Energy Metabolism and Aerobic Respiratory Chain of Vitreoscilla sp. C1: Comparison with β-Proteobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Metabolic Profiling of Carbon/Energy Pathways and Respiratory Chains in β-Proteobacteria
2.2. Vitreoscilla Membrane Preparation
2.3. Oximetry
3. Results and Discussion
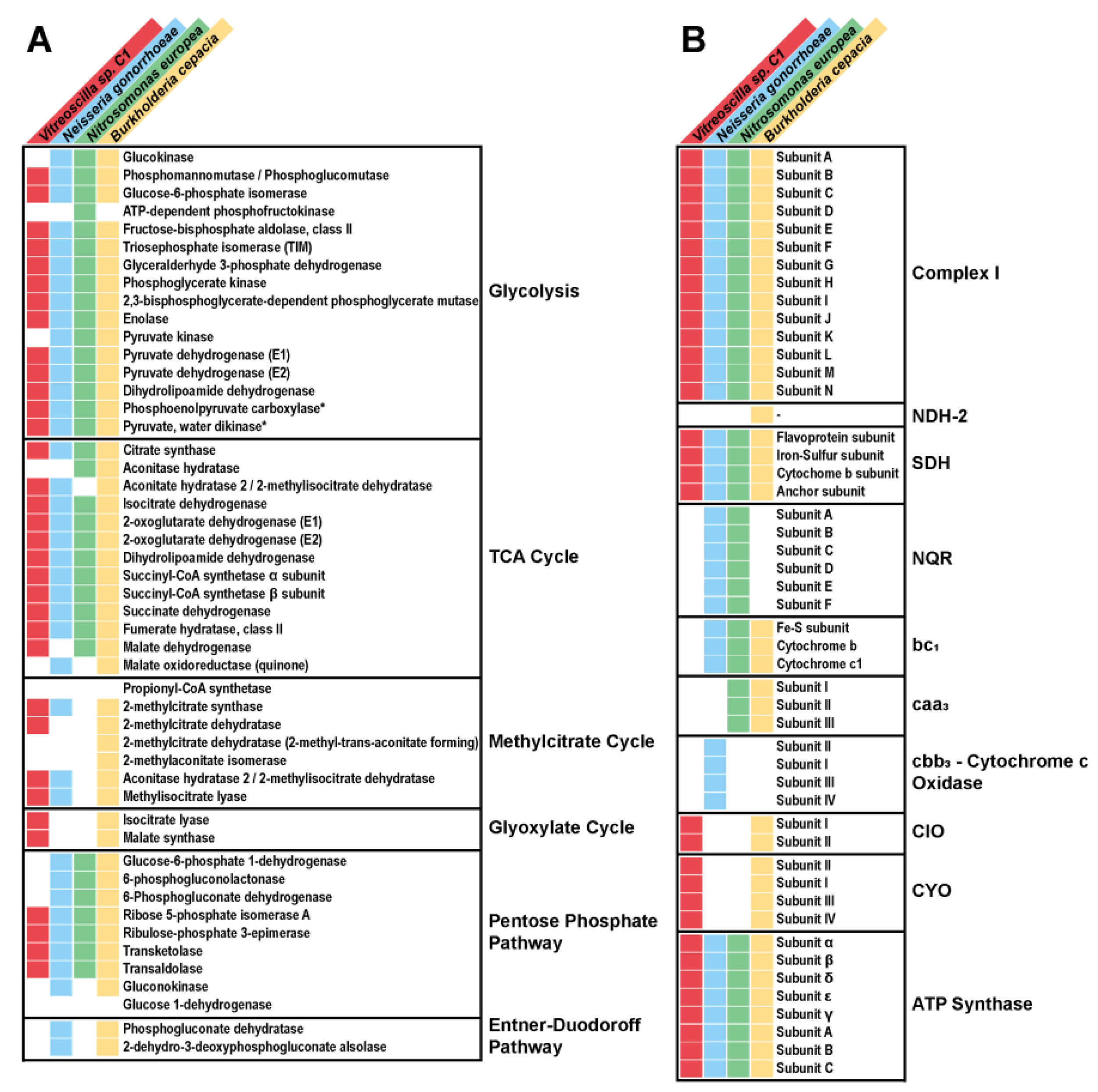
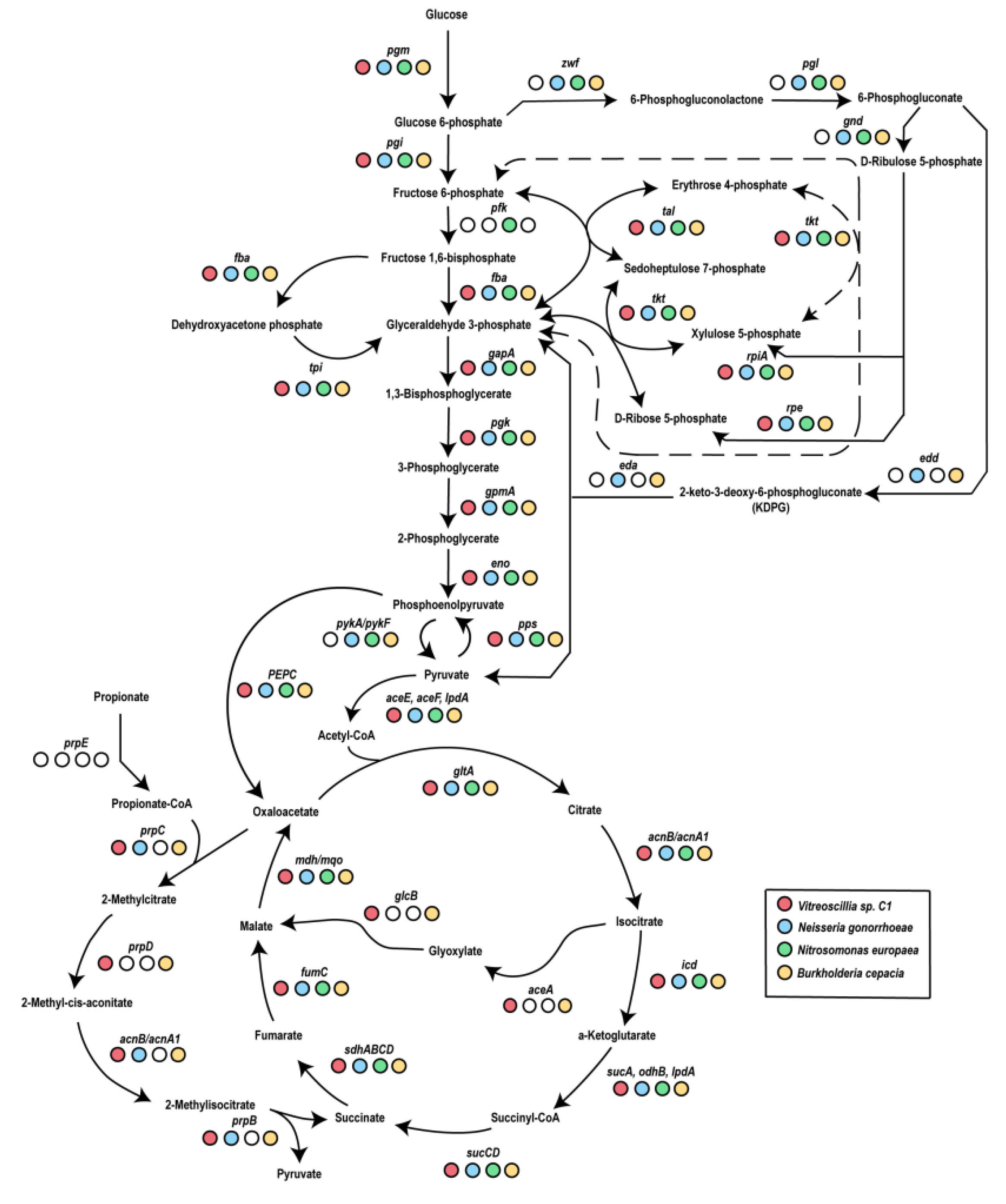
3.1. Vitreoscilla sp. C1 Central Carbon Metabolism
- Glycolysis.
- The Entner–Doudoroff pathway.
- The pentose phosphate pathway.
- The Krebs cycle.
- The glyoxylate pathway.
- The aerobic respiratory chain.
- (1)
- Phosphofructokinase-I, which is essential for glycolysis.
- (2)
- The enzymes required for the Entner–Doudoroff pathway.
- (3)
- The entire oxidative branch of the pentose phosphate pathway (Figure 2).
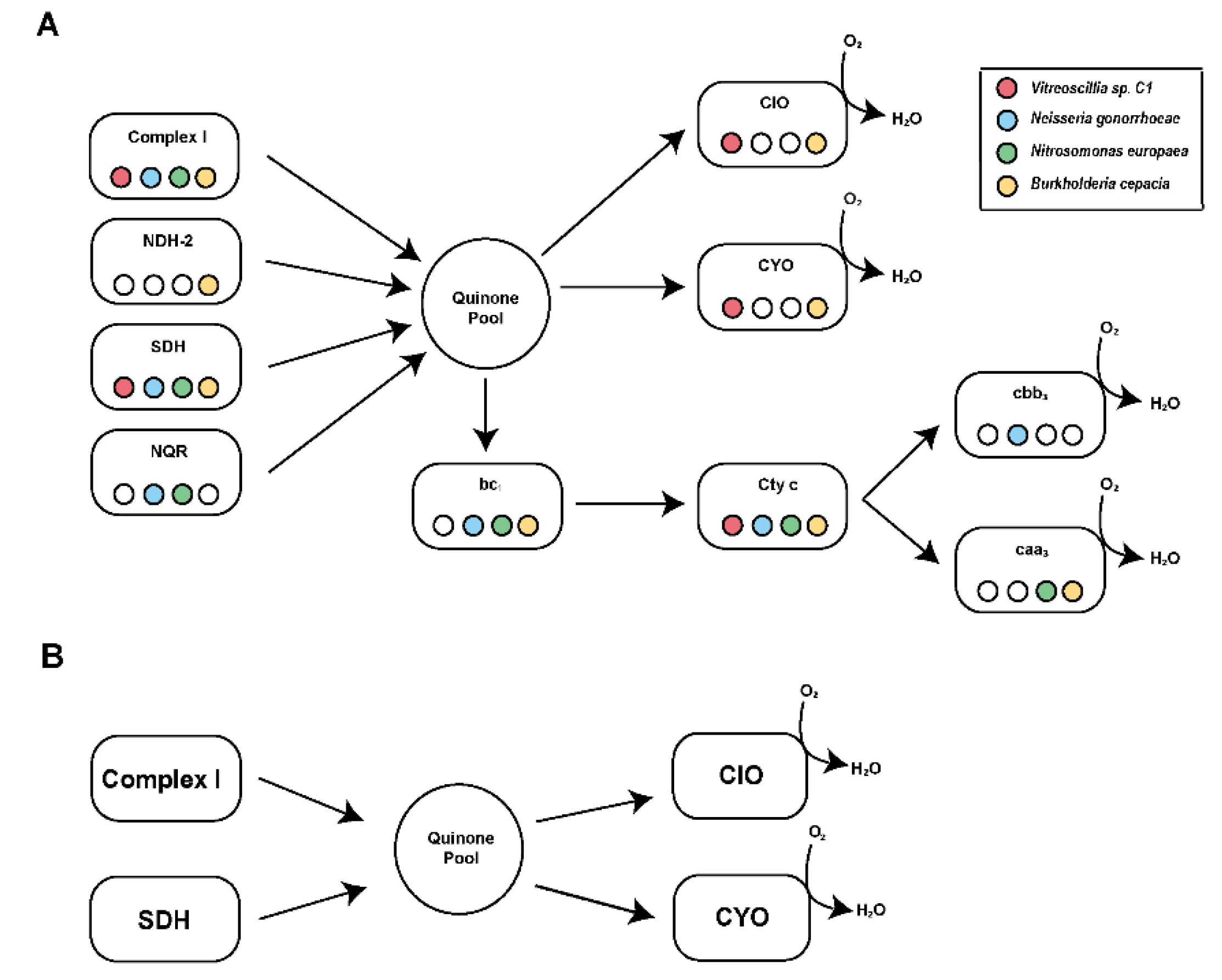
3.2. Identification and Functional Characterization of Vitreoscilla sp. C1 Respiratory Chain Complexes
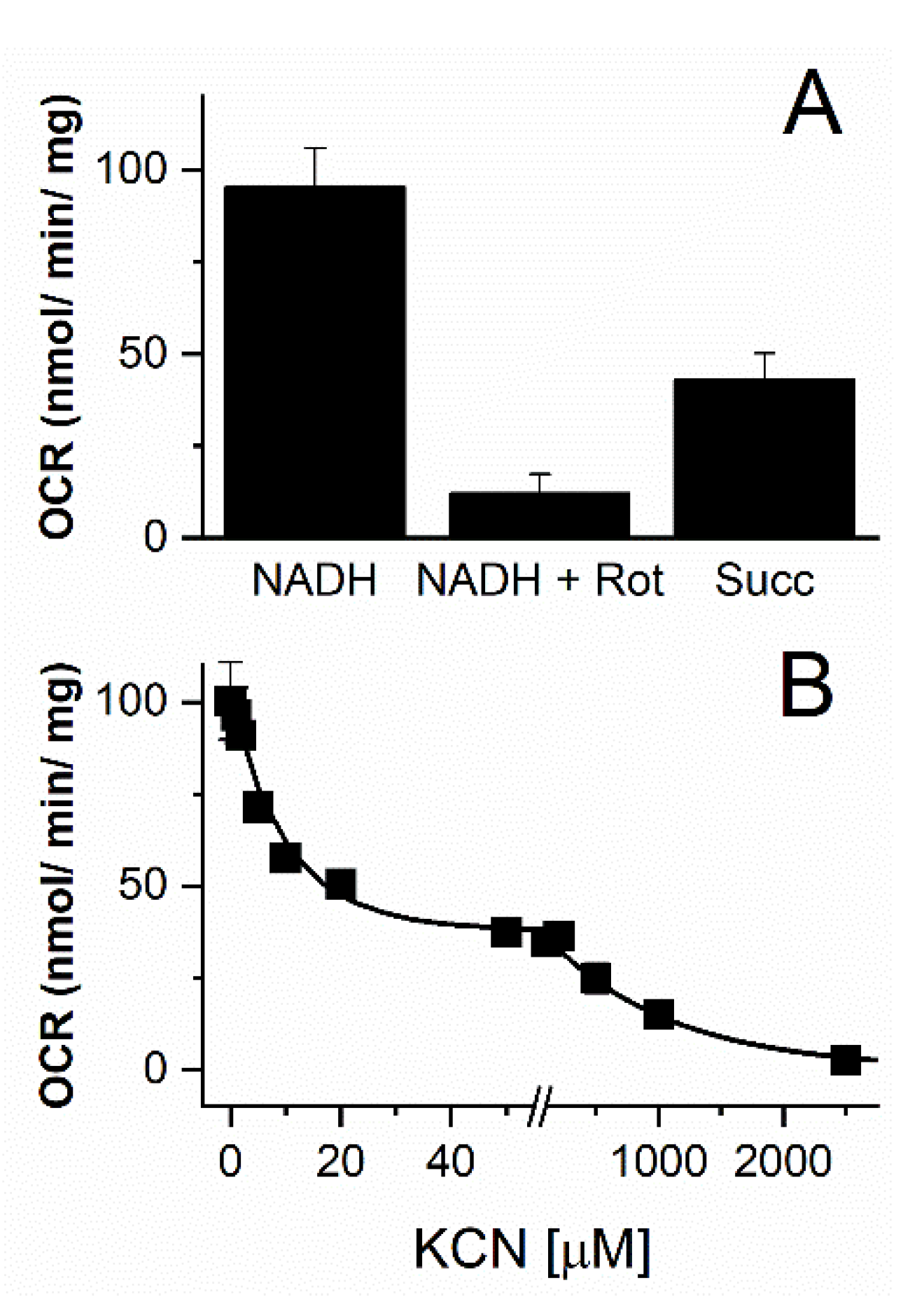
3.3. Functional Characterization of the Vitreoscilla sp. C1 Respiratory Chain
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDTA | Ethylenediaminetetraacetic acid |
| HEPES | 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KO | KEGG Orthology |
| NADH | β-Nicotinamide adenine dinucleotide |
| TCA | Tricarboxylic acid cycle |
References
- Pringsheim, E.G. The Vitreoscillaceae: A Family of Colourless, Gliding, Filamentous Organisms. J. Gen. Microbiol. 1951, 5, 124–149. [Google Scholar] [CrossRef]
- Strohl, W.R.; Schmidt, T.M.; Lawry, N.H.; Mezzino, M.J.; Larkin, J.M. Characterization of Vitreoscilla Beggiatoides and Vitreoscilla Filiformis Sp. Nov., Nom. Rev., and Comparison with Vitreoscilla Stercoraria and Beggiatoa Alba. Int. J. Syst. Bacteriol. 1986, 36, 302–313. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Matsubara, H.; Webster, D.A. Primary Sequence of a Dimeric Bacterial Haemoglobin from Vitreoscilla. Nature 1986, 322, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Efiok, B.J.; Webster, D.A. A Cytochrome That Can Pump Sodium Ion. Biochem. Biophys. Res. Commun. 1990, 173, 370–375. [Google Scholar] [CrossRef]
- Webster, D.A.; Hackett, D.P. The Purification and Properties of Cytochrome o from Vitreoscilla. J. Biol. Chem. 1966, 241, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.D.; Kallio, P.T. Bacterial Hemoglobins and Flavohemoglobins: Versatile Proteins and Their Impact on Microbiology and Biotechnology. FEMS Microbiol. Rev. 2003, 27, 525–545. [Google Scholar] [CrossRef]
- Vinogradov, S.N.; Hoogewijs, D.; Bailly, X.; Arredondo-Peter, R.; Gough, J.; Dewilde, S.; Moens, L.; Vanfleteren, J.R. A Phylogenomic Profile of Globins. BMC Evol. Biol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Ramandeep; Hwang, K.W.; Raje, M.; Kim, K.J.; Stark, B.C.; Dikshit, K.L.; Webster, D.A. Vitreoscilla Hemoglobin. Intracellular Localization and Binding to Membranes. J. Biol. Chem. 2001, 276, 24781–24789. [Google Scholar] [CrossRef]
- Park, K.-W.; Kim, K.-J.; Howard, A.J.; Stark, B.C.; Webster, D.A. Vitreoscilla Hemoglobin Binds to Subunit I of Cytochrome Bo Ubiquinol Oxidases. J. Biol. Chem. 2002, 277, 33334–33337. [Google Scholar] [CrossRef]
- Lin, J.-M.; Stark, B.C.; Webster, D.A. Effects of Vitreoscilla Hemoglobin on the 2,4-Dinitrotoluene (2,4-DNT) Dioxygenase Activity of Burkholderia and on 2,4-DNT Degradation in Two-Phase Bioreactors. J. Ind. Microbiol. Biotechnol. 2003, 30, 362–368. [Google Scholar] [CrossRef]
- Kaur, R.; Pathania, R.; Sharma, V.; Mande, S.C.; Dikshit, K.L. Chimeric Vitreoscilla Hemoglobin (Vhb) Carrying a Flavoreductase Domain Relieves Nitrosative Stress in Escherichia Coli: New Insight into the Functional Role of Vhb. Appl. Environ. Microbiol. 2002, 68, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Duk, B.T.; Singh, S.; Akbas, M.Y.; Webster, D.A.; Stark, B.C.; Dikshit, K.L. Redox-Mediated Interactions of VHb (Vitreoscilla Haemoglobin) with OxyR: Novel Regulation of VHb Biosynthesis under Oxidative Stress. Biochem. J. 2010, 426, 271–280. [Google Scholar] [CrossRef]
- Khosla, C.; Bailey, J.E. Heterologous Expression of a Bacterial Haemoglobin Improves the Growth Properties of Recombinant Escherichia Coli. Nature 1988, 331, 633–635. [Google Scholar] [CrossRef]
- Khosravi, M.; Webster, D.A.; Stark, B.C. Presence of the Bacterial Hemoglobin Gene Improves Alpha-Amylase Production of a Recombinant Escherichia Coli Strain. Plasmid 1990, 24, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Stark, B.C.; Pagilla, K.R.; Dikshit, K.L. Recent Applications of Vitreoscilla Hemoglobin Technology in Bioproduct Synthesis and Bioremediation. Appl. Microbiol. Biotechnol. 2015, 99, 1627–1636. [Google Scholar] [CrossRef]
- Abanoz, K.; Stark, B.C.; Akbas, M.Y. Enhancement of Ethanol Production from Potato-Processing Wastewater by Engineering Escherichia Coli Using Vitreoscilla Haemoglobin. Lett. Appl. Microbiol. 2012, 55, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.A.; Pagilla, K.R.; Stark, B.C. Engineering of Nitrosomonas Europaea to Express Vitreoscilla Hemoglobin Enhances Oxygen Uptake and Conversion of Ammonia to Nitrite. AMB Express 2015, 5, 43. [Google Scholar] [CrossRef]
- Arnaldos, M.; Kunkel, S.A.; Stark, B.C.; Pagilla, K.R. Enhanced Heme Protein Expression by Ammonia-Oxidizing Communities Acclimated to Low Dissolved Oxygen Conditions. Appl. Microbiol. Biotechnol. 2013, 97, 10211–10221. [Google Scholar] [CrossRef]
- Arnaldos, M.; Kunkel, S.A.; Stark, B.C.; Pagilla, K.R. Characterization of Heme Protein Expressed by Ammonia-Oxidizing Bacteria under Low Dissolved Oxygen Conditions. Appl. Microbiol. Biotechnol. 2014, 98, 3231–3239. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG Database. In ‘In Silico’ Simulation of Biological Processes; John Wiley & Sons: Hoboken, NJ, USA, 2002; pp. 91–103. ISBN 978-0-470-85789-2. [Google Scholar]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Qiu, Y.-Q. KEGG Pathway Database. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 1068–1069. ISBN 978-1-4419-9863-7. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.A.; Hackett, D.P. Respiratory Chain of Colorless Algae II. Cyanophyta. Plant Physiol. 1966, 41, 599–605. [Google Scholar] [CrossRef]
- Gonzales-Prevatt, V.; Webster, D.A. Purification and Properties of NADH-Cytochrome o Reductase from Vitreoscilla. J. Biol. Chem. 1980, 255, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Rosas-Lemus, M.; Patel, D.; Fang, X.; Tuz, K.; Juárez, O. Dynamic Energy Dependency of Chlamydia Trachomatis on Host Cell Metabolism during Intracellular Growth: Role of Sodium-Based Energetics in Chlamydial ATP Generation. J. Biol. Chem. 2018, 293, 510–522. [Google Scholar] [CrossRef]
- Veseli, I.A.; Mascarenhas dos Santos, A.C.; Juárez, O.; Stark, B.C.; Pombert, J.-F. Complete Genome Sequence of Vitreoscilla Sp. Strain C1, Source of the First Bacterial Hemoglobin. Microbiol. Resour. Announc. 2018, 7, e00922-18. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Hebeler, B.H.; Morse, S.A. Physiology and Metabolism of Pathogenic Neisseria: Tricarboxylic Acid Cycle Activity in Neisseria Gonorrhoeae. J. Bacteriol. 1976, 128, 192–201. [Google Scholar] [CrossRef]
- Morse, S.A.; Stein, S.; Hines, J. Glucose Metabolism in Neisseria Gonorrhoeae. J. Bacteriol. 1974, 120, 702–714. [Google Scholar] [CrossRef]
- Winter, D.B.; Morse, S.A. Physiology and Metabolism of Pathogenic Neisseria: Partial Characterization of the Respiratory Chain of Neisseria Gonorrhoeae. J. Bacteriol. 1975, 123, 631–636. [Google Scholar] [CrossRef]
- Corregido, M.C.; Asención Diez, M.D.; Iglesias, A.Á.; Piattoni, C.V. New Pieces to the Carbon Metabolism Puzzle of Nitrosomonas Europaea: Kinetic Characterization of Glyceraldehyde-3 Phosphate and Succinate Semialdehyde Dehydrogenases. Biochimie 2019, 158, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.; Bergmann, D.; Arciero, D.; Hooper, A.B. Electron Transfer during the Oxidation of Ammonia by the Chemolithotrophic Bacterium Nitrosomonas Europaea. Biochim. Biophys. Acta 2000, 1459, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Sass, A.; Bazzini, S.; De Roy, K.; Udine, C.; Messiaen, T.; Riccardi, G.; Boon, N.; Nelis, H.J.; Mahenthiralingam, E.; et al. Biofilm-Grown Burkholderia Cepacia Complex Cells Survive Antibiotic Treatment by Avoiding Production of Reactive Oxygen Species. PLoS ONE 2013, 8, e58943. [Google Scholar] [CrossRef]
- Lefebre, M.D.; Flannagan, R.S.; Valvano, M.A. A Minor Catalase/Peroxidase from Burkholderia Cenocepacia Is Required for Normal Aconitase Activity. Microbiology 2005, 151, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Pessi, G.; Braunwalder, R.; Grunau, A.; Omasits, U.; Ahrens, C.H.; Eberl, L. Response of Burkholderia Cenocepacia H111 to Micro-Oxia. PLoS ONE 2013, 8, e72939. [Google Scholar] [CrossRef]
- Jyoti, P.; Shree, M.; Joshi, C.; Prakash, T.; Ray, S.K.; Satapathy, S.S.; Masakapalli, S.K. The Entner-Doudoroff and Nonoxidative Pentose Phosphate Pathways Bypass Glycolysis and the Oxidative Pentose Phosphate Pathway in Ralstonia Solanacearum. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The Complete Genome Sequence of Escherichia Coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete Genome Sequence of Pseudomonas Aeruginosa PAO1, an Opportunistic Pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef]
- Reyes-Prieto, A.; Barquera, B.; Juárez, O. Origin and Evolution of the Sodium -Pumping NADH: Ubiquinone Oxidoreductase. PLoS ONE 2014, 9, e96696. [Google Scholar] [CrossRef]
- Liang, P.; Fang, X.; Hu, Y.; Yuan, M.; Raba, D.A.; Ding, J.; Bunn, D.C.; Sanjana, K.; Yang, J.; Rosas-Lemus, M.; et al. The Aerobic Respiratory Chain of Pseudomonas Aeruginosa Cultured in Artificial Urine Media: Role of NQR and Terminal Oxidases. PLoS ONE 2020, 15, e0231965. [Google Scholar] [CrossRef]
- Lümmen, P. Complex I Inhibitors as Insecticides and Acaricides. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1998, 1364, 287–296. [Google Scholar] [CrossRef]
- Ernster, L.; Dallner, G.; Azzone, G.F. Differential Effects of Rotenone and Amytal on Mitochondrial Electron and Energy Transfer. J. Biol. Chem. 1963, 238, 1124–1131. [Google Scholar] [CrossRef]
- Di Tomaso, G.; Fedi, S.; Carnevali, M.; Manegatti, M.; Taddei, C.; Zannoni, D. The Membrane-Bound Respiratory Chain of Pseudomonas Pseudoalcaligenes KF707 Cells Grown in the Presence or Absence of Potassium Tellurite. Microbiology 2002, 148, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.; Brzezinski, P.; von Ballmoos, C. The Proton Pumping Bo Oxidase from Vitreoscilla. Sci. Rep. 2019, 9, 4766. [Google Scholar] [CrossRef]
- Grund, T.N.; Radloff, M.; Wu, D.; Goojani, H.G.; Witte, L.F.; Jösting, W.; Buschmann, S.; Müller, H.; Elamri, I.; Welsch, S.; et al. Mechanistic and Structural Diversity between Cytochrome Bd Isoforms of Escherichia Coli. Proc. Natl. Acad. Sci. USA 2021, 118, e2114013118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, P.T.; Hu, Y.; Mascarenhas dos Santos, A.C.; Liang, P.; Stark, B.C.; Tuz, K.; Juárez, O. Energy Metabolism and Aerobic Respiratory Chain of Vitreoscilla sp. C1: Comparison with β-Proteobacteria. Microbiol. Res. 2025, 16, 94. https://doi.org/10.3390/microbiolres16050094
Nguyen PT, Hu Y, Mascarenhas dos Santos AC, Liang P, Stark BC, Tuz K, Juárez O. Energy Metabolism and Aerobic Respiratory Chain of Vitreoscilla sp. C1: Comparison with β-Proteobacteria. Microbiology Research. 2025; 16(5):94. https://doi.org/10.3390/microbiolres16050094
Chicago/Turabian StyleNguyen, Paul T., Yuyao Hu, Anne Caroline Mascarenhas dos Santos, Pingdong Liang, Benjamin C. Stark, Karina Tuz, and Oscar Juárez. 2025. "Energy Metabolism and Aerobic Respiratory Chain of Vitreoscilla sp. C1: Comparison with β-Proteobacteria" Microbiology Research 16, no. 5: 94. https://doi.org/10.3390/microbiolres16050094
APA StyleNguyen, P. T., Hu, Y., Mascarenhas dos Santos, A. C., Liang, P., Stark, B. C., Tuz, K., & Juárez, O. (2025). Energy Metabolism and Aerobic Respiratory Chain of Vitreoscilla sp. C1: Comparison with β-Proteobacteria. Microbiology Research, 16(5), 94. https://doi.org/10.3390/microbiolres16050094





