Gut Bacteria-Based Cancer Therapy and Anti-Solid Tumor Mechanisms
Abstract
1. Introduction
2. Gut Bacteria Used in Cancer Therapy
3. Anticancer Mechanism of Gut Bacteria
3.1. Modulation of the Tumor Microenvironment
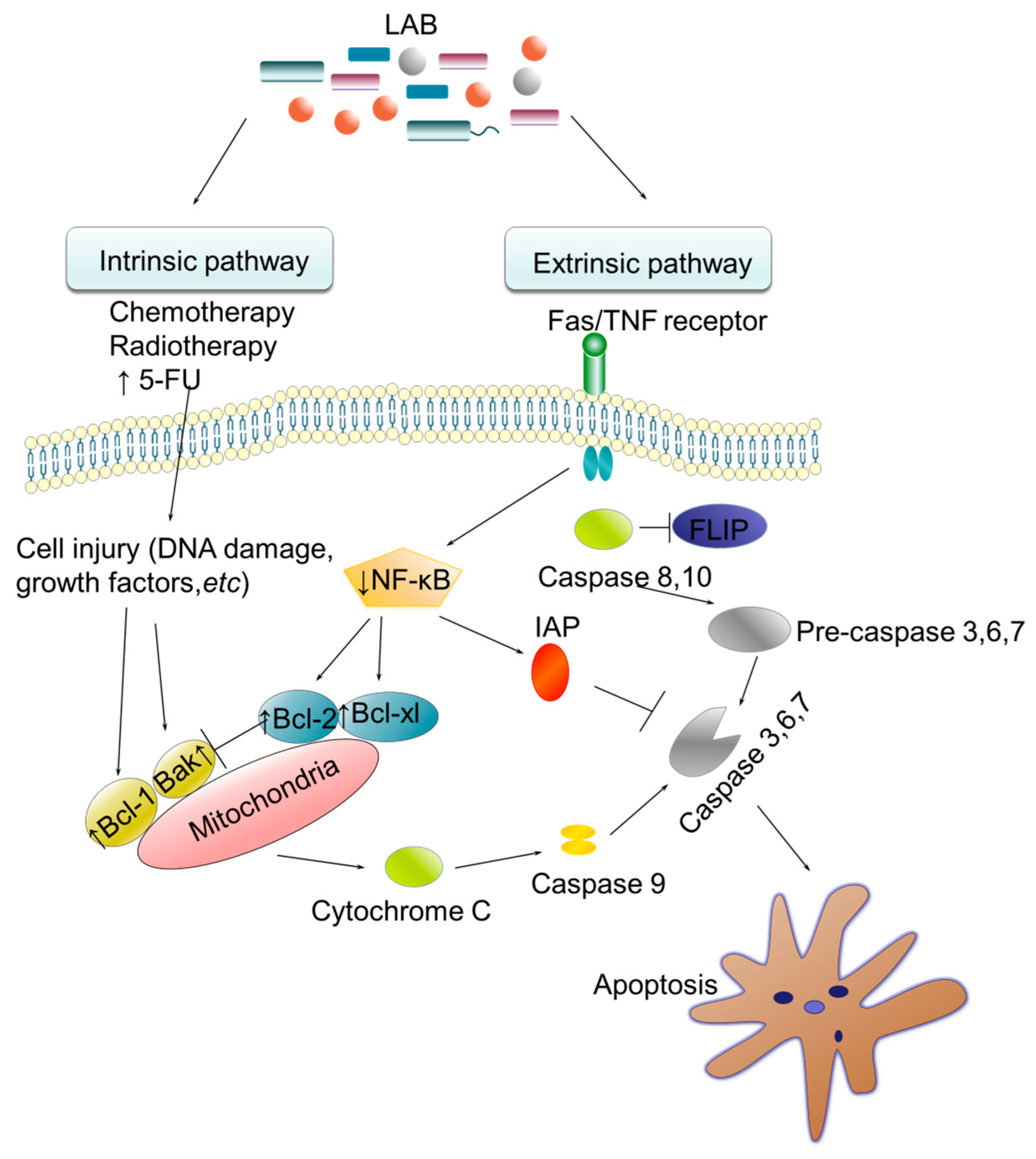
3.1.1. TME Modulation by Lactobacillus
3.1.2. TME Modulation by Clostridium
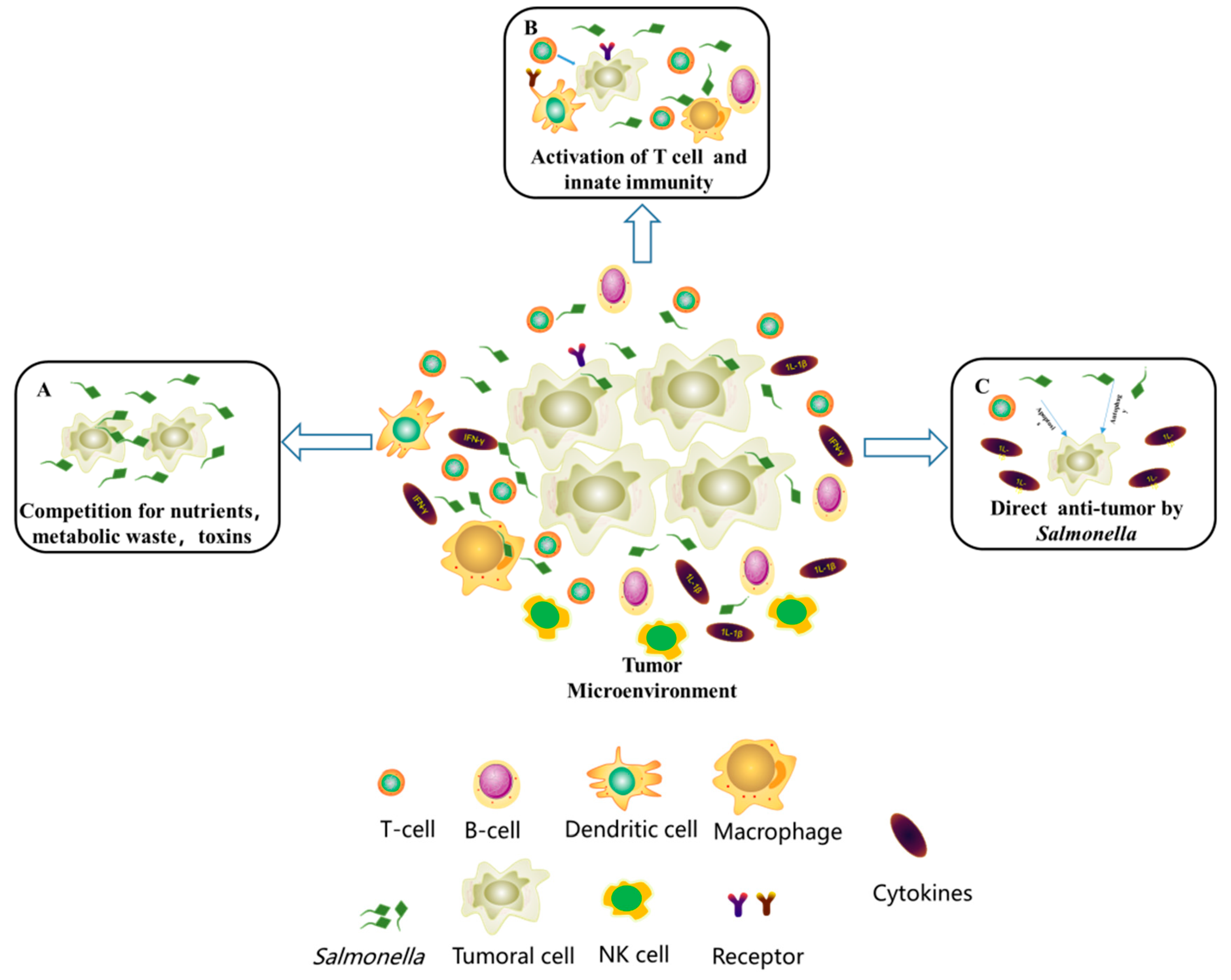
3.2. Competitive Inhibition
Competitive Inhibition by Salmonella
3.3. Activation of Immune Cells
3.3.1. Activation of Immune Cells by Listeria
3.3.2. Activation of Immune Cells by the Gut Microbiome
3.4. Vectors for Gene Therapy
3.4.1. Escherichia as a Vector for Gene Therapy
3.4.2. Bifidobacterium as a Vector for Gene Therapy
3.5. Microbial Anticancer Biomolecules
Production of Anticancer Biomolecules by Bacillus
4. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Feng, Z.-C.; Li, C.; Yang, X.; Ma, M.-T.; Rong, P.-F. Salmonella-mediated cancer therapy: An innovative therapeutic strategy. J. Cancer 2019, 10, 4765. [Google Scholar] [CrossRef] [PubMed]
- Gurbatri, C.R.; Arpaia, N.; Danino, T. Engineering bacteria as interactive cancer therapies. Science 2022, 378, 858–864. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Pontone, M.; Toma, L.; Ensoli, F. Biofilm producing Salmonella typhi: Chronic colonization and development of gallbladder cancer. Int. J. Mol. Sci. 2017, 18, 1887. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.; Wang, Z.; Zhou, J.; Tian, X.; Li, N. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J. Clin. Pathol. 2004, 57, 1273–1277. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.-Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Kenneth, M.J.; Tsai, H.-C.; Fang, C.-Y.; Hussain, B.; Chiu, Y.-C.; Hsu, B.-M. Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer. J. Adv. Res. 2023, 52, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef]
- Curran, C.S.; Rasooly, A.; He, M.; Prickril, B.; Thurin, M.; Sharon, E. Report on the NCI microbial-based cancer therapy conference. Cancer Immunol. Res. 2018, 6, 122–126. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Gan, R.-Y.; Zhao, C.-N.; Meng, X.; Li, H.-B. Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 60, 1025–1037. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef]
- Shui, L.; Yang, X.; Li, J.; Yi, C.; Sun, Q.; Zhu, H. Gut microbiome as a potential factor for modulating resistance to cancer immunotherapy. Front. Immunol. 2020, 10, 2989. [Google Scholar] [CrossRef]
- Bradley, K.C.; Finsterbusch, K.; Schnepf, D.; Crotta, S.; Llorian, M.; Davidson, S.; Fuchs, S.Y.; Staeheli, P.; Wack, A. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019, 28, 245–256.e244. [Google Scholar] [CrossRef]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the gut microbiome in obesity and diabetes mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef]
- Ghorbani, E.; Avan, A.; Ryzhikov, M.; Ferns, G.; Khazaei, M.; Soleimanpour, S. Role of Lactobacillus strains in the management of colorectal cancer: An overview of recent advances. Nutrition 2022, 103, 111828. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, A.; Ghazvini, K.; Khazaei, M.; Hasanian, S.M.; Avan, A.; Soleimanpour, S. The use of Clostridium in cancer therapy: A promising way. Rev. Res. Med. Microbiol. 2022, 33, 121–127. [Google Scholar] [CrossRef]
- Al-Saafeen, B.H.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Integration of Salmonella into combination cancer therapy. Cancers 2021, 13, 3228. [Google Scholar] [CrossRef]
- Selvanesan, B.C.; Chandra, D.; Quispe-Tintaya, W.; Jahangir, A.; Patel, A.; Meena, K.; Alves Da Silva, R.A.; Friedman, M.; Gabor, L.; Khouri, O. Listeria delivers tetanus toxoid protein to pancreatic tumors and induces cancer cell death in mice. Sci. Transl. Med. 2022, 14, eabc1600. [Google Scholar] [CrossRef]
- Osek, J.; Wieczorek, K. Listeria monocytogenes—How this pathogen uses its virulence mechanisms to infect the hosts. Pathogens 2022, 11, 1491. [Google Scholar] [CrossRef]
- Thakur, B.K.; Malaise, Y.; Choudhury, S.R.; Neustaeter, A.; Turpin, W.; Streutker, C.; Copeland, J.; Wong, E.O.; Navarre, W.W.; Guttman, D.S. Dietary fibre counters the oncogenic potential of colibactin-producing Escherichia coli in colorectal cancer. Nat. Microbiol. 2025, 10, 855–870. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut microbiota modulation in the context of immune-related aspects of Lactobacillus spp. and Bifidobacterium spp. in gastrointestinal cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- Oscarsson, J.; Bao, K.; Shiratsuchi, A.; Grossmann, J.; Wolski, W.; Aung, K.M.; Lindholm, M.; Johansson, A.; Mowsumi, F.R.; Wai, S.N. Bacterial symbionts in oral niche use type VI secretion nanomachinery for fitness increase against pathobionts. iScience 2024, 27, 109650. [Google Scholar] [CrossRef]
- Maroufi, N.F.; Rashidi, M.R.; Vahedian, V.; Akbarzadeh, M.; Fattahi, A.; Nouri, M. Therapeutic potentials of Apatinib in cancer treatment: Possible mechanisms and clinical relevance. Life Sci. 2020, 241, 117106. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E. Tumor acidity as evolutionary spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Shon, Y.; Kim, J.; Oh, Y.-K. Selective activation of anticancer chemotherapy by cancer-associated fibroblasts in the tumor microenvironment. J. Natl. Cancer Inst. 2017, 109, djw186. [Google Scholar] [CrossRef]
- Yang, X.; Da, M.; Zhang, W.; Qi, Q.; Zhang, C.; Han, S. Role of Lactobacillus in cervical cancer. Cancer Manag. Res. 2018, 10, 1219–1229. [Google Scholar] [CrossRef]
- Heydari, Z.; Rahaie, M.; Alizadeh, A.M.; Agah, S.; Khalighfard, S.; Bahmani, S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the expression of microRNAs 135b, 26b, 18a and 155, and their involving genes in mice colon cancer. Probiotics Antimicrob. Proteins 2019, 11, 1155–1162. [Google Scholar] [CrossRef]
- Yasuda, S.; Horinaka, M.; Sakai, T. Sulforaphane enhances apoptosis induced by Lactobacillus pentosus strain S-PT84 via the TNFα pathway in human colon cancer cells. Oncol. Lett. 2019, 18, 4253–4261. [Google Scholar] [CrossRef]
- Jacouton, E.; Chain, F.; Sokol, H.; Langella, P.; Bermudez-Humaran, L.G. Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front. Immunol. 2017, 8, 1553. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef]
- Mowday, A.M.; Guise, C.P.; Ackerley, D.F.; Minton, N.P.; Lambin, P.; Dubois, L.J.; Theys, J.; Smaill, J.B.; Patterson, A.V. Advancing clostridia to clinical trial: Past lessons and recent progress. Cancers 2016, 8, 63. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef]
- Möse, J.R.; Möse, G. Oncolysis by clostridia. I. Activity of Clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma. Cancer Res. 1964, 24, 212–216. [Google Scholar]
- Mohr, U.; Boldingh, W.H.; Emminger, A.; Behagel, H. Oncolysis by a new strain of Clostridium. Cancer Res. 1972, 32, 1122–1128. [Google Scholar] [PubMed]
- Agrawal, N.; Bettegowda, C.; Cheong, I.; Geschwind, J.-F.; Drake, C.G.; Hipkiss, E.L.; Tatsumi, M.; Dang, L.H.; Diaz, L.A., Jr.; Pomper, M. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl. Acad. Sci. USA 2004, 101, 15172–15177. [Google Scholar] [CrossRef] [PubMed]
- Theys, J.; Landuyt, W.; Nuyts, S.; Van Mellaert, L.; Van Oosterom, A.; Lambin, P.; Anné, J. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther. 2001, 8, 294–297. [Google Scholar] [CrossRef]
- Patyar, S.; Joshi, R.; Byrav, D.P.; Prakash, A.; Medhi, B.; Das, B. Bacteria in cancer therapy: A novel experimental strategy. J. Biomed. Sci. 2010, 17, 21. [Google Scholar] [CrossRef]
- Jiang, S.-N.; Park, S.-H.; Lee, H.J.; Zheng, J.H.; Kim, H.-S.; Bom, H.-S.; Hong, Y.; Szardenings, M.; Shin, M.G.; Kim, S.-C. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol. Ther. 2013, 21, 1985–1995. [Google Scholar] [CrossRef]
- Sznol, M.; Lin, S.L.; Bermudes, D.; Zheng, L.-M.; King, I. Use of preferentially replicating bacteria for the treatment of cancer. J. Clin. Investig. 2000, 105, 1027–1030. [Google Scholar] [CrossRef]
- Hoffman, R.M. Tumor-targeting Salmonella typhimurium A1-R: An overview. Bact. Ther. Cancer Methods Protoc. 2016, 1409, 1–8. [Google Scholar]
- Barak, Y.; Schreiber, F.; Thorne, S.H.; Contag, C.H.; DeBeer, D.; Matin, A. Role of nitric oxide in Salmonella typhimurium-mediated cancer cell killing. BMC Cancer 2010, 10, 146. [Google Scholar] [CrossRef]
- Grille, S.; Moreno, M.; Bascuas, T.; Marqués, J.M.; Muñoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella enterica serovar T yphimurium immunotherapy for B-cell lymphoma induces broad anti-tumour immunity with therapeutic effect. Immunology 2014, 143, 428–437. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, C.-L.; Shiau, A.-L. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin. Cancer Res. 2008, 14, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Martinoli, C.; Petrovska, L.; Chiodoni, C.; Transidico, P.; Bronte, V.; Longhi, R.; Colombo, M.P.; Dougan, G.; Rescigno, M. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005, 65, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- Hackl, H.; Charoentong, P.; Finotello, F.; Trajanoski, Z. Computational genomics tools for dissecting tumour–immune cell interactions. Nat. Rev. Genet. 2016, 17, 441–458. [Google Scholar] [CrossRef]
- Blake, S.J.; Wolf, Y.; Boursi, B.; Lynn, D.J. Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. 2024, 24, 308–325. [Google Scholar] [CrossRef]
- Tangney, M.; Gahan, C.G. Listeria monocytogenes as a vector for anti-cancer therapies. Curr. Gene Ther. 2010, 10, 46–55. [Google Scholar] [CrossRef]
- Van Pijkeren, J.P.; Morrissey, D.; Monk, I.R.; Cronin, M.; Rajendran, S.; O’Sullivan, G.C.; Gahan, C.G.; Tangney, M. A novel Listeria monocytogenes-based DNA delivery system for cancer gene therapy. Hum. Gene Ther. 2010, 21, 405–416. [Google Scholar] [CrossRef]
- Pillich, H.; Puri, M.; Chakraborty, T. ActA of Listeria monocytogenes and its manifold activities as an important listerial virulence factor. Actin Cytoskelet. Bact. Infect. 2017, 399, 113–132. [Google Scholar]
- O’Riordan, M.; Yi, C.H.; Gonzales, R.; Lee, K.-D.; Portnoy, D.A. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 13861–13866. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.; Vicente, D.; Hoffman, K.; Wei, S.C. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.A.; Ramirez, T.; Russell, N.C.; Moye, L.A. Coley toxins immunotherapy: A retrospective review. Altern. Ther. Health Med. 1999, 5, 42. [Google Scholar]
- Ermak, G. Emerging Medical Technologies; World Scientific Publishing Company: Chennai, India, 2015. [Google Scholar]
- Kaji, E.H.; Leiden, J.M. Gene and stem cell therapies. JAMA 2001, 285, 545–550. [Google Scholar] [CrossRef]
- Xie, X.; Guo, J.; Kong, Y.; Xie, G.X.; Li, L.; Lv, N.; Xiao, X.; Tang, J.; Wang, X.; Liu, P. Targeted expression of Escherichia coli purine nucleoside phosphorylase and Fludara® for prostate cancer therapy. J. Gene Med. 2011, 13, 680–691. [Google Scholar] [CrossRef]
- Watanabe, M.; Nasu, Y.; Kashiwakura, Y.; Kusumi, N.; Tamayose, K.; Nagai, A.; Sasano, T.; Shimada, T.; Daida, H.; Kumon, H. Adeno-associated virus 2-mediated intratumoral prostate cancer gene therapy: Long-term maspin expression efficiently suppresses tumor growth. Hum. Gene Ther. 2005, 16, 699–710. [Google Scholar] [CrossRef]
- Onion, D.; Patel, P.; Pineda, R.G.; James, N.; Mautner, V. Antivector and tumor immune responses following adenovirus-directed enzyme prodrug therapy for the treatment of prostate cancer. Hum. Gene Ther. 2009, 20, 1249–1258. [Google Scholar] [CrossRef]
- Tai, C.-K.; Wang, W.; Lai, Y.-H.; Logg, C.R.; Parker, W.B.; Li, Y.-F.; Hong, J.S.; Sorscher, E.J.; Chen, T.C.; Kasahara, N. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 2010, 17, 614–623. [Google Scholar] [CrossRef]
- Swain, A.L.; Jaskólski, M.; Housset, D.; Rao, J.; Wlodawer, A. Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Proc. Natl. Acad. Sci. USA 1993, 90, 1474–1478. [Google Scholar] [CrossRef]
- Lee, J.-H.; O’Sullivan, D.J. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, G.-F.; Fan, Y.-R.; Liu, W.-H.; Liu, X.-J.; Wang, J.-J.; Xu, G.-X. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: Selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003, 10, 105–111. [Google Scholar] [CrossRef]
- Fu, G.-F.; Li, X.; Hou, Y.-Y.; Fan, Y.-R.; Liu, W.-H.; Xu, G.-X. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther. 2005, 12, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.T.; Taniguchi, S.; Aoki, K.; Baba, T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980, 40, 2061–2068. [Google Scholar]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef]
- Wei, H.; Chen, L.; Lian, G.; Yang, J.; Li, F.; Zou, Y.; Lu, F.; Yin, Y. Antitumor mechanisms of bifidobacteria. Oncol. Lett. 2018, 16, 3–8. [Google Scholar]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. WJG 2014, 20, 7878. [Google Scholar] [CrossRef]
- Jouda, J.-B.; Mbazoa, C.D.; Sarkar, P.; Bag, P.K.; Wandji, J. Anticancer and antibacterial secondary metabolites from the endophytic fungus Penicillium sp. CAM64 against multi-drug resistant Gram-negative bacteria. Afr. Health Sci. 2016, 16, 734–743. [Google Scholar] [CrossRef]
- Kumavath, R.N. Novel antimicrobial and anticancer drugs from bacteria. In Antimicrobials; CRC Press: Boca Raton, FL, USA, 2015; pp. 160–171. [Google Scholar]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Tamanna, U.; Asaduzzaman, M.; Nargis, S.; Mozammel, M. Bacillus spp.: Attractive sources of anti-cancer and anti-proliferative biomolecules. Microb. Bioact. 2018, 1, 33–45. [Google Scholar]
- Chen, Y.-T.; Yuan, Q.; Shan, L.-T.; Lin, M.-A.; Cheng, D.-Q.; Li, C.-Y. Antitumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp. isolated from Ophiopogon japonicus. Oncol. Lett. 2013, 5, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Kumar, M.; Thippeswamy, B.; Vasanth Raj, P. Cytotoxicity and anticancer studies of Bacillus cereus and Bacillus pumilus metabolites targeting human cancer cells. Appl. Biochem. Microbiol. 2014, 50, 619–623. [Google Scholar] [CrossRef]
- Seerangaraj, V.; Suruli, K.; Vijayakumar, U.; Meganathan, B.; Seerangara, V.; Selvam, S.; Rajendran, V.; Selvaraj, J. Isolation and characterization of bioactive compounds for Bacillus cereus and Bacillus subtilis from Oreochromis mossambicus and Labeo rohita. Int. J. Pharm. Sci. Rev. Res 2017, 43, 71–77. [Google Scholar]
- Aboul-Ela, H.M.; Shreadah, M.A.; Abdel-Monem, N.M.; Yakout, G.A.; van Soest, R.W. Isolation, cytotoxic activity and phylogenetic analysis of Bacillus sp. bacteria associated with the red sea sponge Amphimedon ochracea. Adv. Biosci. Biotechnol. 2012, 3, 815–823. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Han, Z.; Lai, P.Y.; Guo, X.; Zhang, X.; Lin, W.; Qian, P.-Y. Five new amicoumacins isolated from a marine-derived bacterium Bacillus subtilis. Mar. Drugs 2012, 10, 319–328. [Google Scholar] [CrossRef]
- Ramasubburayan, R.; Sumathi, S.; Bercy, D.M.; Immanuel, G.; Palavesam, A. Antimicrobial, antioxidant and anticancer activities of mangrove associated bacterium Bacillus subtilis subsp. subtilis RG. Biocatal. Agric. Biotechnol. 2015, 4, 158–165. [Google Scholar] [CrossRef]
- El-Sersy, N.A.; Abdelwahab, A.E.; Abouelkhiir, S.S.; Abou-Zeid, D.M.; Sabry, S.A. Antibacterial and anticancer activity of ε-poly-L-lysine (ε-PL) produced by a marine Bacillus subtilis sp. J. Basic Microbiol. 2012, 52, 513–522. [Google Scholar] [CrossRef]
- Kameda, Y.; Matsui, K.; Kato, H.; Yamada, T.; Sagai, H. Antitumor activity of Bacillus natto. III. Isolation and characterization of a cytolytic substance on Ehrlich ascites carcinoma cells in the culture medium of Bacillus natto KMD 1126. Chem. Pharm. Bull. 1972, 20, 1551–1557. [Google Scholar] [CrossRef]
- Kameda, Y.; Ouhira, S.; Matsui, K.; Kanatomo, S.; Hase, T.; Atsusaka, T. Antitumor activity of Bacillus natto. V. Isolation and characterization of surfactin in the culture medium of Bacillus natto KMD 2311. Chem. Pharm. Bull. 1974, 22, 938–944. [Google Scholar] [CrossRef]
- Dahech, I.; Belghith, K.S.; Belghith, H.; Mejdoub, H. Partial purification of a Bacillus licheniformis levansucrase producing levan with antitumor activity. Int. J. Biol. Macromol. 2012, 51, 329–335. [Google Scholar] [CrossRef]
- Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Shin, H.J. Ieodoglucomides A and B from a marine-derived bacterium Bacillus licheniformis. Org. Lett. 2012, 14, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.V.; Kumar, V.; Rajendran, V.; Saran, S.; Ghosh, P.C.; Saxena, R.K. Purification and characterization of a novel and robust L-asparaginase having low-glutaminase activity from Bacillus licheniformis: In vitro evaluation of anti-cancerous properties. PLoS ONE 2014, 9, e99037. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasser, S.M.; Yahya, S.M.; Mohamed, W.F.; Asker, M.M.; Shady, H.M.A.; Mahmoud, M.G.; Gadallah, M.A. Antitumor exopolysaccharides derived from novel marine bacillus: Isolation, characterization aspect and biological activity. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 1847. [Google Scholar]
- Chatterjee, P.; Kouzi, S.A.; Pezzuto, J.M.; Hamann, M.T. Biotransformation of the antimelanoma agent betulinic acid by Bacillus megaterium ATCC 13368. Appl. Environ. Microbiol. 2000, 66, 3850–3855. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 2011, 1, 5. [Google Scholar] [CrossRef]
- Parthiban, K.; Vignesh, V.; Thirumurugan, R. Characterization and in vitro studies on anticancer activity of exopolymer of Bacillus thuringiensis S13. Afr. J. Biotechnol. 2014, 13, 2137–2144. [Google Scholar]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317–322. [Google Scholar] [CrossRef]
- Pettit, G.R.; Arce, P.M.; Chapuis, J.-C.; Macdonald, C.B. Antineoplastic agents. 600. from the south pacific ocean to the silstatins. J. Nat. Prod. 2015, 78, 510–523. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, S.Y.; Kim, Y.H.; Kim, M.; Lee, S.J. Cytotoxicity and apoptosis induction of Bacillus vallismortis BIT-33 metabolites on colon cancer carcinoma cells. J. Appl. Microbiol. 2008, 104, 796–807. [Google Scholar] [CrossRef]
- Ma, E.L.; Choi, Y.J.; Choi, J.; Pothoulakis, C.; Rhee, S.H.; Im, E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer 2010, 127, 780–790. [Google Scholar] [CrossRef]
- Kumar, S.N.; Nambisan, B.; Kumar, B.D.; Vasudevan, N.G.; Mohandas, C.; Cheriyan, V.T.; Anto, R.J. Antioxidant and anticancer activity of 3, 5-dihydroxy-4-isopropylstilbene produced by Bacillus sp. N strain isolated from entomopathogenic nematode. Arch. Pharmacal Res. 2013, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Hua, H.M.; Pei, Y.H.; Yao, X.S. Three new cytotoxic cyclic acylpeptides from marine Bacillus sp. Chem. Pharm. Bull. 2004, 52, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Trischman, J.A.; Jensen, P.R.; Fenical, W. Halobacillin: A cytotoxic cyclic acylpeptide of the iturin class produced by a marine Bacillus. Tetrahedron Lett. 1994, 35, 5571–5574. [Google Scholar] [CrossRef]
- Alrumman, S.; Mostafa, Y.; Al-Izran, K.A.; Alfaifi, M.; Taha, T.; Elbehairi, S. Production and anticancer activity of an L-asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci. Rep. 2019, 9, 3756. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Nam, S.H.; Seo, W.T.; Yun, H.D.; Hong, S.Y.; Kim, M.K.; Cho, K.M. The production of surfactin during the fermentation of cheonggukjang by potential probiotic Bacillus subtilis CSY191 and the resultant growth suppression of MCF-7 human breast cancer cells. Food Chem. 2012, 131, 1347–1354. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Shakambari, G.; Birendranarayan, A.K.; Lincy, M.J.A.; Rai, S.K.; Ahamed, Q.T.; Ashokkumar, B.; Saravanan, M.; Mahesh, A.; Varalakshmi, P. Hemocompatible glutaminase free L-asparaginase from marine Bacillus tequilensis PV9W with anticancer potential modulating p53 expression. RSC Adv. 2016, 6, 25943–25951. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Nadumane, V.K. Overexpression of p53 and Bax mediating apoptosis in cancer cell lines induced by a bioactive compound from Bacillus endophyticus JUPR15. Process Biochem. 2018, 73, 170–179. [Google Scholar] [CrossRef]
- Prokhorova, I.V.; Akulich, K.A.; Makeeva, D.S.; Osterman, I.A.; Skvortsov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Yusupova, G.; Yusupov, M.M.; Dmitriev, S.E. Amicoumacin A induces cancer cell death by targeting the eukaryotic ribosome. Sci. Rep. 2016, 6, 27720. [Google Scholar] [CrossRef]
- Arnold, L., Jr.; Dagan, A.; Gutheil, J.; Kaplan, N. Antineoplastic activity of poly (L-lysine) with some ascites tumor cells. Proc. Natl. Acad. Sci. USA 1979, 76, 3246–3250. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Ngai, S.-C.; Goh, B.-H.; Chan, K.-G.; Lee, L.-H.; Chuah, L.-H. Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front. Pharmacol. 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.A.; Fortes, Z.B.; da Cunha, M.A.; Sarilmiser, H.K.; Dekker, A.M.B.; Öner, E.T.; Dekker, R.F.; Khaper, N. Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int. J. Biol. Macromol. 2017, 102, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Khan, A.A.; Khurshid, M.; Kalam, M.A.; Jain, S.K.; Singhal, P.K. Recent developments in L-asparaginase discovery and its potential as anticancer agent. Crit. Rev. Oncol. Hematol. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Pierpoint, L. Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial agent. Med. Res. Rev. 2012, 32, 1292–1327. [Google Scholar] [CrossRef]
- Katayama, H.; Kusaka, Y.; Yokota, H.; Akao, T.; Kojima, M.; Nakamura, O.; Mekada, E.; Mizuki, E. Parasporin-1, a novel cytotoxic protein from Bacillus thuringiensis, induces Ca2+ influx and a sustained elevation of the cytoplasmic Ca2+ concentration in toxin-sensitive cells. J. Biol. Chem. 2007, 282, 7742–7752. [Google Scholar] [CrossRef]
- Abe, Y.; Inoue, H.; Ashida, H.; Maeda, Y.; Kinoshita, T.; Kitada, S. Glycan region of GPI anchored-protein is required for cytocidal oligomerization of an anticancer parasporin-2, Cry46Aa1 protein, from Bacillus thuringiensis strain A1547. J. Invertebr. Pathol. 2017, 142, 71–81. [Google Scholar] [CrossRef]
- Ito, A.; Sasaguri, Y.; Kitada, S.; Kusaka, Y.; Kuwano, K.; Masutomi, K.; Mizuki, E.; Akao, T.; Ohba, M. A Bacillus thuringiensis crystal protein with selective cytocidal action to human cells. J. Biol. Chem. 2004, 279, 21282–21286. [Google Scholar] [CrossRef]
- Yamashita, S.; Katayama, H.; Saitoh, H.; Akao, T.; Park, Y.S.; Mizuki, E.; Ohba, M.; Ito, A. Typical three-domain Cry proteins of Bacillus thuringiensis strain A1462 exhibit cytocidal activity on limited human cancer cells. J. Biochem. 2005, 138, 663–672. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Okamura, S.; Saitou, H.; Akao, T.; Mizuki, E. Three Cry toxins in two types from Bacillus thuringiensis strain M019 preferentially kill human hepatocyte cancer and uterus cervix cancer cells. Biosci. Biotechnol. Biochem. 2010, 74, 494–498. [Google Scholar] [CrossRef]
- Makarov, A.A.; Kolchinsky, A.; Ilinskaya, O.N. Binase and other microbial RNases as potential anticancer agents. BioEssays 2008, 30, 781–790. [Google Scholar] [CrossRef]
- Hajare, S.N.; Subramanian, M.; Gautam, S.; Sharma, A. Induction of apoptosis in human cancer cells by a Bacillus lipopeptide bacillomycin D. Biochimie 2013, 95, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, X.; Lei, S.; Shao, D.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Sun, H. Iturin A-like lipopeptides from Bacillus subtilis trigger apoptosis, paraptosis, and autophagy in Caco-2 cells. J. Cell. Physiol. 2019, 234, 6414–6427. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Aslam, M.; Saffarzadeh, M.; Kolpakov, A.; Zelenikhin, P.; Preissner, K.T.; Ilinskaya, O.N. Internalization of Bacillus intermedius ribonuclease (BINASE) induces human alveolar adenocarcinoma cell death. Toxicon 2013, 69, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fuge, O.; Vasdev, N.; Allchorne, P.; Green, J.S. Immunotherapy for bladder cancer. Res. Rep. Urol. 2015, 7, 65–79. [Google Scholar]



| Species | Cell Lines | Dosage, IC50 (μg/mL) | Bioactive Agents [82] | References |
|---|---|---|---|---|
| Bacillus Amyloliquefaciens (MD-bl) | i. MC-4 ii. SGC-7901 | i. 19,600 ii. 26,800 | Exopolysaccharide | [83] |
| Bacillus cereus | i. HepG2 ii. Hep2 | i. 225.4 ii. 152.2 | ND | [84] |
| Bacillus cereus SVSK2 | i. MCF7 ii. HeLa | i. 150 ii. 300 | Silicic acid, diethyl bis (trimethylsilyl) ester | [85] |
| Bacillus subtilis FS05 | i. HepG2 ii. HCT iii. MCF | i. 10.42 ii. 4.3 iii. 75.5 | ND | [86] |
| Bacillus subtilis SVSK5 | i. MCF7 ii. HeLa | i. 150 ii. 300 | Eicosane, Pentacosane, Phthalic Acid | [85] |
| Bacillus subtilis B1779 | HeLa | i. 33.60 μM ii. 4.32 μM | i. Amicoumacin A ii. Bacilosarcin B | [87] |
| Bacillus subtilis subsp. subtilis RG | MCF-7 | 46.64 | ND | [88] |
| Bacillus subtilis SDNS | i. HelaS3 ii. HepG2 | i. 77.2% ii. 56.2% | ε-Poly-L-lysine | [89] |
| B. subtilis var. natto KMD 1126 | EAC | 10% | Surfactin | [90] |
| B. subtilis var. KMD 2311 | EAC | 20% | Surfactin | [91] |
| Bacillus licheniformis | HepG2 | 200 mg/mL | Levan | [92] |
| Bacillus licheniformis 09IDYM23 | i. NCI-H23 ii. NUGC3 | i. 25.18 ii. 17.78 | Ieodoglucomide B | [93] |
| Bacillus licheniformis RAM-8 | i. Jurkat clone E6-1 ii. MCF-7 iii. K-562 | i. 0.22 IU ii. 0.78 IU iii. 0.153 IU | L-asparaginase | [94] |
| Bacillus megaterium SAmt17 | HepG2 | 218 | EPS | [95] |
| Bacillus megaterium ATCC 13368 | Mel-2 | 0.1–0.3 | Betulinic acid metabolites | [96] |
| Bacillus flexus | HepG2 | 372 | Exopolysaccharide | [95] |
| Bacillus sp. BS3 | Mammary epithelial carcinoma | 0.25 | Biosurfactant | [97] |
| Bacillus safensis PDRV | i. HepG2 ii. HCT iii. MCF | i. 46.9 ii. 28.6 iii. 721.3 | ND | [86] |
| Bacillus thuringiensis S13 | A549 | 133.27 | Exopolymer | [98] |
| Bacillus mojavensis B0621A | HL-60 | 100 100 1.6 mM | Andanteiso-C17 fengycin B Mojavensin A iso-C16 fengycin B | [99] |
| Bacillus silvestris | i. BXPC-3 ii. MCF-7 iii. SF-268 iv. NCI-H460 v. KM20L2 | 10−4–10−5 | Bacillistatins 1 and 2 | [100] |
| Bacillus vallismortis BIT-33 | i. HT-29, ii. SW480 iii. HCT116 | 10 | PCC | [101] |
| Bacillus polyfermenticus | i. HT-29 ii. DLD-1 iii. Caco-2 | i. 56% ii. 33% iii. 95% | ND | [102] |
| Bacillus sp. N | HeLa | 25 | 3,5-Dihydroxy-4-isopropylstilbene | [103] |
| Marine Bacillus sp. | HCT-116 | 0.68, 1.6, 1.3 mg/mL | Mixirins A, B and C | [104] |
| Marine Bacillus sp. CND-914 | HCT-116 | 0.98 | Halobacillin | [105] |
| Marine Bacillus sp. BF1-3 | i. HepG2 ii. HCT iii. MCF-7 | i. 13.2 ii. 9.3 iii. 12.2 | ND | [86] |
| Bacillus licheniformis KKU-KH14 | i. HepG-2 ii. MCF-7 iii. HCT-116 | i. 11.66 ii. 14.55 iii. 17.02 | L-asparaginase | [106] |
| Bacillus subtilis CSY191 | MCF-7 | 10 | Surfactin | [107] |
| Bacillus thuringiensis RSK CAS4 | i. HEp-2 ii. A549 iii. Vero cell lines | i. 480 ii. 115 iii. 320 | EPS | [108] |
| Bacillus tequilensis PV9W | HeLa | 0.036 ± 0.009 IU | L-asparaginase | [109] |
| Bacillus endophyticus JUPR15 | i. HeLa ii. HepG2 iii. MCF-7 | i. 13.21 ii. 6.53 iii. 8.21 | ND | [110] |
| Bioactive Agents | Mode of Action | References |
|---|---|---|
| Exopolysaccharide | Causes morphological abnormalities and mitochondrial dysfunction in tumor cells leading to apoptosis | [83] |
| Amicoumacin A | Inhibits mRNA translation | [111] |
| ε-Poly-L-lysine | Causes morphological changes and growth inhibition | [112] |
| Surfactin | Inhibits tumor growth, cell cycle arrest, apoptosis, and metastasis arrest | [113] |
| Levan | Increases oxidative stress and apoptosis | [114] |
| L-asparaginase | Causes nutritional deficiencies and inhibits protein synthesis resulting in apoptosis | [115] |
| Ieodoglucomide B | Inhibits tumor cell growth | [93] |
| Bacillistatins 1 and 2 | Inhibits tumor cell growth | [100] |
| Mixirins A, B, and C | Inhibits tumor cell growth | [116] |
| Parasporin 1 | Activates apoptotic signaling pathway by binding with Beclin 1 receptor and increasing Ca2+ influx | [117] |
| Parasporin 2 | Permeabilizes the plasma membrane through GPI-anchored protein | [118] |
| Parasporin 3 | Causes pore formation | [119] |
| Parasporin 4 | Induces cholesterol-independent pore formation | [120] |
| Parasporin 6 | Causes swelling of cells and vacuole formation | [121] |
| RNase | Catalyzes RNA degradation and inhibits protein synthesis | [122] |
| Bacillomycin D | Increases apoptosis | [123] |
| Iturin A-like lipopeptide | Upregulates apoptotic genes bax and bad expression and downregulates antiapoptotic gene bcl-2 expression | [124] |
| Binase | Increases cellular permeability for macromolecules and induces apoptosis | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yu, X.-M.; Yang, S.-T.; Zhou, W.-W. Gut Bacteria-Based Cancer Therapy and Anti-Solid Tumor Mechanisms. Microbiol. Res. 2025, 16, 92. https://doi.org/10.3390/microbiolres16050092
Zhang T, Yu X-M, Yang S-T, Zhou W-W. Gut Bacteria-Based Cancer Therapy and Anti-Solid Tumor Mechanisms. Microbiology Research. 2025; 16(5):92. https://doi.org/10.3390/microbiolres16050092
Chicago/Turabian StyleZhang, Tianzhu, Xiao-Mei Yu, Shang-Tian Yang, and Wen-Wen Zhou. 2025. "Gut Bacteria-Based Cancer Therapy and Anti-Solid Tumor Mechanisms" Microbiology Research 16, no. 5: 92. https://doi.org/10.3390/microbiolres16050092
APA StyleZhang, T., Yu, X.-M., Yang, S.-T., & Zhou, W.-W. (2025). Gut Bacteria-Based Cancer Therapy and Anti-Solid Tumor Mechanisms. Microbiology Research, 16(5), 92. https://doi.org/10.3390/microbiolres16050092








