Abstract
Soil, rhizosphere, and plant-associated microorganisms can enhance plant growth and health. A genomic analysis of these microbes revealed the key characteristics contributing to their beneficial effects. Following a field survey in Panama, four bacterial isolates with plant growth-promoting traits (PGPT) in rice (Oryza sativa L.) were identified. In this study, we sequenced, assembled, and annotated the genomes of Lysinibacillus fusiformis C6 and 24, and Bacillus cereus D23 and 59. The C6 genome was 4,754,472 bp long with 10 contigs, 37.62% guanine-cytosine (GC) content, and 4657 coding sequences (CDS). The 24 genome was 4,683,219 bp with five contigs, 37.65% GC content, and 4550 CDS. The D23 genome was 6,199,908 bp long with 18 contigs, 34.84% GC content, and 6141 CDS. The 59 genome was 6,194,462 bp with 21 contigs, 34.87% GC content, and 6122 CDS. Digital DNA–DNA hybridization (dDDH) and average nucleotide identity (ANI) confirmed that C6 and 24 belong to Lysinibacillus fusiformis, whereas D23 and 59 belong to the Bacillus cereus species. Further results revealed that these bacteria contained genes characteristic of plant growth-promoting bacteria, such as siderophore, phytohormone auxin (IAA) production, and nitrogen-fixing abilities that promote plant growth. Moreover, the antiSMASH database identified gene clusters involved in secondary metabolite production (biosynthetic gene clusters), such as betalactone, NRPS-like, NRP-siderophore, terpene, and RiPP-like clusters. Moreover, diverse and novel biosynthetic clusters (BCGs) have included non-ribosomal peptides (NRPs), polyketides (PKs), bacteriocins, and ribosomally synthesized and post-transcriptionally modified peptides (RiPPs). This work offers new insights into the genomic basis of the studied strains’ plant growth-promoting capabilities.
1. Introduction
Rice (Oryza sativa L.) is a basic nutrition source for much of the global population, especially those in Asia, Latin America, and Africa [1,2]. Since global demand is increasing, boosting rice production is necessary, but current yield trajectories indicate that these current agricultural practices may soon become infeasible and that the expansion of agricultural land may be necessary [3,4].
Crops require specific nutrients for growth, but natural nutrient availability often falls short of meeting the global food demand. Climate change further exacerbates this challenge, posing significant threats to agricultural production and global food security [5,6]. The essential growth-limiting nutrients in rice crops are nitrogen, phosphorus, potassium, and iron (important for chlorophyll synthesis) [7]. More significant cloud cover and changing weather systems can limit available sunlight and influence photosynthesis, as well as nutrient uptake, especially of nitrogen and phosphorus, which would ultimately affect crop yields and overall plant health. Synthetic fertilizers and genetically modified crops have been used to address these issues [8,9]. However, excessive use of chemical fertilizers has been linked to soil degradation, water pollution, and biodiversity loss, further challenging sustainable food production [10,11]. Concerns persist regarding their long-term environmental impact [12,13,14].
Plant growth-promoting bacteria (PGPB) offer a sustainable alternative to chemical fertilizers, minimizing environmental risks while stimulating plant growth and maintaining soil fertility [15]. PGPB not only enhances nutrient availability but also contributes to plant disease suppression and stress tolerance, making them a viable solution for sustainable agriculture [16,17]. Among the diverse bacterial genera exhibiting PGP activities [18], the Bacillaceae family, particularly Bacillus and Lysinibacillus species, stand out due to their robust nature and diverse functional capabilities in soil ecology and agriculture [19,20]. These bacteria inhabit the rhizosphere and produce beneficial substances such as hormones, siderophores, and phosphate-solubilizing compounds, thus promoting plant growth [21]. Additionally, PGPB can enhance plant disease resistance and increase crop yields [22,23]. Emerging research highlights their potential to improve stress tolerance and plant defense mechanisms [24,25,26]. For example, certain PGPB strains, such as those isolated from the rhizosphere of pepper plants, have shown antagonistic activity against phytopathogens including Phytophthora capsici and Fusarium species, demonstrating their role as biocontrol agents [27]. Moreover, species such as Bacillus amyloliquefaciens contribute to plant resilience by modulating phytohormone responses and enhancing resistance to abiotic stress [28]. To mitigate the impacts of climate change, soil degradation, and water scarcity, PGPBs are increasingly used as bioinoculants in agricultural formulations [29,30].
In Panama, rice is a key component of the national diet and agricultural sector [31,32]. However, challenges such as climate change and disease pressures threaten sustainable production. The use of PGPB is a promising alternative to reduce dependence on agrochemicals while enhancing yield [33,34]. The effectiveness of PGPB effectiveness is influenced by ecological and geographical conditions [35]. Therefore, the identification and characterization of native PGPB strains is crucial for each region [35,36].
This study investigates the plant growth-promoting potential of Lysinibacillus fusiformis and Bacillus cereus strains isolated from Panamanian rice fields. Our primary aim was to identify the genetic determinants of PGP activity through whole-genome sequencing and analysis. These insights will contribute to a better understanding of PGPB and facilitate their application in sustainable agriculture.
2. Materials and Methods
2.1. Source and Information of the Microbial Isolates
The Instituto de Innovación Agropecuaria de Panamá (IDIAP), a Panamanian government agency, provided four bacterial isolates, previously identified and characterized biochemically, both in vitro and in vivo, for plant growth-promoting traits (Figure S1 and Table S1). These isolates originated from soil samples collected in two distinct regions within central Panama: two isolates (C6 and D23) were obtained from Tonosí, located in Los Santos Province, and two isolates (24 and 59) were obtained from Antón, in Coclé Province. The original soil samples were obtained from plots used in rice cultivation under a 100% certified organic production system exhibiting similar physicochemical properties (sandy loam, pH 5.8–6).
An earlier, unpublished study conducted by IDIAP isolated and characterized these bacteria. The methodology applied included soil sampling, serial dilutions on Ashby agar, and subsequent screening for plant growth-promoting traits. Specifically, the isolates were assessed for nitrogen fixation, indole-3-acetic acid (IAA) production (using the method described by Özdal et al. [37]), siderophore production (following the protocols of [38,39]), and phosphate solubilization. Microbial activity in the original soils was evaluated using CO2 evolution and dehydrogenase activity measurements. Finally, a quantitative analysis of phytosiderophore and IAA production was performed using spectrophotometry. All assays were conducted in triplicate. The resulting bacterial strains were preserved at 4 °C and −80 °C for long-term storage.
2.2. Whole-Genome Sequencing (WGS) by Illumina MiSeq and Oxford Nanopore MinION
Bacterial DNA was extracted using a Nucleospin® Tissue extraction kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s protocol. The extracted DNA samples were then divided into two, and each portion was sequenced using either Illumina or Oxford Nanopore Technologies (ONT) products. The Illumina library generated 2 × 250 bp paired-end reads using the Nextera XT DNA Sample Prep Kit (Illumina, San Diego, CA, USA) and was sequenced on an Illumina MiSeq platform. The ONT library was prepared using the Rapid Barcoding Kit SQK-RBK110.96 and sequenced on the MinION platform according to the manufacturer’s recommendations at the sequencing laboratory of the Genomic and Proteomic Department at the Gorgas Memorial Institute, using the MinKNOW software v.5.4.3.
2.3. Whole-Genome Assembly
The quality of the Illumina reads was verified using FastQC [40], and the ONT reads were verified using nanoPLOT [41]. As a quality-control step before assembly, we filtered out poor-quality reads using fastp v0.20.1 [42] (short reads) and Filtlong v0.2.1 [43] (long reads), with the parameters min_length 1000, keep_percent 90, and target_bases 500,000,000, to filter the long reads by either removing the worst 10% or retaining 500 Mb in total, which resulted in fewer reads.
Following quality filtering and trimming, fastq reads were de novo assembled using Unicycler v.0.5.0 [44] with default parameters for hybrid assembly (short + long reads). Assembly quality was assessed using multiple metrics. QUAST v.4.4 [45] was used to evaluate the standard assembly statistics (e.g., contig number, N50, L50). Genome completeness and contamination were estimated using CheckM v.1.2.1 [46] and a bacteria-specific marker set. This multifaceted approach provides a robust evaluation of assembly quality.
2.4. Genome-Based Taxonomic and Phylogenetic Analysis
To accurately assign the taxonomy of the sequenced isolates, we used the Genome Taxonomy Database Toolkit (GTDB-Tk) v1.7.0 [47] as well as whole-genome-based phylogeny with the Type (Strain) Genome Server (TYGS) [48] from the Leibniz Institute DSMZ (German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany). Moreover, TYGS (accessed on 5 August 2024) was used to find the best-matching-type strains for each genome and to subsequently calculate precise distances using the Genome Blast Distance Phylogeny approach (GBDP) under the algorithm ‘coverage’ and distance formula d5 [49]. In addition, the TYGS was used to determine pairwise digital DNA–DNA hybridization (dDDH [50]) values among isolated strains and published Bacillus and Lysinibacillus type strains. Finally, average nucleotide identity (ANI) was calculated using the orthologous ANI algorithm in BLASTN, implemented in the standalone program Orthologous Average Nucleotide Identity Tool (OAT) (https://www.ezbiocloud.net/tools/orthoani, accessed on 6 August 2024) [51].
2.5. Genome Annotation and Identification of Genes Associated with Plant Growth-Promoting Traits
Gene annotation was performed using Bakta web v1.7.0 [52] and the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) v6.9 [53] for the general genome annotation provided by NCBI RefSeq. Annotated protein sequences of each genome were analyzed through the PLant-associated BActeria web resource (PLaBAse) [54,55] to assess their plant growth-promoting traits (PGPTs) using the PGPT-Pred tool, which employs the BLASTP+HMMER Aligner/Mapper for gene prediction. The cutoff e-values and identity thresholds used in BLASTP+HMMER alignments were determined using the PGPT-Pred tool’s internal scoring system, which prioritizes high-confidence matches based on domain architecture and sequence similarity to known PGP genes. The functional annotation of PGP genes was based on the curated dataset of PLaBAse.
In addition, to quantify the number of genes/factors occurring in the genomes of the four strains involved in functions relevant to plant–microbe interactions, the PIFAR-Pred tool was used on the annotated protein files from each isolate. BLASTP+HMMER Aligner/Mapper was used to identify genetic factors involved in the interaction of bacteria with host plants, including genes related to adhesion, antibiotic production, biofilm formation, detoxification, exopolysaccharide secretion (EPSs), bacterial lipopolysaccharides (LPSs), microbe-associated molecular patterns (MAMPs), multidrug resistances (MDRs), plant cell wall-degrading enzymes (PCWDEs), phytohormones, phytotoxins, pigmentation, proteases, siderophores, type III effectors, and volatile compound production in the four rice-bacteria genomes under study.
2.6. Identification and Analysis of Biosynthetic Gene Clusters (BGCs)
For genome-wide detection and annotation of secondary metabolite BCGs produced by isolated strains of L. fusiformis and B. cereus, FASTA formats of the C6, 24, D23 and 59 genomes were uploaded to the AntiSMASH server version 7.0 [56,57] (https://antismash.secondarymetabolites.org, accessed on 5 August 2024) as data input. Default parameters (i.e., relaxed detection strictness) and the use of all additional analyses were selected. A Minimum Information about a Biosynthetic Gene cluster (MIBiG) comparison was used to compare the detected clusters with reference genomes. Finally, to summarize the number of BGCs identified in the samples, a matrix plot was created using RAWGraphs [58].
3. Results and Discussion
3.1. Genomic Assembling Metrics and Annotation of the Isolates
Genome assemblies were deposited in NCBI under the Bioproject PRJNA1209869 (BioSamples: SAMN46231022, SAMN46231023, SAMN46231024, and SAMN46231025). The hybrid assembly produced nearly complete genomes comprising 5 to 20 contigs, with genome sizes ranging from 4.6 to 6.1 Mbp. Genome completeness ranged from 99.17% to 99.34%, with minimal contamination levels (0.31% to 1.32%). The GC content varied from 34.84% to 37.65%. PGAP annotation of the draft genomes yielded the following gene counts: C6, 4812 total genes, with 4662 protein-coding; D23, 6296 total, 5820 protein-coding; 24, 4680 total, 4489 protein-coding; and 59, 6290 total, 5750 protein-coding (Table 1). These results confirm the quality of the genome assemblies and annotations. Genome completeness ranged from 99.17% to 99.34%, with minimal contamination levels (0.31% to 1.32%). The GC content varied from 34.84% to 37.65%. Prokaryotic Genome Annotation Pipeline (PGAP) annotation of the draft genomes yielded the following gene counts: C6, 4812 total genes, with 4662 protein-coding; D23, 6296 total, 5820 protein-coding; 24, 4680 total, 4489 protein-coding; and 59, 6290 total, 5750 protein-coding (Table 1). These results confirm the quality of the genome assemblies and annotations.

Table 1.
Genome quality metrics and annotation of C6, D23, 24, and 59 isolates.
3.2. Taxonomic Annotation
Taxonomic classification using GTDB-tk identified isolates D23 and 59 as Bacillus cereus, whereas isolates C6 and 24 were classified as Lysinibacillus fusiformis. Both species belong to the Bacillaceae family, and are rod-shaped, Gram-positive, spore-forming bacteria commonly found in natural environments [59]. These isolates’ assembly sizes and GC contents align with previously sequenced genomes of B. cereus and L. fusiformis [59,60,61,62]. Genome size and GC content are important factors that influence microbial adaptability to diverse environments [63]. Larger genomes often correlate with greater adaptability, as they enable the encoding of additional metabolic pathways and stress-response mechanisms [64]. Conversely, smaller genomes can provide energy-efficiency advantages, particularly in specialized niches, as reported in earlier studies [65,66].
3.3. Phylogenomic Classification, ANI, and dDDH Analysis
Phylogenomic analysis using Type (Strain) Genome Server (TYGS), based on the MASH algorithm [67], placed isolates C6 and 24 within the same cluster as L. fusiformis ATCC 7055T, and isolates D23 and 59 were closely related to B. cereus ATCC 14579T (Figure 1). The genomic characteristics of our isolates were also compared to those of closely related reference strains to assess their genomic similarities and potential unique features. The genome sizes of L. fusiformis strains C6 and 24 are comparable to L. fusiformis ATCC 7055T but smaller than those of other Lysinibacillus species, such as L. xylanilyticus and L. agricola. Similarly, the GC content of both C6 and 24 was identical to that of L. fusiformis ATCC 7055T and within the typical range observed in Lysinibacillus species. The number of protein-coding genes in our strains aligns with that in other L. fusiformis strains. For B. cereus, the genome sizes of strains D23 and 59 were larger than those of B. cereus ATCC 14579T, whereas their GC content was slightly lower than that of the reference. However, the number of protein-coding genes in both isolates exceeded that in the reference strain, suggesting potential differences in gene content. A comparative genomic analysis of different bacterial isolates indicates that the number of protein-coding genes in various bacterial isolates can exceed that of reference strains, driven by factors such as genetic variability, the presence of unique virulence factors, and adaptations to specific environments [68,69]. These comparisons provide further context for our isolates within their respective species and highlight possible strain-specific adaptations.
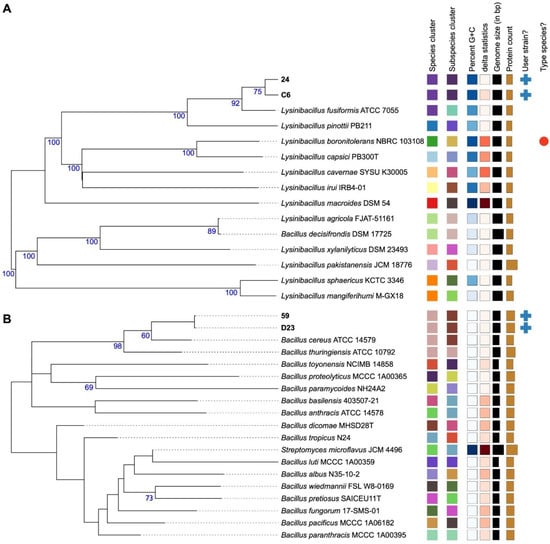
Figure 1.
Type (Strain) Genome Server (TYGS) phylogram results for bacterial strains based on genomic data. Based on the TYGS results, phylogenomic trees showing the relationship between (A) C6 and 24 and (B) D23 and 59 strains with related type strain genomes. Branches were annotated using GBDP pseudo-bootstrap support values of >60% from 100 replications. The tree was rooted at its midpoint. Leaf labels were annotated by affiliation to species and subspecies clusters, genomic G + C content, and δ values, as well as genome sequence length, number of proteins, and strain. The color of the squares indicates that a group of strains belongs to the same cluster, as defined by each of the parameters indicated at the top of the squares.
To further confirm their taxonomic affiliation, average nucleotide identity (ANI) and digital DNA–DNA hybridization (dDDH) analyses were performed against reference databases. The ANI values for isolates C6 and 24 were 97.30% and 97.33%, respectively, when compared to L. fusiformis ATCC 7055T, whereas isolates D23 and 59 displayed ANI values of 97.71% and 97.75%, respectively, against B. cereus ATCC 14579T. The corresponding dDDH values further corroborated these findings, with values exceeding 76% for all the isolates (Table 2). Both ANI and dDDH values were above the established species thresholds of 95% and 70%, respectively, confirming the species-level classification of the isolates [49,70,71]. However, it is important to acknowledge that although these values confirm species-level assignment, they do not prevent the possibility of intraspecific variability. Significant genetic diversity can exist within bacterial species [72], potentially leading to variations in phenotypic traits, including those relevant to plant-growth promotion.

Table 2.
Genome distance measurements of four isolates against type strain.
The bacterial genera Bacillus and Lysinibacillus have been widely described as plant growth-promoting bacteria (PGPB). In particular, the Bacillus genus is known for its adaptability to diverse ecosystems, including aquatic, terrestrial, and extreme environments [73,74]. Many Bacillus species produce a variety of bioactive compounds that promote plant growth and protect against pathogens [26]. For instance, inoculation with Bacillus cereus strains has been shown to improve growth and yield in cereals such as soybean, maize, rice, and wheat [75,76]. Species such as Bacillus subtilis can effectively colonize plant roots and promote growth through stable interactions [77,78,79]. Similarly, Bacillus licheniformis has demonstrated considerable colonization abilities in tomato and pepper plants, functioning as an effective biofertilizer without interfering with greenhouse or field-management practices [80].
In contrast, Lysinibacillus is a genus that has been previously grouped with Bacillus. Certain Lysinibacillus species have been identified as nutrient enhancers and growth promoters in plants [81]. Several studies have reported their roles as biological control agents and plant-growth promoters in jack beans, paprika, and tomatoes [82,83,84]. Moreover, the ability of Lysinibacillus to form endospores makes it a promising candidate for microbial product formulation, ensuring stability and effectiveness in agricultural applications [85]. Furthermore, their efficacy in poor soils is improved by interactions with soil amendments such as biochar [86,87,88]. Along with Bacillus, they play key roles in diverse soils by promoting plant growth through nutrient solubilization, phytohormone production, and stress mitigation [89,90,91], including improving phosphorus availability and alleviating stress in contaminated soils [83].
3.4. Identification of Genetic Factors Involved in Plant–Bacteria Interactions
The potential plant growth-promoting traits of the four isolates were computationally analyzed through annotation against the PLaBAse database using the PGPT-Pred tool, which revealed many genes related to plant interactions. The genomes of L. fusiformis strain C6 and 24 contained approximately 2600 PGPT genes, whereas the genomes of B. cereus strains D23 and 59 exhibited a slightly higher count, averaging around 2800 genes. Most identified genes in both species strains produced indirect effects such as colonization of the plant system, competitive exclusion, and involvement in stress control and biocontrol (Figure 2 and Supplementary File S1). This aligns with the well-documented role of PGPB in creating a favorable rhizosphere environment, thereby promoting plant health and growth [15]. Among the direct effects, the main categories of genes are involved in biofertilization, bioremediation, and phytohormone/plant-signal production.
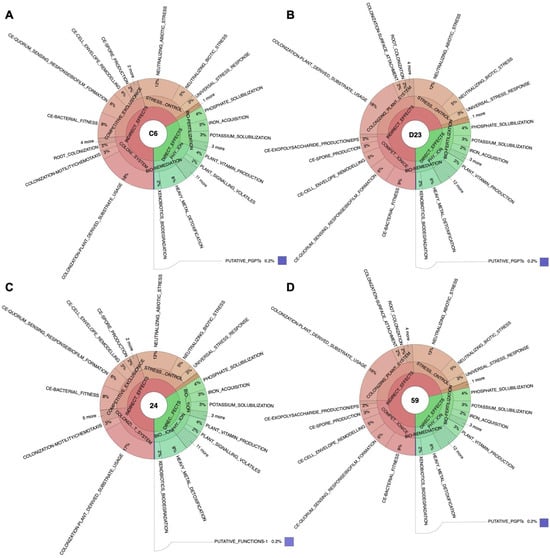
Figure 2.
Krona plot representation of the major plant growth-promoting traits found in (A) C6, (B) D23, (C) 24, and (D) 59. Identification of plant growth promoting traits (PGPTs) was performed using BLASTP and HMMER annotation against PGPT-BASE. The depth of annotation is shown to level three of six, excluding pathways, gene names, and accession numbers.
A comparative analysis indicated that L. fusiformis strains displayed higher gene counts related to biofertilization and bioremediation, highlighting their potential as powerful biofertilizers, which is consistent with studies showing their ability to solubilize insoluble minerals such as potassium, iron, and zinc [92,93,94]. Due to their ubiquity and ability to survive under adverse conditions, they are assumed to effectively degrade pesticides and therefore play an important role in the bioremediation of pesticide contamination sites. Organophosphate pesticides can be detoxified by Bacillus spp. through the enzymatic activity of carboxylesterases, resulting in the hydrolysis of the carboxylester bonds [95]. Singh et al. [96,97] isolated an organophosphate-degrading bacterium, designated Lysinibacillus sp. strain KB1 and Bacillus cereus strain PU, Brevibacillus sp. strain KB2, from agricultural soils and studied bioremediation in soil contaminated with organophosphate. In contrast, B. cereus strains exhibited significantly more genes associated with plant immune-response stimulation and competitive exclusion traits, supporting their role as biocontrol agents capable of inducing plant immunity [98,99]. Specifically, some exclusive genes from B. cereus involved in immune-response stimulation, such as entE, psmE, dhbE, basE, angE, aebE, and pchC, are involved in the salicylic acid biosynthesis. This plant hormone plays a key role in systemic acquired resistance (SAR), a defense response that provides long-lasting protection against a broad spectrum of pathogens [100]. Both species demonstrate colonization and stress-control genes, which are fundamental for their persistence in the rhizosphere environment [59,62]. These findings align with the established understanding that plant growth-promoting bacteria (PGPB) employ diverse mechanisms to support plant health by enhancing nutrient availability, producing growth-promoting compounds, and defending against pathogens.
In general, PGPT-Pred analysis identified key genes in B. cereus and L. fusiformis that are linked to plant growth promotion. In B. cereus, genes involved in carbon dioxide fixation and RuBisCo regulation suggest potential autotrophic capabilities. Additionally, iron acquisition genes and stress-response regulators indicate efficient nutrient uptake and environmental adaptation. Similarly, L. fusiformis harbors genes for CO2 fixation, iron transport, and nitrogen acquisition, thereby reinforcing its role in plant nutrient availability.
These findings align with well-established plant growth-promoting mechanisms, including phytohormone production, nitrogen fixation, and phosphate solubilization [101,102]. Genes associated with indole-3-acetic acid (IAA) biosynthesis and 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity may enhance root development and stress tolerance [103,104]. Furthermore, genes related to siderophore production contribute to iron availability, whereas phosphate solubilization genes improve nutrient uptake [105,106].
In addition, the PIFAR-Pred web-based tool identified several genetic factors involved in plant–bacteria interactions. A comparative analysis revealed that the number of annotated genes involved in these interactions for Lysinibacillus fusiformis strains C6 and 24 were very similar, ranging from 280 to 287. In contrast, Bacillus cereus strains D23 and 59 exhibited slightly higher gene counts, ranging from 380 to 385. Despite this difference, the same gene clusters were identified in both species for critical functions such as adhesion, detoxification, exopolysaccharide (EPS) production, hormone biosynthesis, lipopolysaccharides (LPS), multidrug resistance (MDR), metabolism, movement, plant cell-wall-degrading enzymes (PCWDE), pigment production, proteases, toxin synthesis, and volatiles production (Figure 3 and Supplementary File S2). This confirmed the close relatedness between the four bacterial strains.
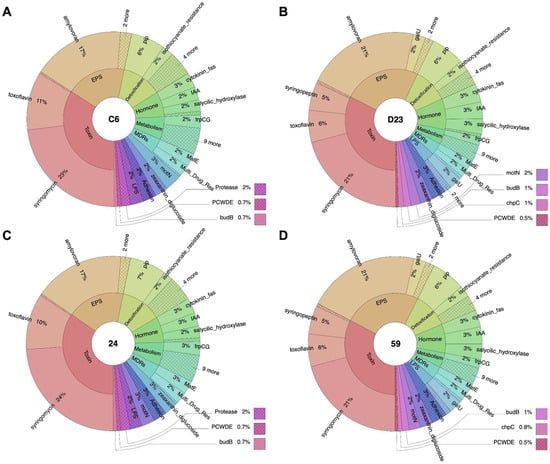
Figure 3.
The percentage of genetic factors involved in plant-bacteria interactions detected in the genomes of strains (A) C6, (B) D23, (C) 24, and (D) 59, as identified by the PIFAR-Pred web-based tool.
As expected, the profile of genetic factors in L. fusiformis strains differed from that in B. cereus, with more remarkable similarities observed within strains of the same species. For instance, fewer genes related to toxin production, EPS production, hormone biosynthesis, metabolism, and pigment synthesis were detected in L. fusiformis strains C6 and 24 compared to B. cereus strains D23 and 59. In contrast, L. fusiformis strains possess an additional protease gene known as High-temperature Requirement A (HtrA), and B. cereus possesses an additional asparagine synthetase gene, asnB. HtrA aids bacteria in surviving stress and influences important proteins such as E-cadherin, fibronectin, and proteoglycans, although it is not a direct cause of disease [107]. In Gram-positive bacteria, HtrAs have been shown to regulate virulence and biofilm formation [108,109]. In contrast, asnB is associated with virulence and drug resistance in various pathogenic bacteria [110,111].
3.5. Genome Mining for BGCs Encoding Bioactive Compounds
Bioinformatic analysis using AntiSMASH identified 37 biosynthetic gene clusters (BGCs) across the four genomes. L. fusiformis strains C6 and 24 contained 7 and 6 BGCs, respectively, while B. cereus strains D23 and 59 exhibited a higher number, with 12 BGCs each. BGCs are genomic regions that encode the enzymes required for the biosynthesis of specific secondary metabolites. The identified clusters included diverse types such as betalactones containing protease inhibitors (betalactones), non-ribosomal peptide synthetase (NRPS), NRPS-independent, IucA/ucC-like siderophores (NI-siderophore), NRPS-like fragment (NRPS-like), non-ribosomal peptide metallophores (NRPS-metallophores), terpene (terpene), Linear azol(in)e-containing peptides (LAP), type I PKS (Polyketide synthase, T1PKS), type III PKS (T3PKS), other unspecified ribosomally synthesized and post-translationally modified peptide products (RiPP, RiPP-like), RRE-element-containing cluster (RRE-containing), and agrD-like cyclic lactone autoinducer peptides (cyclic-lactone autoinducer) (Figure 4, Table 3).
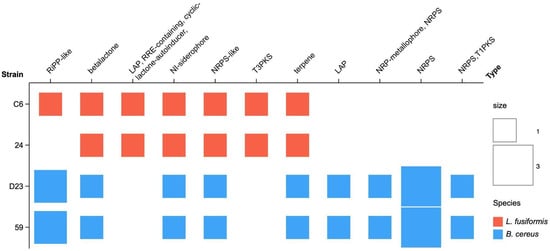
Figure 4.
The number of biosynthetic gene clusters (BGCs) detected by antiSMASH in the isolated strains. Red and blue light colors refer to taxonomic annotations based on the species using the Genome Taxonomy Database. The size of the squares indicates the number of BGCs.

Table 3.
Biosynthetic gene clusters identified using AntiSMASH. Nucleotide positions within each genome are reported.
Interestingly, most predicted BGCs shared less than 50% similarity with known clusters in the MiBIG database, underscoring their novelty. However, specific BGCs, such as NI-siderophore, NRPS-metallophore, and NRPS-T1PKS, exhibited significant similarity to well-characterized biosynthetic pathways, including petrobactin (100%), bacillibactin (85%), and zwittermicin A (100%). Additionally, coding regions for fengycin and bogorol A biosynthesis were detected within NRPS gene clusters. These secondary metabolites are known for their antimicrobial properties, suggesting a competitive advantage for the strains in suppressing bacterial and fungal pathogens in the rhizosphere, particularly exemplified by zwittermicin A (ZmA), which exhibits notable biological activities, including antiprotist activity, antibiotic effects against both Gram-positive and Gram-negative bacteria, and antifungal properties [112]. Moreover, ZmA enhances the effectiveness of Bacillus thuringiensis toxin proteins in insect control, contributing to its potential as a biocontrol agent [113].
Previous research has shown that Bacillus and Lysinibacillus strains harbor a diverse array of BGCs responsible for producing antimicrobial compounds that play critical roles in ecological adaptation and biocontrol activities against phytopathogens [114,115,116,117]. These strains exhibit significant potential as biocontrol agents and plant-growth promoters, with species- or clade-specific metabolites such as lipopeptides, siderophores, lantibiotics, bacteriocins, and polyketides contributing to enhanced plant health and defense [118]. For instance, Bacillus and Lysinibacillus strains effectively combat rice pathogens like Rhizoctonia solani by producing antifungal compounds and activating plant defense mechanisms, reducing disease incidence [119,120,121].
Furthermore, Bacillus species isolated from diverse environments, including the Indo-Gangetic plains and the Qinghai–Tibetan plateau, demonstrate notable plant growth-promoting activities. These include the production of indole-3-acetic acid (IAA), siderophores, and phosphate-solubilizing compounds, which significantly enhance rice growth and yield [119,122,123]. This biosynthetic versatility is particularly evident in strains like Lysinibacillus fusiformis and Bacillus cereus, underscoring their agricultural potential for improving crop productivity and resilience in various ecological settings.
The identification of Bacillus cereus as a plant growth-promoting bacterium requires careful consideration of its potential toxin production. While B. cereus is known for producing enterotoxins and emetic toxins, these traits are not universally present across all strains [124,125]. Strain-level differences in toxin gene presence and expression are well documented [126]. To ensure their safe and reliable use, further in-depth analysis is essential. Furthermore, as recent research indicates, strain-specific safety assessments are essential for B. cereus agricultural applications [127]. Before potential commercialization, a comprehensive risk assessment, including toxin analysis and virulence factor evaluation, is essential to ensure biosafety [127]. It is imperative that these safety assessments, including toxin gene analysis and phenotypic assays, are conducted as part of future research to definitively establish the safety profile of our B. cereus isolate before any agricultural application is considered.
This study highlights the potential for utilizing native microorganisms as biofertilizers in Panamanian rice fields. Given the financial burden of traditional fertilization practices, with costs reaching USD 278.14 per hectare in 2020 (Panamanian Department of Agriculture, MIDA 2020), and considering the critical role of nitrogen in rice productivity, our research provides a foundation for sustainable agriculture. In Panama, soil analysis is a crucial tool for determining optimal fertilizer doses, with applications varying across agroclimatic zones, soil types, and rice varieties. The genomic presence of genes associated with plant growth-promoting traits, particularly those related to atmospheric nitrogen fixation by diazotrophic organisms, indicates that these native bacteria could serve as a sustainable substitute for traditional chemical fertilizers like urea. Atmospheric nitrogen fixation is a fundamental biochemical process, and harnessing it through native microorganisms could reduce dependence on synthetic nitrogen sources while enhancing soil health and agricultural sustainability.
4. Conclusions
The genomic analysis of Lysinibacillus fusiformis isolates C6 and 24, along with Bacillus cereus isolates D23 and 59, revealed their potential as plant growth-promoting bacteria. The identification of genes involved in biofertilization, siderophore production, stress tolerance, and bioremediation highlights their utility as functional bioinoculants. Furthermore, the analysis revealed the presence of key genes linked to CO2 fixation, iron acquisition, and stress-response regulation, which reinforces their potential for improved nutrient uptake and environmental adaptation. Additionally, the discovery of biosynthetic gene clusters underscores their ability to produce bioactive compounds that may enhance plant health and competitiveness in the rhizosphere. These findings warrant further studies on applying these strains in sustainable agriculture, particularly for improving Oryza sativa L. cultivation in Panama.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16050095/s1, Figure S1: Siderophore production, plant growth and development of Oriza sativa L. treated with PGPBs; Table S1: In vitro root measures of plant growth promoting bacterial strains; File S1: Krona data table of PGPT-Pred tool results from PLaBAse; Krona data table of PIFAR-Pred tool results from PLaBAse.
Author Contributions
Conceptualization, R.H. and A.A.M.; Data curation, C.A.; Formal analysis, C.A.; Funding acquisition, R.H.; Investigation, C.A., R.H. and A.A.M.; Methodology, C.A., R.H., J.L.C., B.B., O.C., C.G., J.G. and A.M.; Project administration, R.H.; Resources, A.A.M.; Supervision, A.A.M.; Visualization, C.A.; Writing—original draft, C.A. and R.H.; Writing—review and editing, C.A., R.H., J.L.C., B.B., O.C., C.G., J.G., A.M. and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaría Nacional de Ciencia, Tecnología e Innovación (grant number FID17-048 and FID23-030) and Instituto de Innovación Agropecuaria de Panamá.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The assembled genomes have been deposited to NCBI GenBank under the Bioproject accession number PRJNA1209869, and the Biosample accession numbers: SAMN46231022, SAMN46231023, SAMN46231024, and SAMN46231025.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Chen, W.; Zhao, X. Understanding Global Rice Trade Flows: Network Evolution and Implications. Foods 2023, 12, 3298. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An Overview of Global Rice Production, Supply, Trade, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; He, Y.; Liu, F.; Feng, X.; Zhang, J. Assessment of the Vigor of Rice Seeds by near-Infrared Hyperspectral Imaging Combined with Transfer Learning. RSC Adv. 2020, 10, 44149–44158. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Parry, M.L. Climate Change and World Agriculture; Routledge: London, UK, 2019; ISBN 9781000692778. [Google Scholar]
- Kumari, A.; Lakshmi, G.A.; Krishna, G.K.; Patni, B.; Prakash, S.; Bhattacharyya, M.; Singh, S.K.; Verma, K.K. Climate Change and Its Impact on Crops: A Comprehensive Investigation for Sustainable Agriculture. Agronomy 2022, 12, 3008. [Google Scholar] [CrossRef]
- Shrestha, J.; Kandel, M.; Subedi, S.; Shah, K.K. Role of Nutrients in Rice (Oryza sativa L.): A Review. Agrica 2020, 9, 53. [Google Scholar] [CrossRef]
- Lightfoot, D.A. Developing Crop Varieties with Improved Nutrient-Use Efficiency. In Engineering Nitrogen Utilization in Crop Plants; Shrawat, A., Zayed, A., Lightfoot, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–11. ISBN 9783319929583. [Google Scholar]
- Thapa, S.; Bhandari, A.; Ghimire, R.; Xue, Q.; Kidwaro, F.; Ghatrehsamani, S.; Maharjan, B.; Goodwin, M. Managing Micronutrients for Improving Soil Fertility, Health, and Soybean Yield. Sustain. Sci. Pract. Policy 2021, 13, 11766. [Google Scholar] [CrossRef]
- Davis, A.G.; Huggins, D.R.; Reganold, J.P. Linking Soil Health and Ecological Resilience to Achieve Agricultural Sustainability. Front. Ecol. Environ. 2023, 21, 131–139. [Google Scholar] [CrossRef]
- Gomiero, T. Soil Degradation, Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Penuelas, J.; Coello, F.; Sardans, J. A Better Use of Fertilizers Is Needed for Global Food Security and Environmental Sustainability. Agric. Food Secur. 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Kremer, R.J. Impacts of Genetically Engineered Crops on the Soil Microbiome, Biological Processes, and Ecosystem Services. In GMOs: Implications for Biodiversity Conservation and Ecological Processes; Chaurasia, A., Hawksworth, D.L., Pessoa de Miranda, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 129–147. ISBN 9783030531836. [Google Scholar]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Chapter 2-Influence of Synthetic Fertilizers and Pesticides on Soil Health and Soil Microbiology. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. ISBN 9780081030172. [Google Scholar]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) Integrated Phytotechnology: A Sustainable Approach for Remediation of Marginal Lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef] [PubMed]
- Tchuisseu Tchakounté, G.V.; Berger, B.; Patz, S.; Fankem, H.; Ruppel, S. Community Structure and Plant Growth-Promoting Potential of Cultivable Bacteria Isolated from Cameroon Soil. Microbiol. Res. 2018, 214, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Abedinzadeh, M.; Etesami, H.; Alikhani, H.A. Characterization of Rhizosphere and Endophytic Bacteria from Roots of Maize (Zea mays L.) Plant Irrigated with Wastewater with Biotechnological Potential in Agriculture. Biotechnol. Rep. Amst. 2019, 21, e00305. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Mendoza, J.L.; Sangoquiza-Caiza, C.A.; Campaña-Cruz, D.F.; Yánez-Guzmán, C.F. Use of Biofertilizers in Agricultural Production. In Technology in Agriculture; IntechOpen: London, UK, 2021. [Google Scholar]
- Mandic-Mulec, I.; Stefanic, P.; van Elsas, J.D. Ecology of Bacillaceae. Microbiol. Spectr. 2015, 3, TBS-0017-2013. [Google Scholar] [CrossRef]
- Naureen, Z.; Rehman, N.U.; Hussain, H.; Hussain, J.; Gilani, S.A.; Al Housni, S.K.; Mabood, F.; Khan, A.L.; Farooq, S.; Abbas, G.; et al. Exploring the Potentials of Lysinibacillus Sphaericus ZA9 for Plant Growth Promotion and Biocontrol Activities against Phytopathogenic Fungi. Front. Microbiol. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Malik, K.A.; Bilal, R.; Mehnaz, S.; Rasul, G.; Mirza, M.S.; Ali, S. Association of Nitrogen-Fixing, Plant-Growth-Promoting Rhizobacteria (PGPR) with Kallar Grass and Rice. In Opportunities for Biological Nitrogen Fixation in Rice and Other Non-Legumes: Papers presented at the Second Working Group Meeting of the Frontier Project on Nitrogen Fixation in Rice held at the National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan, 13–15 October 1996; Ladha, J.K., de Bruijn, F.J., Malik, K.A., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 37–44. ISBN 9789401171137. [Google Scholar]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Ruiu, L. Plant-Growth-Promoting Bacteria (PGPB) against Insects and Other Agricultural Pests. Agronomy 2020, 10, 861. [Google Scholar] [CrossRef]
- Abdelkrim, S.; Abid, G.; Chaieb, O.; Taamalli, W.; Mannai, K.; Louati, F.; Jebara, M.; Jebara, S.H. Plant Growth Promoting Rhizobacteria Modulates the Antioxidant Defense and the Expression of Stress Responsive Genes Providing Pb Accumulation and Tolerance of Grass Pea. Res. Sq. 2022, 30, 10789–10802. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Admassie, M.; Wold-Hawariat, Y.; Alemu, T. Characterization and Diversity of Plant Growth Promoting Endophytic and Rhizosphere Bacteria Isolated from theRhizosphere and Tissues of Pepper and Their Effect on Plant Growth Promotion and Disease Suppression of Phytophthora Capsici. Res. Sq. 2021, 1–19. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus Amyloliquefaciens Confers Tolerance to Various Abiotic Stresses and Modulates Plant Response to Phytohormones through Osmoprotection and Gene Expression Regulation in Rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M.; et al. Bioinoculants-Natural Biological Resources for Sustainable Plant Production. Microorganisms 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- del Orozco-Mosqueda, M.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Fontanelli, M.M.; Kowalskys, I.; Gómez, G.; Rigotti, A.; Cortés, L.Y.; García, M.Y.; Pareja, R.G.; Herrera-Cuenca, M.; Fisberg, M.; et al. Total and Whole Grain Intake in Latin America: Findings from the Multicenter Cross-Sectional Latin American Study of Health and Nutrition (ELANS). Eur. J. Nutr. 2022, 61, 489–501. [Google Scholar] [CrossRef]
- Singh, V.; Zhou, S.; Ganie, Z.; Valverde, B.; Avila, L.; Marchesan, E.; Merotto, A.; Zorrilla, G.; Burgos, N.; Norsworthy, J.; et al. Rice Production in the Americas. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 137–168. ISBN 9783319475165. [Google Scholar]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The Role of Plant Growth Promoting Bacteria in Improving Nitrogen Use Efficiency for Sustainable Crop Production: A Focus on Wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of Plant Growth-Promoting Bacteria in Sustainable Agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Armada, E.; Leite, M.F.A.; Medina, A.; Azcón, R.; Kuramae, E.E. Native Bacteria Promote Plant Growth under Drought Stress Condition without Impacting the Rhizomicrobiome. FEMS Microbiol. Ecol. 2018, 94, fiy092. [Google Scholar] [CrossRef]
- Aviles, C.F.A.; Acosta, C.B.C.; de los Santos Villalobos, S.; Santoyo, G.; Cota, F.I.P. Characterization of Native Plant Growth-Promoting Bacteria (PGPB) and Their Effect on the Development of Maize (Zea mays L.). Biotecnia 2022, 24, 15–22. [Google Scholar] [CrossRef]
- Özdal, M.; Gür Özdal, Ö.; Sezen, A.; Algur, Ö.F. Biosynthesis of Indole-3-Acetic Acid by Bacillus Cereus Immobilized Cells. Cumhur. Sci. J. 2016, 37, 212. [Google Scholar] [CrossRef]
- Milagres, A.M.; Machuca, A.; Napoleão, D. Detection of Siderophore Production from Several Fungi and Bacteria by a Modification of Chrome Azurol S (CAS) Agar Plate Assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-P. A Simple Double-Layered Chrome Azurol S Agar (SD-CASA) Plate Assay to Optimize the Production of Siderophores by a Potential Biocontrol Agent Bacillus. Afr. J. Microbiol. Res. 2011, 5, 4321–4327. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 12 March 2023).
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wick, R.; Menzel, P. Filtlong: Quality Filtering Tool for Long Reads. 2019. Available online: https://github.com/rrwick/Filtlong (accessed on 10 February 2023).
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A Toolkit to Classify Genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Auch, A.F.; von Jan, M.; Klenk, H.-P.; Göker, M. Digital DNA-DNA Hybridization for Microbial Species Delineation by Means of Genome-to-Genome Sequence Comparison. Stand. Genom. Sci. 2010, 2, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An Improved Algorithm and Software for Calculating Average Nucleotide Identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and Standardized Annotation of Bacterial Genomes via Alignment-Free Sequence Identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Martínez-García, P.M.; López-Solanilla, E.; Ramos, C.; Rodríguez-Palenzuela, P. Prediction of Bacterial Associations with Plants Using a Supervised Machine-Learning Approach. Environ. Microbiol. 2016, 18, 4847–4861. [Google Scholar] [CrossRef]
- Patz, S.; Gautam, A.; Becker, M.; Ruppel, S.; Rodríguez-Palenzuela, P.; Huson, D.H. PLaBAse: A Comprehensive Web Resource for Analyzing the Plant Growth-Promoting Potential of Plant-Associated Bacteria. bioRxiv 2021, 2021, 12.13.472471. [Google Scholar]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; Association for Computing Machinery: New York, NY, USA, 2017; pp. 1–5. [Google Scholar]
- Zeng, Q.; Xie, J.; Li, Y.; Gao, T.; Xu, C.; Wang, Q. Comparative Genomic and Functional Analyses of Four Sequenced Bacillus Cereus Genomes Reveal Conservation of Genes Relevant to Plant-Growth-Promoting Traits. Sci. Rep. 2018, 8, 17009. [Google Scholar] [CrossRef]
- Pudova, D.S.; Lutfullin, M.T.; Shagimardanova, E.I.; Hadieva, G.F.; Shigapova, L.; Toymentseva, A.A.; Kabanov, D.A.; Mardanova, A.M.; Vologin, S.G.; Sharipova, M.R. Draft Genome Sequence Data of Lysinibacillus Fusiformis Strain GM, Isolated from Potato Phyllosphere as a Potential Probiotic. Data Brief 2018, 21, 2504–2509. [Google Scholar] [CrossRef]
- Smith, B.R.; Unckless, R.L. Draft Genome Sequence of Lysinibacillus Fusiformis Strain Juneja, a Laboratory-Derived Pathogen of Drosophila Melanogaster. Genome Announc. 2018, 6, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Passera, A.; Rossato, M.; Oliver, J.S.; Battelli, G.; Shahzad, G.-I.-R.; Cosentino, E.; Sage, J.M.; Toffolatti, S.L.; Lopatriello, G.; Davis, J.R.; et al. Characterization of Lysinibacillus Fusiformis Strain S4C11: In Vitro, in Planta, and in Silico Analyses Reveal a Plant-Beneficial Microbe. Microbiol. Res. 2021, 244, 126665. [Google Scholar] [CrossRef] [PubMed]
- Šmarda, P.; Bureš, P.; Horová, L.; Leitch, I.J.; Mucina, L.; Pacini, E.; Tichý, L.; Grulich, V.; Rotreklová, O. Ecological and Evolutionary Significance of Genomic GC Content Diversity in Monocots. Proc. Natl. Acad. Sci. USA 2014, 111, E4096–E4102. [Google Scholar] [CrossRef] [PubMed]
- Ranea, J.A.G.; Buchan, D.W.A.; Thornton, J.M.; Orengo, C.A. Evolution of Protein Superfamilies and Bacterial Genome Size. J. Mol. Biol. 2004, 336, 871–887. [Google Scholar] [CrossRef]
- Mijakovic, I.; Grangeasse, C.; Stülke, J. Regulatory Potential of Post-Translational Modifications in Bacteria; Frontiers Media SA: Lausanne, Switzerland, 2015; ISBN 9782889196104. [Google Scholar]
- Martínez-Cano, D.J.; Reyes-Prieto, M.; Martínez-Romero, E.; Partida-Martínez, L.P.; Latorre, A.; Moya, A.; Delaye, L. Evolution of Small Prokaryotic Genomes. Front. Microbiol. 2014, 5, 742. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast Genome and Metagenome Distance Estimation Using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Vebø, H.C.; Solheim, M.; Snipen, L.; Nes, I.F.; Brede, D.A. Comparative Genomic Analysis of Pathogenic and Probiotic Enterococcus Faecalis Isolates, and Their Transcriptional Responses to Growth in Human Urine. PLoS ONE 2010, 5, e12489. [Google Scholar] [CrossRef]
- Solanki, S.; Devi, D. Analysis of Virulence Genes of Staphylococcus Aureus, Streptococcus. E. coli Isolated from Bovine Subclinical Mastitis. Indian J. Anim. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Thompson, C.C.; Chimetto, L.; Edwards, R.A.; Swings, J.; Stackebrandt, E.; Thompson, F.L. Microbial Genomic Taxonomy. BMC Genom. 2013, 14, 913. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S.; et al. Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Van Rossum, T.; Ferretti, P.; Maistrenko, O.M.; Bork, P. Diversity within Species: Interpreting Strains in Microbiomes. Nat. Rev. Microbiol. 2020, 18, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Gat, D.; Mazar, Y.; Cytryn, E.; Rudich, Y. Origin-Dependent Variations in the Atmospheric Microbiome Community in Eastern Mediterranean Dust Storms. Environ. Sci. Technol. 2017, 51, 6709–6718. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Wang, T.; Wang, J.; Li, L.; Wang, X.; Liu, L.; Wen, T. Adaptive Mechanisms of Bacillus to near Space Extreme Environments. Sci. Total Environ. 2023, 886, 163952. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.M.; Umesh, M.; Priyanka, K.; Preethi, K. Isolation of Plant Growth-Promoting Bacillus Cereus from Soil and Its Use as a Microbial Inoculant. Arab. J. Sci. Eng. 2021, 46, 151–161. [Google Scholar] [CrossRef]
- Kulkova, I.; Dobrzyński, J.; Kowalczyk, P.; Bełżecki, G.; Kramkowski, K. Plant Growth Promotion Using Bacillus Cereus. Int. J. Mol. Sci. 2023, 24, 9759. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D.; Basak, B.B.; Meena, R.S. Potassium Uptake by Crops as Well as Microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 267–280. ISBN 9788132227762. [Google Scholar]
- Jaiswal, D.K.; Verma, J.P.; Prakash, S.; Meena, V.S.; Meena, R.S. Potassium as an Important Plant Nutrient in Sustainable Agriculture: A State of the Art. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 21–29. ISBN 9788132227762. [Google Scholar]
- Jha, Y.; Subramanian, R.B. Regulation of Plant Physiology and Antioxidant Enzymes for Alleviating Salinity Stress by Potassium-Mobilizing Bacteria. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 149–162. ISBN 9788132227762. [Google Scholar]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of Biofertilizer Containing N-Fixer, P and K Solubilizers and AM Fungi on Maize Growth: A Greenhouse Trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Aguirre-Monroy, A.M.; Santana-Martínez, J.C.; Dussán, J. Lysinibacillus Sphaericus as a Nutrient Enhancer during Fire-Impacted Soil Replantation. Appl. Environ. Soil Sci. 2019, 2019, 3075153. [Google Scholar] [CrossRef]
- Kumar Sahu, P.; Shivaprakash, M.K.; Karaba, N.; Mallesha, B.C.; Subbarayappa, C.T.; Brahmaprakash, G.P. Effect of Bacterial Endophytes Lysinibacillus Sp. on Plant Growth and Fruit Yield of Tomato (Solanum Lycopersicum). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3399–3408. [Google Scholar] [CrossRef]
- Martínez, S.A.; Dussán, J. Lysinibacillus Sphaericus Plant Growth Promoter Bacteria and Lead Phytoremediation Enhancer with Canavalia Ensiformis. Environ. Prog. Sustain. Energy 2018, 37, 276–282. [Google Scholar] [CrossRef]
- Sodiq, A.H.; Setiawati, M.R.; Santosa, D.A.; Widayat, D. The Effect of Bio-Fertilizer Applications Bacillus Cereus and Lysinibacillus Sp On Paprika Plants (Capsicum annuum L.) On Plant Nutrient Content and Cultivation Media. IOP Conf. Ser. Earth Environ. Sci. 2022, 978, 012032. [Google Scholar] [CrossRef]
- Gibbons, S.M.; Gilbert, J.A. Microbial Diversity--Exploration of Natural Ecosystems and Microbiomes. Curr. Opin. Genet. Dev. 2015, 35, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Bertola, M.; Mattarozzi, M.; Sanangelantoni, A.M.; Careri, M.; Visioli, G. PGPB Colonizing Three-Year Biochar-Amended Soil: Towards Biochar-Mediated Biofertilization. J. Soil Sci. Plant Nutr. 2019, 19, 841–850. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Dhawi, F.; Hess, A. Poor-Soil Rhizosphere Enriched with Different Microbial Activities Influence the Availability of Base Elements. Open J. Ecol. 2017, 7, 495–502. [Google Scholar] [CrossRef]
- Caniato, M.M.; de Catarino, A.M.; Sousa, T.F.; da Silva, G.F.; Bichara, K.P.d.A.; da Cruz, J.C.; de Assis, L.A.G.; Hanada, R.E. Diversity of Bacterial Strains in Biochar-Enhanced Amazon Soil and Their Potential for Growth Promotion and Biological Disease Control in Tomato. Acta Amazon. 2020, 50, 278–288. [Google Scholar] [CrossRef]
- Rafique, M.; Sultan, T.; Ortas, I.; Chaudhary, H.J. Enhancement of Maize Plant Growth with Inoculation of Phosphate-Solubilizing Bacteria and Biochar Amendment in Soil. Soil Sci. Plant Nutr. 2017, 63, 460–469. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Y. The Effect of Salt-Dissolving Growth-Promoting Rhizobacteria on the Growth of Pepper. Front. Sustain. Dev. 2023, 3, 62–67. [Google Scholar] [CrossRef]
- Hilário, S.; Gonçalves, M.F.M.; Matos, I.; Rangel, L.F.; Sousa, J.A.; Santos, M.J.; Ayra-Pardo, C. Comparative Genomics Reveals Insights into the Potential of Lysinibacillus Irui as a Plant Growth Promoter. Appl. Microbiol. Biotechnol. 2024, 108, 370. [Google Scholar] [CrossRef]
- Ahsan, N.; Shimizu, M. Lysinibacillus Species: Their Potential as Effective Bioremediation, Biostimulant, and Biocontrol Agents. Rev. Agric. Sci. 2021, 9, 103–116. [Google Scholar] [CrossRef]
- Majeed, R.E. Isolation and Diagnosis of Lysinibacillus Fusiformis Obtained from Soil and Its Use as Biofertilizer in Wheat Crop. SABRAO J. Breed. Genet. 2024, 56, 1207–1218. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, J.; Singh, K. Bioremediation of Malathion in Soil by Mixed Bacillus Culture. Adv. Biosci. Biotechnol. 2013, 4, 674–678. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, J.; Singh, K. Biodegradation of Malathion by Brevibacillus Sp. Strain KB2 and Bacillus Cereus Strain PU. World J. Microbiol. Biotechnol. 2012, 28, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, J.; Singh, K. Transformation of Malathion by Lysinibacillus Sp. Isolated from Soil. Biotechnol. Lett. 2012, 34, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Madriz-Ordeñana, K.; Pazarlar, S.; Jørgensen, H.J.L.; Nielsen, T.K.; Zhang, Y.; Nielsen, K.L.; Hansen, L.H.; Thordal-Christensen, H. The Bacillus Cereus Strain EC9 Primes the Plant Immune System for Superior Biocontrol of Fusarium Oxysporum. Plants 2022, 11, 687. [Google Scholar] [CrossRef]
- Mishra, A.K.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef]
- Mayer, E.; Dörr de Quadros, P.; Fulthorpe, R. Plantibacter Flavus, Curtobacterium Herbarum, Paenibacillus Taichungensis, and Rhizobium Selenitireducens Endophytes Provide Host-Specific Growth Promotion of Arabidopsis Thaliana, Basil, Lettuce, and Bok Choy Plants. Appl. Environ. Microbiol. 2019, 85, e00383-19. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, H.; Gao, J.; Portieles, R.; Du, L.; Gao, X.; Borroto Nordelo, C.; Borrás-Hidalgo, O. Endophytic Bacillus Altitudinis Strain Uses Different Novelty Molecular Pathways to Enhance Plant Growth. Front. Microbiol. 2021, 12, 692313. [Google Scholar] [CrossRef]
- Heydarian, Z.; Yu, M.; Gruber, M.; Glick, B.R.; Zhou, R.; Hegedus, D.D. Inoculation of Soil with Plant Growth Promoting Bacteria Producing 1-Aminocyclopropane-1-Carboxylate Deaminase or Expression of the Corresponding acdS Gene in Transgenic Plants Increases Salinity Tolerance in Camelina Sativa. Front. Microbiol. 2016, 7, 1966. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernandez, A.G.; Glick, B.R.; Rossi, M.J. The Extreme Plant-Growth-Promoting Properties of Pantoea Phytobeneficialis MSR2 Revealed by Functional and Genomic Analysis. Environ. Microbiol. 2020, 22, 1341–1355. [Google Scholar] [CrossRef]
- Leite, M.; Pereira, A.; Souza, A.; Andrade, P.; Barbosa, M.; Andreote, F.; Freire, F.; Sobral, J. Potentially Diazotrophic Endophytic Bacteria Associated to Sugarcane Are Effective in Plant Growth-Promotion. J. Exp. Agric. Int. 2018, 21, 1–15. [Google Scholar] [CrossRef]
- Thomas, J.; Kim, H.R.; Rahmatallah, Y.; Wiggins, G.; Yang, Q.; Singh, R.; Glazko, G.; Mukherjee, A. RNA-Seq Reveals Differentially Expressed Genes in Rice (Oryza sativa) Roots during Interactions with Plant-Growth Promoting Bacteria, Azospirillum Brasilense. PLoS ONE 2019, 14, e0217309. [Google Scholar] [CrossRef] [PubMed]
- Wessler, S.; Schneider, G.; Backert, S. Bacterial Serine Protease HtrA as a Promising New Target for Antimicrobial Therapy? Cell Commun. Signal. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Bolken, T.C.; Jones, K.F.; Zeller, G.O.; Hruby, D.E. Conserved DegP Protease in Gram-Positive Bacteria Is Essential for Thermal and Oxidative Tolerance and Full Virulence in Streptococcus Pyogenes. Infect. Immun. 2001, 69, 5538–5545. [Google Scholar] [CrossRef]
- Chao, Y.; Bergenfelz, C.; Sun, R.; Han, X.; Achour, A.; Hakansson, A.P. The Serine Protease HtrA Plays a Key Role in Heat-Induced Dispersal of Pneumococcal Biofilms. Sci. Rep. 2020, 10, 22455. [Google Scholar] [CrossRef]
- Ren, H.; Liu, J. AsnB Is Involved in Natural Resistance of Mycobacterium Smegmatis to Multiple Drugs. Antimicrob. Agents Chemother. 2006, 50, 250–255. [Google Scholar] [CrossRef]
- Qian, G.; Liu, C.; Wu, G.; Yin, F.; Zhao, Y.; Zhou, Y.; Zhang, Y.; Song, Z.; Fan, J.; Hu, B.; et al. AsnB, Regulated by Diffusible Signal Factor and Global Regulator Clp, Is Involved in Aspartate Metabolism, Resistance to Oxidative Stress and Virulence in Xanthomonas Oryzae Pv. Oryzicola. Mol. Plant Pathol. 2013, 14, 145–157. [Google Scholar] [CrossRef]
- Rogers, E.W.; Dalisay, D.S.; Molinski, T.F. (+)-Zwittermicin A: Assignment of Its Complete Configuration by Total Synthesis of the Enantiomer and Implication of D-Serine in Its Biosynthesis. Angew. Chem. Int. Ed Engl. 2008, 47, 8086–8089. [Google Scholar] [CrossRef]
- Broderick, N.A.; Goodman, R.M.; Handelsman, J.; Raffa, K.F. Effect of Host Diet and Insect Source on Synergy of Gypsy Moth (Lepidoptera: Lymantriidae) Mortality to Bacillus Thuringiensis Subsp. Kurstaki by Zwittermicin A. Environ. Entomol. 2003, 32, 387–391. [Google Scholar] [CrossRef]
- Yin, Q.-J.; Ying, T.-T.; Zhou, Z.-Y.; Hu, G.-A.; Yang, C.-L.; Hua, Y.; Wang, H.; Wei, B. Species-Specificity of the Secondary Biosynthetic Potential in Bacillus. Front. Microbiol. 2023, 14, 1271418. [Google Scholar] [CrossRef]
- Jähne, J.; Le Thi, T.T.; Blumenscheit, C.; Schneider, A.; Pham, T.L.; Le Thi, P.T.; Blom, J.; Vater, J.; Schweder, T.; Lasch, P.; et al. Novel Plant-Associated Brevibacillus and Lysinibacillus Genomospecies Harbor a Rich Biosynthetic Potential of Antimicrobial Compounds. Microorganisms 2023, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus Velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.D.A.; Bach, E.; da Silva Moreira, F.; Müller, A.R.; Rangel, C.P.; Wilhelm, C.M.; Barth, A.L.; Passaglia, L.M.P. Antifungal Potential against Sclerotinia Sclerotiorum (Lib.) de Bary and Plant Growth Promoting Abilities of Bacillus Isolates from Canola (Brassica napus L.) Roots. Microbiol. Res. 2021, 248, 126754. [Google Scholar] [CrossRef]
- van Santen, J.A.; Poynton, E.F.; Iskakova, D.; McMann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; McCadden, C.A.; et al. The Natural Products Atlas 2.0: A Database of Microbially-Derived Natural Products. Nucleic Acids Res. 2022, 50, D1317–D1323. [Google Scholar] [CrossRef]
- Devi, S.; Sharma, S.; Tiwari, A.; Bhatt, A.K.; Singh, N.K.; Singh, M.; Kaushalendra; Kumar, A. Screening for Multifarious Plant Growth Promoting and Biocontrol Attributes in Bacillus Strains Isolated from Indo Gangetic Soil for Enhancing Growth of Rice Crops. Microorganisms 2023, 11, 1085. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of Plant Growth Promotion Properties and Induction of Antioxidative Defense Mechanism by Tea Rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Shabanamol, S.; Divya, K.; George, T.K.; Rishad, K.S.; Sreekumar, T.S.; Jisha, M.S. Characterization and in Planta Nitrogen Fixation of Plant Growth Promoting Endophytic Diazotrophic Lysinibacillus Sphaericus Isolated from Rice (Oryza sativa). Physiol. Mol. Plant Pathol. 2018, 102, 46–54. [Google Scholar] [CrossRef]
- Ali, Q.; Ayaz, M.; Mu, G.; Hussain, A.; Yuanyuan, Q.; Yu, C.; Xu, Y.; Manghwar, H.; Gu, Q.; Wu, H.; et al. Revealing Plant Growth-Promoting Mechanisms of Bacillus Strains in Elevating Rice Growth and Its Interaction with Salt Stress. Front. Plant Sci. 2022, 13, 994902. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-A.; Song, J.; Choe, S.; Jang, G.; Kim, Y. Plant Growth-Promoting Rhizobacterium Bacillus Megaterium Modulates the Expression of Antioxidant-Related and Drought-Responsive Genes to Protect Rice (Oryza sativa L.) from Drought. Front. Microbiol. 2024, 15, 1430546. [Google Scholar] [CrossRef]
- Logan, N.A. Bacillus and Relatives in Foodborne Illness. J. Appl. Microbiol. 2012, 112, 417–429. [Google Scholar] [CrossRef]
- Adeleke, B.; Ayangbenro, A.; Babalola, O. Genomic Analysis of Endophytic Bacillus Cereus T4S and Its Plant Growth-Promoting Traits. Plants 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and Pathogenesis of Bacillus Cereus Infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Märtlbauer, E.; Dietrich, R.; Luo, H.; Ding, S.; Zhu, K. Multifaceted Toxin Profile, an Approach toward a Better Understanding of probiotic Bacillus Cereus. Crit. Rev. Toxicol. 2019, 49, 342–356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).