interfaces 2024, 16, 65553–65564. [Google Scholar] [CrossRef] [PubMed]
- Jangid, H.; Joshi, H.C.; Dutta, J.; Ahmad, A.; Alshammari, M.B.; Hossain, K.; Pant, G.; Kumar, G. Advancing Food Safety with Biogenic Silver Nanoparticles: Addressing Antimicrobial Resistance, Sustainability, and Commercial Viability. Food Chem. X 2025, 26, 102298. [Google Scholar] [CrossRef] [PubMed]
- Maloufi, M.; Djelad, A.; Mokhtar, A.; Reguig, K.; Abdelkrim, M.; Kebir-medjhouda, Z.A.; Ghamnia, M. Fabrication and Characterization of Cellulose-Based Packaging Films with Polyethylene Glycol and Silver Nanoparticles for Enhanced Antimicrobial Efficacy. Int. J. Biol. Macromol. 2025, 308, 142381. [Google Scholar] [CrossRef]
- Plaeyao, K.; Talodthaisong, C.; Yingyuen, W.; Kaewbundit, R. Biodegradable Antibacterial Food Packaging Based on Carboxymethyl Cellulose from Sugarcane Bagasse/Cassava Starch/Chitosan/Gingerol Extract Stabilized Silver Nanoparticles (Gin-AgNPs) and Vanillin as Cross-Linking Agent. Food Chem. 2025, 466, 142102. [Google Scholar] [CrossRef]
- Erdem, O.; Mutlu, A.; Can, A. Production and Characterization of Eco-Composite Polylactic Acid Films Doped with Carob Pod Powder/Silver Nanoparticles and Their Potential Utilization in Packaging Applications. J. Polym. Environ. 2025, 33, 730–742. [Google Scholar] [CrossRef]
- Zhu, Y.; Pang, X.; Zhang, W.; Zhang, C.; Zhang, B.; Fu, J.; Zhao, H.; Han, W. Green Synthesis of Silver Nanoparticles Using Persimmon Polysaccharides for Enhanced Polysaccharide-Based Film Performance. Food Res. Int. 2025, 209, 116252. [Google Scholar] [CrossRef]
- Nguyen, K.T.C.; Tran, Q.K.; Nguyen, A.T.L.; Dang, L.L.P.; Le, H.N.; Do, K.D.; Duong, Q.X.; Do, H.H.; Nguyen, V.D.; Ngo, T.H.A. Nanoarchitectonics of Antibacterial Bio-Packaging Film from Grapefruit Peel-Derived Low Methoxy Pectin Integrated with Silver Nanoparticles. J. Polym. Res. 2025, 32, 90. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, F.; Hou, Y.; Li, Z.; Yuan, D.; Liu, C.; Hu, F.; Zhao, R.; Wang, H.; Liu, W.; et al. Bacterial Cellulose-Based Pickering Emulsions Reinforced with Silver and Silica Nanoparticles for Advanced Antibacterial and Hydrophobic Food Packaging Solutions. Carbohydr. Polym. 2025, 355, 123357. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life. Polymers 2024, 16, 941. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Venkatesan, R.; Madhavan, A.A. Development and Characterization of Antimicrobial Nisin/MMT K10/AgNPs Nanocomposite Coatings on Oxygen Plasma Surface-Modified Polypropylene for Food Packaging Applications. Food Bioprocess Technol. 2024, 18, 3553–3565. [Google Scholar] [CrossRef]
- Musino, D.; Peyron, S.; Capron, I. Versatile Biobased Biocidal Nanomaterial for a Safer-by-Design Coated Food Packaging. J. Appl. Polym. Sci. 2024, 141, e55856. [Google Scholar] [CrossRef]
- El Guerraf, A.; Jadi, S.B.; Ziani, I.; Dalli, M.; Sher, F.; Bazzaoui, M.; Bazzaoui, E.A. Multifunctional Smart Conducting Polymers—Silver Nanocomposites-Modified Biocellulose Fibers for Innovative Food Packaging Applications. Ind. Eng. Chem. Res. 2023, 62, 4540–4553. [Google Scholar] [CrossRef]
- Li, D.; Xue, R. Nanostructured Materials for Smart Food Packaging: Integrating Preservation and Antimicrobial Properties. Alex. Eng. J. 2025, 124, 446–461. [Google Scholar] [CrossRef]
- Vasilev, O.; Hayles, A.; Campbell, D.; Jaarsma, R.; Johnson, L.; Vasilev, K. Nanoscale Antibacterial Coatings Incorporating Silver Nanoparticles Derived by Plasma Techniques—A State-of-the-Art Perspective. Mater. Today Chem. 2024, 41, 102341. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Engineered Nanoparticles in Aquatic Systems: Toxicity and Mechanism of Toxicity in Fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver Nanoparticles Induce Mitochondrial Protein Oxidation in Lung Cells Impacting Cell Cycle and Proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Justin, M.; James, F.A.; Calsis, R.F.; Juan-corpuz, L.M. De Silver Nanoparticle-Infused Hydrogels for Biomedical Applications: A Comprehensive Review. J. Chin. Chem. Soc. 2025, 72, 124–162. [Google Scholar] [CrossRef]
- Nešovic, K.; Miškovic-Stankovic, V. A Comprehensive Review of the Polymer-Based Hydrogels with Electrochemically Synthesized Silver Nanoparticles for Wound Dressing Applications. Polym. Eng. Sci. 2020, 60, 1393–1419. [Google Scholar] [CrossRef]
- Azizi-lalabadi, M.; Garavand, F.; Mahdi, S. Incorporation of Silver Nanoparticles into Active Antimicrobial Nanocomposites: Release Behavior, Analyzing Techniques, Applications and Safety Issues. Adv. Colloid Interface Sci. 2021, 293, 102440. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Popescu-pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, T.; Art, T.; Delguste, C. Silver Nanoparticles as Antimicrobial Agents in Veterinary Medicine: Current Applications and Future Perspectives. Nanomaterials 2025, 15, 202. [Google Scholar] [CrossRef]




| NPs | Size (nm) | Shape | Coating | Effect | Ref |
|---|---|---|---|---|---|
| AgNPs | 17.16 ± 1.94 | Spherical | Polyurethane (PU) nanofiber | AgNPs concentrations of 0.5, 2, and 4 wt% were tested; 4 wt% PU/AgNPs nanofibers exhibited the most ZOI against E. coli (18.2 ± 0.9 mm) and Staphylococcus aureus (16.1 ± 0.7 mm). | [21] |
| 18 ± 4.32 | Spherical | Polyhexamethylene biguanidine (PHMBG) embedded on chitosan thiourea/polyvinyl alcohol (PVA) nanofibers | Notable antimicrobial efficacy against S. aureus and Pseudomonas aeruginosa at 3 wt% Ag/PHMBG concentration, with animal studies showing faster wound healing. | [22] | |
| 8 ± 2 | Spherical | Oleylamine (OA) as NPs capping agent coated on textile of surgical masks | Ag@OA-coated textiles (2.9–47.1 μg) showed increasing anti-SARS-CoV-2 activity, reaching up to 100% inactivation after 10 min. | [23] | |
| 15 to 118 | Spherical | Polyvinyl butyral (PVB) | The nanocomposite coatings (AgNP-PVB with AgNP concentrations from 150 to 1000 ppm) were effective in reducing the activity of SARS-CoV-2 virus, with 1000 ppm coating showing the highest antiviral efficacy. | [24] | |
| 123 | Spherical, flake, triangle, wire, and rod-shaped | Cotton fabrics | Cotton fabric composites with AgNPs (250–2000 µg/mL) showed antimicrobial activity against S. aureus and E. coli at all concentrations. | [25] | |
| 4.8 ± 2.4 | Spherical | Chloroxylenol-carboxyethylchitosan (CECSX) | The resulting CECSX-AgNP-coated surgical suture surfaces demonstrated outstanding antimicrobial properties against E. coli, S. aureus, and A. baumanii with MIC values of 25, 12.5, and 1.56 mg/L, respectively. | [26] | |
| 4 | Spherical | Polylactic acid (PLA) | TiO2 surfaces coated with AgNPs and PLA microfibers exhibited antimicrobial activity against S. aureus. | [27] | |
| 20 | Spherical | Urushiol-based polybenzoxazine (UOHP) | UOHP composite coatings with 0.05–1.0 wt% AgNPs showed antimicrobial activity against bacteria and marine microalgae, even at 0.05 wt%. | [28] | |
| 194 ± 55 | Spherical | Polydopamine on open-cell polyurethane foams | Complete microbicidal effect against C. albicans and E. coli after 3 h of contact time and after 24 h of contact time for S. aureus. | [29] | |
| - | Uniform shape | Acacia gum/chitosan nanogel | AgNPs@acacia gum/chitosan nanogel coatings achieved 6-log reduction in E. coli, K. pneumoniae, E. faecalis, and B. subtilis at 150–200 mg/L. | [30] | |
| - | - | Cotton fabric is treated with perfluorooctyltriethoxysilane | Superior antibacterial properties against E. coli. | [31] | |
| 72.1 to 159.2 | - | Kappa-carrageenan on cotton fabric | AgNPs/kappa-carrageenan coating significantly improved antibacterial activity against S. aureus. | [32] | |
| 37.91 ± 2.35 | Spherical | Sodium dodecyl sulfate (SDS) | SDS-coated AgNPs exhibit superior antibacterial activity against S. aureus, Listeria monocytogenes, Salmonella Typhimurium, Yersinia enterocolitica, Enterococcus faecalis, E. faecium, E. coli, and Klebsiella pneumoniae with the MIC of 78.125 µg/mL. | [33] | |
| 85.37 to 117.51 | Spherical | Calliandra surinamensis (Cs) leaf extract | Cs-AgNPs effectively inhibit biofilm formation on medical catheters at concentrations of 30 µM and 50 µM. | [34] | |
| 12.18 | - | Polymethyl methacrylate (PMMA), an acrylic resin | Nystatin-coated AgNPs in PMMA effectively inhibited C. albicans growth and showed considerable antibacterial activity against Streptococcus mutans. The MIC of AgNPs/PMMA extracts ranged from 220 to 323 µL/mL, while the MBC ranged from 234 to 389 µL/mL against S. mutans. | [35] | |
| 6.5 | - | Dialdehyde-modified TEMPO-oxidized cellulose nanofibers | Paper sheets coated with nanocellulose/AgNPs suspensions containing 120 µg Ag/g effectively inhibited the growth of B. subtilis for at least one month. | [36] | |
| 11 ± 3 | - | Polyurethane (PU) nanofibers | PU embedded with 1–10% heparin-functionalized AgNPs (0.3–1.7% Ag) exhibited potent antibacterial activity against S. aureus and Salmonella Typhimurium. | [37] | |
| 50–100 | Spherical | Collagen-coated polycaprolactone (PCL) nanofiber | The AgNP-functionalized membranes significantly reduced biofilm formation in P. aeruginosa and Vancomycin-resistant Enterococcus. The MICs were 50 µg/mL for P. aeruginosa, 100 µg/mL for vancomycin-resistant Enterococcus, and above 400 µg/mL for S. aureus. | [38] | |
| 10–30 | Spherical | Mesoporous silica (MS) loaded with octadecylamine (ODA) and coated with polydopamine (PDA) | ODA/Ag-PDA@MS nanomaterial exhibited excellent bactericidal performance (against P. aeruginosa and B. subtilis), fully inhibiting growth at 6.3 mg/L within 72 h and effectively preventing biofouling. | [39] | |
| 55 ± 5 | Cube-shaped | Stearyl methacrylate (SM) and vinyl pyrrolidone | - | [40] | |
| CaO2@SiO2/AgNPs | 89 ± 9.6 | Quasi-spherical | Silicon dioxide-coated calcium peroxide (CaO2@SiO2) | CaO2@SiO2/AgNPs hydrogels exhibited enhanced antibacterial activity against S. aureus and E. coli, with bactericidal efficiency of over 99% at a concentration of 40 μg/mL. | [41] |
| Alginate-Chitosan Loaded with AgNPs | between 30 and 50 | Spherical | Eugenol/Quercetin | Antibacterial activity against E. coli, B. subtilis, Klebsiella, and S. aureus. | [42] |
| AgNPs coated with silicon dioxide (Ag@SiO2 NPs) | Aggregates SiO2 NPs 260, AgNPs 890 length | Spherical SiO2NPs and rod-shaped AgNPs | Bisphenol A-glycidyl methacrylate (BisGMA) and Triethylene glycol dimethacrylate (TEGDMA)-based resin | 3 wt% and 5 wt% Ag@SiO2 NPs in a resinous matrix demonstrated a significant reduction in S. mutans biofilm. | [43] |
| SiO2@AgNPs | - | Spherical | Steel | Antibacterial activity against Bacillus subtilis with an MIC of 250 μg/mL. | [44] |
| 28 with an SiO2 shell of 16 | Spherical | Polyurethane-doped films | Films containing AgNPs at concentrations of 4.0 and 8.0 µg/cm2 exhibited a bactericidal effect against E. coli and Salmonella enterica subsp. enterica serovar Choleraesuis and B. cereus. | [45] | |
| Mixed metal oxides (MMOs) of TiO2, ZnO, SiO2, and CuO with AgNPs (MMO–AgNPs) | - | Spherical | Fabric | Excellent antimicrobial properties against E. coli and S. aureus bacteria and bacteriophage viruses, with an MIC of 107 mg/mL. The minimum bactericidal concentration required to reduce 99.9% of S. aureus was 2.5 mg/mL. | [46] |
| Graphene oxide (GO)-AgNPs composite | - | Spherical | Surface | The 20 μg/cm2 GO-AgNPs composite completely prevents P. aeruginosa biofilm formation. | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, E.; Okuthe, G.E. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiol. Res. 2025, 16, 110. https://doi.org/10.3390/microbiolres16060110
Dube E, Okuthe GE. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiology Research. 2025; 16(6):110. https://doi.org/10.3390/microbiolres16060110
Chicago/Turabian StyleDube, Edith, and Grace Emily Okuthe. 2025. "Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control" Microbiology Research 16, no. 6: 110. https://doi.org/10.3390/microbiolres16060110
APA StyleDube, E., & Okuthe, G. E. (2025). Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiology Research, 16(6), 110. https://doi.org/10.3390/microbiolres16060110






