Detoxification of Ustiloxin A by Hydroxylation of Endophytic Fungus Petriella setifera Nitaf10
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Endophytic Fungus Petriella setifera Nitaf10
2.3. Preparation of Buffer Solution
2.4. Preparation of the Cell-Free Extract of P. setifera
2.5. Incubation of Ustiloxin A in the Cell-Free Extract of P. setifera
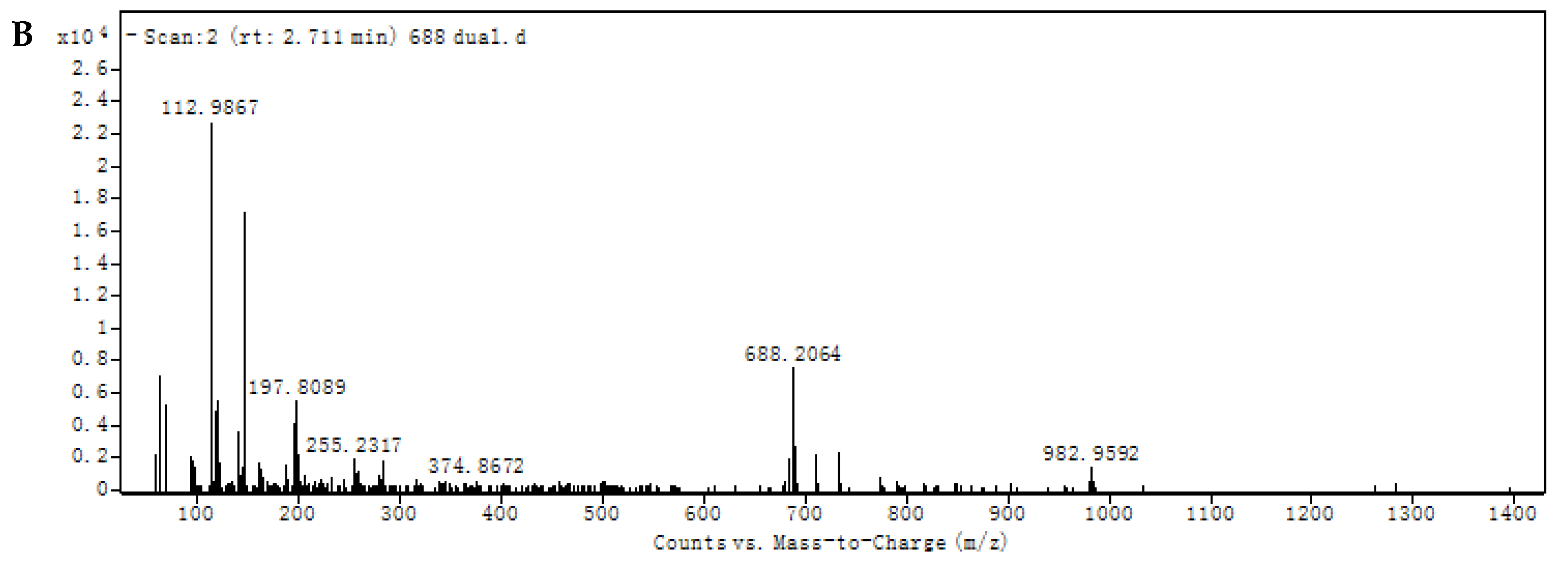
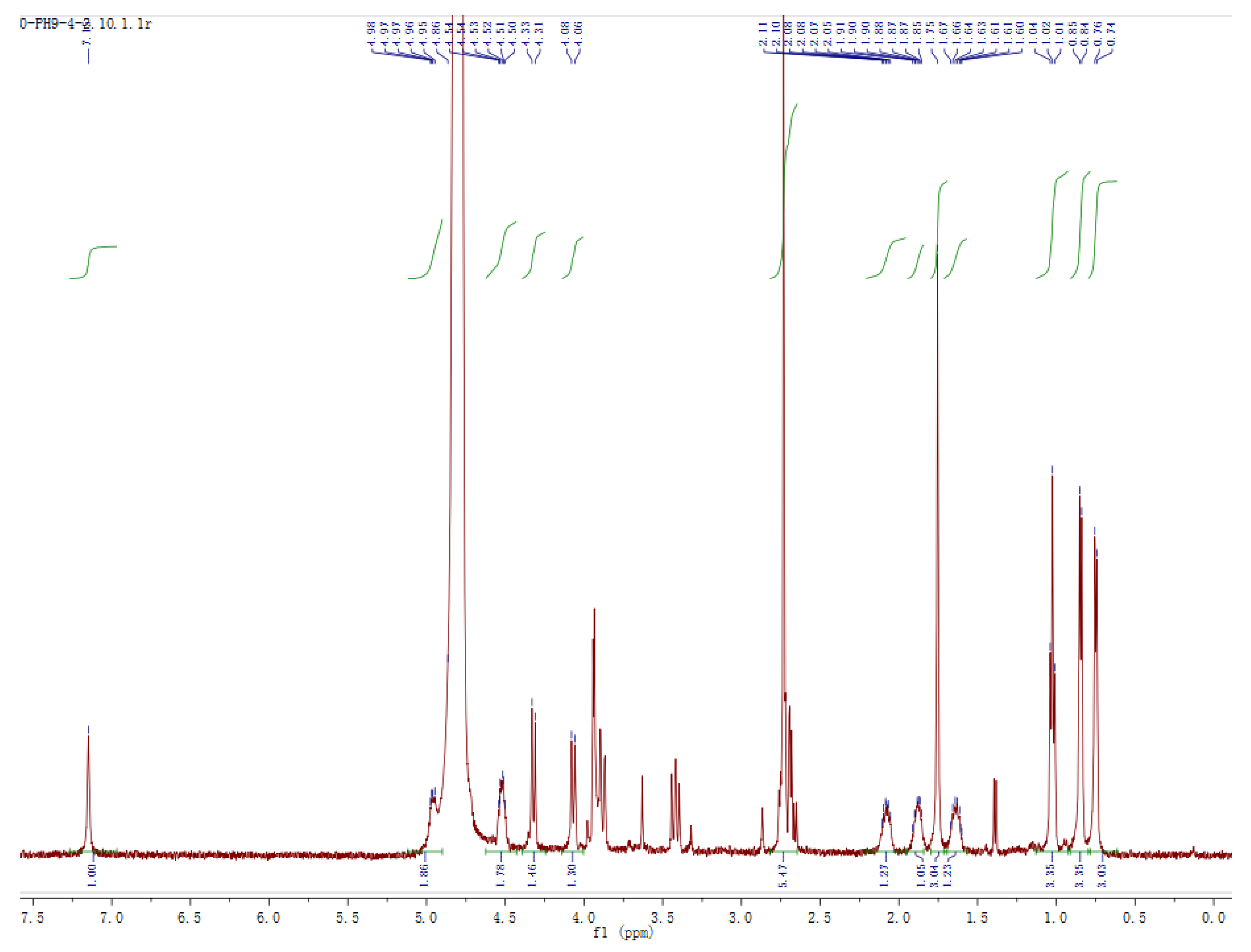
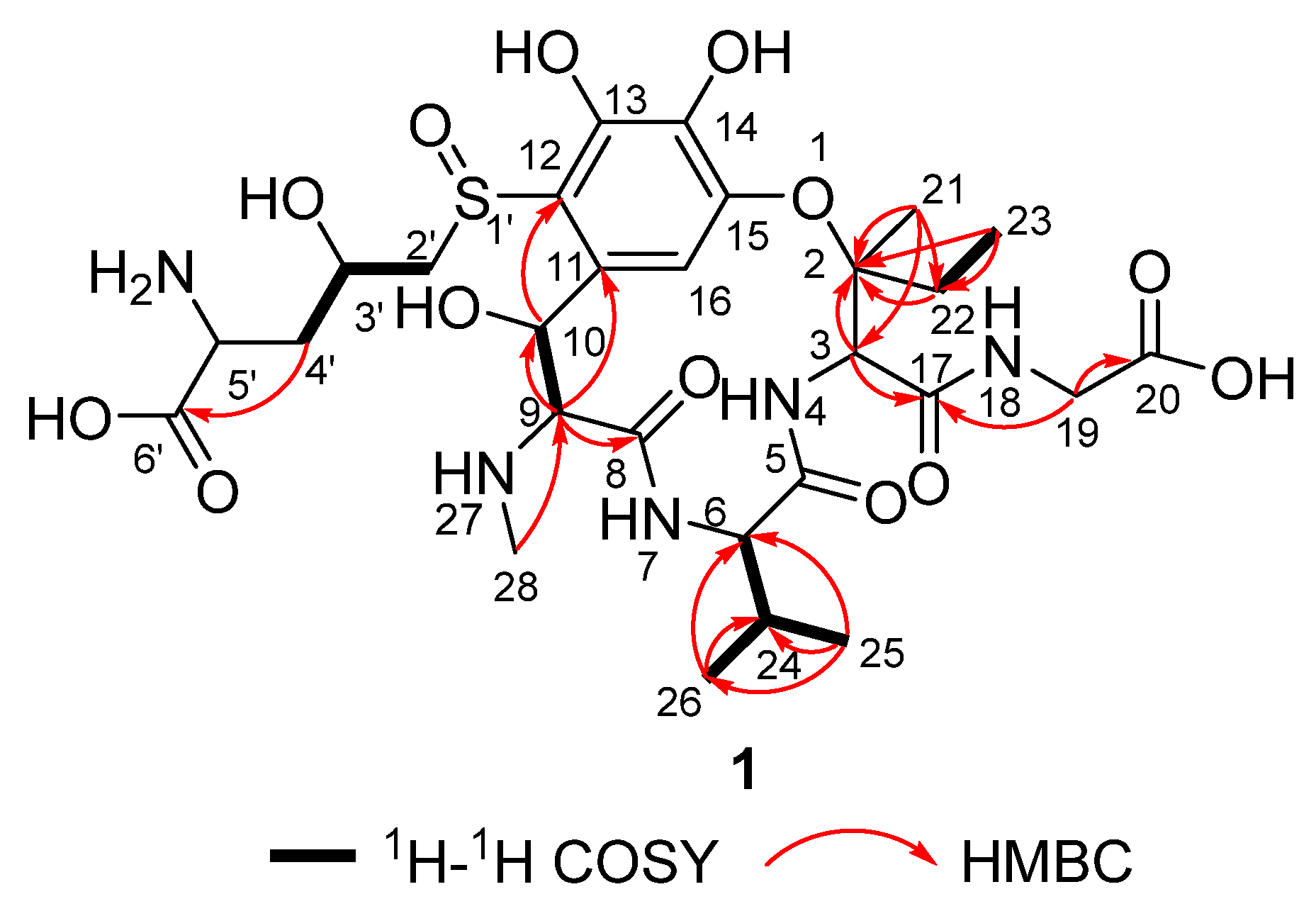
2.6. Isolation and Structural Identification of the Transformed Product 1 of Ustiloxin A
2.7. Cytotoxic Activity Assay
2.8. Phytotoxic Activity Assay
3. Results
3.1. HPLC Analysis of the Transformed Products of Ustiloxin A
3.2. Structural Identification of Transformed Product 1
3.3. Cytotoxic and Phytotoxic Activities of Ustiloxin A and 13-Hydroxy Ustiloxin A (1)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, W.; Fan, J.; Fang, A.; Li, Y.; Tariqjaveed, M.; Li, D.; Hu, D.; Wang, W.-M. Ustilaginoidea virens: Insights into an emerging rice pathogen. Annu. Rev. Phytopathol. 2020, 58, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, J.; Wang, Y.; Li, G.; Li, Y.; Huang, F.; Wang, W. Current understanding on Villosiclava virens, a unique flower-infecting fungus causing rice false smut disease. Mol. Plant Pathol. 2016, 17, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Koiso, Y.; Li, Y.; Iwasaki, S.; Hanaoka, K.; Kobayashi, T.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994, 47, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Lai, D.; Wang, W.; Dai, J.; Zhou, L.; Liu, Y. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins 2017, 9, 54. [Google Scholar] [CrossRef]
- Lin, X.; Bian, Y.; Mou, R.; Cao, Z.; Cao, Z.; Zhu, Z.; Chen, M. Isolation, identification, and characterization of Ustilaginoidea virens from rice false smut balls with high ustilotoxin production potential. J. Basic Microbiol. 2018, 58, 670–678. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.; Lin, F.; Sun, W.; Meng, J.; Wang, A.; Lai, D.; Zhou, L.; Liu, Y. The contents of ustiloxins A and B along with their distribution in rice false smut balls. Toxins 2016, 8, 262. [Google Scholar] [CrossRef]
- Nakamura, K.-I.; Izumiyama, N.; Ohtsubo, K.-I.; Koiso, Y.; Iwasaki, S.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. “Lupinosis”-like lesions in mice caused by ustiloxin, produced by Ustilaginoidea virens: A morphological study. Nat. Toxins 1994, 2, 22–28. [Google Scholar] [CrossRef]
- Zhang, G.; Li, H.; Liu, S.; Zhou, X.; Lu, M.; Tang, L.; Sun, L. Water extract of rice false smut balls activates Nrf2/HO-1 and apoptosis pathways, causing liver injury. Rice Sci. 2023, 30, 473–485. [Google Scholar]
- Zhang, Y.; Xu, Q.; Sun, Q.; Kong, R.; Liu, H.; Yi, X.; Liang, Z.; Letcher, R.J.; Liu, C. Ustiloxin A inhibits proliferation of renal tubular epithelial cells in vitro and induces renal injury in mice by disrupting structure and respiratory function of mitochondria. J. Hazard. Mater. 2023, 448, 130791. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Zhang, Y.; Kong, R.; Yi, X.; Liu, C. Detection of ustiloxin A in urine by ultra-high-performance liquid chromatography-tandem mass spectrometry coupled with two-step solid-phase extraction. J. Chromatogr. B 2021, 1181, 122916. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Zhang, Y.; Yi, X.; Kong, R.; Cheng, S.; Man, J.; Zheng, L.; Huang, J.; Su, G.; et al. Global distribution of ustiloxins in rice and their male-biased hepatotoxicity. Environ. Pollut. 2022, 301, 118992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, X.; Liu, S.; Ma, Y.; Li, H.; Du, Y.; Cao, Z.; Sun, L. Full-length transcriptomics study of ustiloxins-induced hepatocyte injury. Toxicon 2024, 238, 107604. [Google Scholar] [CrossRef]
- Koiso, Y.; Natori, M.; Iwasaki, S.; Sato, S.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxin: A phytotoxin and a mycotoxin from false smuth balls on rice panicles. Tetrahedron Lett. 1992, 33, 4157–4160. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.T.; Cartwright, R.D.; Sciumbato, G.L. Ustilaginoidea virens infection of rice in Arkansas: Toxicity of false smut galls, their extracts and the ustiloxin fraction. Am. J. Plant Sci. 2014, 5, 3166–3176. [Google Scholar] [CrossRef]
- Fu, R.; Wang, J.; Chen, C.; Gong, X.; Lu, D. Effect of crude toxins of Ustilaginoidea virens on rice seed germination. Afr. J. Microbiol. Res. 2017, 11, 1267–1273. [Google Scholar]
- Li, P.; Gu, G.; Hou, X.; Xu, D.; Dai, J.; Kuang, Y.; Wang, M.; Lai, D.; Zhou, L. Detoxification of ustiloxin A through oxidative deamination and decarboxylation by endophytic fungus Petriella setifera. Toxins 2025, 17, 48. [Google Scholar] [CrossRef]
- Rustamova, N.; Huang, G.; Isokov, M.; Movlanov, J.; Farid, R.; Buston, I.; Xiang, H.; Davranov, K.; Yili, A. Modification of natural compounds through biotransformation process by microorganisms and their pharmacological properties. Fitoterapia 2024, 79, 106227. [Google Scholar] [CrossRef]
- Loi, M.; Francesca, F.; Liuzzi, V.C.; Logrieco, A.F.; Mule, G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef]
- Adegoke, T.V.; Yang, B.; Xing, F.; Tian, X.; Wang, G.; Tai, B.; Si, P.; Hussain, S.; Jahn, I. Microbial enzymes involved in the biotransformation of major mycotoxins. J. Agric. Food Chem. 2023, 71, 35–51. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Peng, Y.; Yu, R.; Yue, Y.; Lai, D.; Zhou, L. Endophytic fungi from Nicotiana tabacum L. and their antibacterial activity. Nat. Prod. Res. Dev. 2015, 27, 1847–1852. [Google Scholar]
- Freitas-Silva, O.; Venancio, A. Ozone applications to prevent and degrade mycotoxin: A review. Drug Metab. Rev. 2010, 42, 612–620. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P. Microbial detoxification of mycotoxins. J. Chem. Ecol. 2013, 39, 907–918. [Google Scholar] [CrossRef]
- Hathout, A.S.; Aly, S.E. Biological detoxification of mycotoxins: A review. Ann. Microbiol. 2014, 64, 905–919. [Google Scholar] [CrossRef]
- Diao, E.; Li, X.; Zhang, Z.; Ma, W.; Ji, N.; Dong, H. Ultraviolet irradiation detoxification of aflatoxins: A review. Trends Food Sci. Technol. 2015, 42, 64–69. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef]
- Wang, N.; Wu, W.; Pan, J.; Long, M. Detoxification strategies for zearalenone using microorganism: A review. Microorgansims 2019, 7, 208. [Google Scholar] [CrossRef]
- Dos Santos, V.H.P.; Neto, D.M.C.; Lacerda, V.; Borges, W.D.; Silva, E.D. Fungal biotransformation: An efficient approach for stereoselective chemical reactions. Curr. Org. Chem. 2020, 24, 2902–2953. [Google Scholar] [CrossRef]
- Birolli, W.G.; Lima, R.N.; Porto, A.L.M. Applications of marine-derived microorganisms and their Enzymes in biocatalysis and biotransformation, the underexplored potentials. Front. Microbiol. 2019, 10, 1453. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Hildebrand, A.; Damm, G.; Rapp, A.; Cramer, B.; Uumpf, H.-U.; Metzler, M. Aromatic hydroxylation is a major metabolic pathway of the mycotoxin zearalenone in vitro. Mol. Nutr. Food Res. 2009, 53, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic secondary metabolites from fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Crutcher, F.K.; Puckhaber, L.S.; Bell, A.A.; Liu, J.; Duke, S.E.; Stipanovic, R.D.; Nichols, R.L. Detoxification of fusaric acid by the soil microbe Mucor rouxii. J. Agric. Food Chem. 2017, 65, 4989–4992. [Google Scholar] [CrossRef] [PubMed]



| Position | Compound (δH, Mult., J in Hz) | |
|---|---|---|
| Ustiloxin A a | 13-Hydroxy Ustiloxin A (1) | |
| 2 | ||
| 3 | 4.83 s | 4.82 s |
| 5 | ||
| 6 | 4.13 d (10.0) | 4.08 d (10.0) |
| 8 | ||
| 9 | 4.28 d (10.0) | 4.33 d (10.0) |
| 10 | 4.96 d (10.0) | 4.96 d (10.0) |
| 11 | ||
| 12 | ||
| 13 | 7.61 s | |
| 14 | ||
| 15 | ||
| 16 | 7.11 s | 7.16 s |
| 17 | ||
| 19 | 3.79 s | 3.95 s |
| 20 | ||
| 21 | 1.77 s | 1.76 s |
| 22 | 2.24 dd (14.2, 7.2); 1.73 dd (14.2, 7.2) | 2.08 dd (14.4, 7.2); 1.65 dd (14.4, 7.2) |
| 23 | 1.09 dd (7.2, 7.2) | 1.03 t (7.2) |
| 24 | 1.92 dd (10.0, 7.0) | 1.89 m |
| 25 | 0.80 d (7.0) | 0.75 d (6.6) |
| 26 | 0.88 d (7.0) | 0.85 d (6.6) |
| 28 | 2.77 s | 2.74 s |
| 2′ | 3.33 dd (13.6, 10.0); 3.04 dd (13.6, 3.0) | 3.86–3.96 overlapped; 3.43 dd (14.0, 10.0) |
| 3′ | 4.39 m | 4.52 m |
| 4′ | 2.22 ddd (15.0, 10.0, 8.0); 2.12 ddd (15.0, 8.0, 3.0) | 2.72 m |
| 5′ | 4.01 dd (8.0, 8.0) | 3.86–3.96 overlapped |
| 6′ | ||
| Compound | IC50 (μmol/L) | ||||
|---|---|---|---|---|---|
| HCT-8 | PANC-1 | HGC-27 | HepG2 | PC9 | |
| Ustiloxin A | 2.81 | 3.59 | 3.62 | 11.94 | 1.85 |
| 13-Hydroxy ustiloxin A | 12.08 | 5.36 | 18.42 | 20.71 | 5.62 |
| Taxol (CK+) | 4.18 × 10−3 | 1.53 × 10−3 | 1.76 × 10−3 | 4.50 × 10−3 | 2.51 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Hou, X.; Gu, G.; Lai, D.; Zhou, L. Detoxification of Ustiloxin A by Hydroxylation of Endophytic Fungus Petriella setifera Nitaf10. Microbiol. Res. 2025, 16, 93. https://doi.org/10.3390/microbiolres16050093
Li P, Hou X, Gu G, Lai D, Zhou L. Detoxification of Ustiloxin A by Hydroxylation of Endophytic Fungus Petriella setifera Nitaf10. Microbiology Research. 2025; 16(5):93. https://doi.org/10.3390/microbiolres16050093
Chicago/Turabian StyleLi, Peng, Xuwen Hou, Gan Gu, Daowan Lai, and Ligang Zhou. 2025. "Detoxification of Ustiloxin A by Hydroxylation of Endophytic Fungus Petriella setifera Nitaf10" Microbiology Research 16, no. 5: 93. https://doi.org/10.3390/microbiolres16050093
APA StyleLi, P., Hou, X., Gu, G., Lai, D., & Zhou, L. (2025). Detoxification of Ustiloxin A by Hydroxylation of Endophytic Fungus Petriella setifera Nitaf10. Microbiology Research, 16(5), 93. https://doi.org/10.3390/microbiolres16050093







