Abstract
Rhizosphere microorganisms effectively exploit nutrient resources within the rhizosphere, while growth-promoting bacteria in this environment play a vital role in regulating soil fertility and enhancing plant health. In this study, we utilized a comprehensive approach that included the isolation, purification, and identification of dominant microorganisms, alongside high-throughput sequencing technology. This methodology was employed to analyze the primary microbial groups and their diversity within the rhizosphere soil of Helichrysum arenarium (L.) Moench in Altay, Xinjiang, China. By isolating bacterial strains from the rhizosphere soil using a dilution coating method, we successfully obtained 43 distinct strains. Subsequently, selective media were employed to screen for growth-promoting characteristics among these isolated strains derived from the rhizosphere soil of H. arenarium (L.) Moench. The results, obtained through high-throughput amplification sequencing, revealed diverse bacterial communities belonging to 35 phyla, 93 orders, 215 families, 324 genera, and 231 species associated with H. arenarium (L.) Moench, as well as fungal communities comprising 14 phyla, 47 orders, 96 families, 204 genera, and 571 species present in the rhizosphere soil. Among these identified communities, Actinobacteriota emerged as the predominant bacterial phylum while Ascomycetes and Mortieromycetes were recognized as the principal fungal phyla found in the rhizospheric soil of H. arenarium (L.) Moench. Analysis of culturable bacteria’s promotion activity within this rhizospheric environment indicated that three strains—S16, S31, and S29—exhibited the highest solubility index for inorganic phosphorus; additionally, the screened strains S7 and S10 demonstrated nitrogen-fixing capabilities. Furthermore, ten strains exhibiting excellent iron-bearing capacities were identified; notably, strain S16 displayed the highest D/d value indicating, its superior iron-bearing capacity. The growth-promoting bacteria were identified as Kocuria rosea, Priestia megaterium, Bacillus mobilis, Bacillus bataviensis, three variants of Bacillus mycoides, Bacillus paramobilis, Bacillus sonorensis, and Alcaligenes faecalis. This study provides a foundational understanding of how microorganisms in the rhizosphere of H. arenarium (L.) Moench influence soil nutrient release and offers valuable insights into enhancing yield and quality cultivation by isolating, screening, and identifying growth-promoting bacteria from rhizosphere soil.
1. Introduction
Helichrysum arenarium (L.) Moench is a perennial herb that belongs to the genus Helichrysum within the Asteraceae family. This species is characterized by its woody and robust rhizome. Its medicinal significance is notable, attributed to the presence of tannins and volatile oils throughout all parts of the plant, which are known to stimulate bile secretion as well as exhibit anti-inflammatory and antioxidant properties. This herb thrives at altitudes ranging from 900 m to 2400 m on earthy hillocks, semi-sandy dunes, slopes of wet saline soils, gravelly terrains, sand dunes, and grasslands, and beneath pine forests [1,2]. The distribution of this species extends across Russia, Europe, Mongolia, and the Xinjiang Province in China. Within Xinjiang Province specifically, it can primarily be found in Altay City, Habahe County, Fuyun County, and Qinghe County. The Altay region is characterized by a continental north-temperate cold climate zone featuring dry and hot summers alongside cold winters. It experiences low precipitation levels coupled with high evaporation rates; there are significant temperature fluctuations between day and night, along with abundant sunlight [3]. The sampling site itself exhibits sandy soil conditions combined with a dry climate marked by considerable diurnal temperature variations and ample sunlight. The primary chemical constituents of H. arenarium (L.) Moench are predominantly flavonoids and polyphenolic compounds, with tannins and volatile oils present throughout the entire herb. Furthermore, the flower sap demonstrates significant effects in promoting bile secretion [4,5]. This plant exhibits a wide array of medicinal properties, including choleretic, hepatoprotective, and antiviral activities. Additionally, its flowers reveal lipid-lowering effects, with identified components such as lcariside F2, icari-side D1, phenethanol-β-vicianoside, benzyl alcohol-β-vicianoside, benzoic acid-β-D-gentiobioside, and phenethanol-β-D-gentiobioside [6,7,8].
The rhizosphere is a multifunctional and dynamic zone in the soil, located approximately 5 mm from the root system, where essential nutrients for plant growth can be sourced from the nutrient sphere [9]. This area serves as a crucial habitat for microorganisms, which play an integral role in the interactions within the plant rhizosphere. Root exudates are produced by various parts of the root system during plant growth. These exudates consist of a range of organic compounds, protons, and inorganic ions that are secreted or released into the surrounding environment [10]. They collectively influence plant growth and development directly, as well as indirectly by affecting the surrounding ecosystem [11]. Research indicates that higher local plant diversity correlates with greater variability in root exudates, which in turn enhances the diversity of soil microbial communities [12]. Plant metabolites play a crucial role in shaping these microbial communities. Studies have demonstrated that root secretions can impact both the activity and species composition of rhizosphere microorganisms [13]; they stimulate microbial growth, enhance their metabolic activity, accelerate nutrient cycling within the rhizosphere, and improve soil nutrient utilization. Through root secretions, plants regulate rhizosphere microflora to promote their own growth effectively [14].
The root system of this plant enhances soil microorganisms through the secretion of various compounds. Rhizosphere microorganisms play a crucial role in regulating plant growth and development by engaging in metabolic activities. Bacteria and fungi are the primary types of rhizosphere microorganisms, with beneficial species promoting plant growth by facilitating soil nutrient cycling, secreting phytohormones, and inhibiting pathogenic bacteria. Conversely, harmful microorganisms can infect plants, leading to stunted growth or even death. Plants depend on the rhizosphere microbial community for their functions by acquiring nutrients from these organisms and under the influence of their activities and composition. The diversity of rhizosphere microbes is essential for maintaining stable soil ecosystems [15,16,17]. Numerous studies have investigated the rhizosphere microbial communities associated with various plants worldwide, resulting in the isolation of functional strains that are widely utilized in biopromotion studies or antagonism research. However, no study has specifically addressed the rhizospheric microorganisms linked to H. arenarium (L.) Moench. This unique plant, found in Xinjiang, possesses significant medicinal value. Moreover, demand for its output is increasing due to a growing market interest in Kozimuke granules—a traditional Kazakh medicine that utilizes it as a raw material. To establish a foundation for the large-scale cultivation of this plant, we conducted screenings for rhizosphere growth-promoting bacteria.
The objective of this study was to investigate the diversity of rhizosphere microorganisms associated with H. arenarium (L.) Moench through high-throughput sequencing techniques. This research lays a foundation for understanding the microbial species present in the rhizosphere, with a particular focus on rhizosphere probiotics and their role in regulating soil fertility and enhancing plant health.
2. Materials and Methods
2.1. Sample Collection
Soil samples were collected from Haba River County, located in the Altay Region of the Xinjiang Uygur Autonomous Region, at an elevation of 737.4 m in late July 2023 (48°18′ N, 86°78′ E) (Figure 1). The samples were obtained utilizing a five-point sampling method. A sample square measuring 20 cm × 20 cm was collected using the five-point sampling method. From the five samples, three exhibiting optimal growth were randomly selected, and soil was collected from the root system at a depth of 0 to 5 mm. A total of 15 samples were collected.

Figure 1.
Sampling site for H. arenarium (L.) Moench soil specimens.
Sample Pre-Treatment
The collected soil samples were meticulously homogenized, passed through a 2 mm sieve, divided into three aliquots, appropriately labeled, and stored at −80 °C and 4 °C in a refrigerator. For the soil collected by the 5-point sampling method, the soil of each point was fully mixed and 3 parallel samples were selected for high-throughput sequencing.
2.2. Cuiture Medium
NA medium: peptone, beef paste powder, NaCl, and Agarose. Inorganic phosphorus culture medium: glucose, (NH4)2SO4, yeast extract powder, NaCl, KCl, MgSO4, FeSO4, MnSO4, GaPO4, and agarose. Mongina medium: glucose, (NH4)2SO4, yeast extract powder, NaCl, KCl, MgSO4, FeSO4, MnSO4, GaCO4, and agarose. Ashby’s Medium: mannitol, CaCl2·2H2O, K2HPO4, MgSO4·7H2O, MoO3, FeCl3, and agar. CAS detection medium: piperazine diethanolsulfonic acid, agarose, peptone, MgSO4·7H2O, CaCl2, and agar. Silicate bacteria medium: agarose, (NH4)2SO4, yeast powder, MgSO4, Na2HPO4, FeSO4, MnSO4, potassium feldspar, and agar.
2.3. Quantification of Soil Nutrient Indicators
The organic matter content was assessed through potassium dichromate oxidation in conjunction with thermal oxidation. The total nitrogen content was determined by utilizing the Kjeldahl method, while hydrolyzable nitrogen content was evaluated using the alkaline diffusion technique. Effective phosphorus content was quantified via colorimetric analysis employing an ultraviolet spectrophotometer, and readily available potassium content was measured using flame photometry [18].
2.4. Analysis of Microbial Amplicons in the Rhizosphere Using High-Throughput Sequencing
The extraction of genomic DNA from soil samples was performed by utilizing the TianGen magnetic bead-based soil genomic DNA extraction kit. This process was followed by an assessment of purity and concentration through 1% agarose gel electrophoresis. Subsequently, PCR amplification was conducted to obtain bacterial 16S rRNA sequences as well as fungal ITS sequences. Sequencing libraries were prepared using the NEB Next® Ultra™ II FS DNA PCR-free Library Prep Kit in accordance with the manufacturer’s guidelines, which included the addition of indexes. Following this, library quantification was carried out using Qubit and real-time PCR methods, while size distribution analysis was performed with a bioanalyzer. After quantification, the libraries were pooled together and subjected to sequencing on the Illumina platform, taking into account both effective library concentration and data requirements.
Mark the species annotation. A distribution histogram of relative abundance was generated in Perl using the SVG function. To visually represent variations in abundance and taxonomic clustering, a heatmap was created in R utilizing the pheatmap function, which incorporated abundance data for the top 35 taxa at each taxonomic rank from every sample. Commonalities and unique characteristics among different samples or groups were illustrated through Venn and flower diagrams, produced in R with the VennDiagram function and in Perl with the SVG function, respectively [19].
2.5. Screening of Rhizosphere Growth-Promoting Bacteria in H. arenarium (L.) Moench
2.5.1. Isolation and Purification of Culturable Bacteria from the Rhizosphere of H. arenarium (L.) Moench
Each rhizosphere soil sample weighed 1 g and was mixed with 9 ml of sterile water using a vortex mixer for a duration of 30 min. The resulting mixture underwent gradient dilutions (10−2, 10−3, 10−4, 10−5, and 10−6) employing the dilution coating method. A suspension of 100 μL was plated onto Nutrient Agar (NA) and a Gao I medium [20], followed by incubation at 28 °C for seven days. Single colonies were subsequently selected from these cultures for purification and enumeration before being stored in a refrigerator maintained at an temperature of four degrees Celsius.
2.5.2. Analysis of Strains’ Phosphorus-Solubility Capacity
The strains obtained were inoculated into both an inorganic phosphorus medium and an organophosphorus medium. Each pure culture strain was introduced into three different media to observe the formation of phosphorus solubilization zones at a temperature of 28 °C over a period of seven days. Strains that exhibited significant phosphate-solubilizing zones were selected based on the measurements taken for both the diameter of the phosphorus solubilization zone (D) and the colony diameter (d). The ratio between these two measurements determined their effectiveness in terms of phosphorus solubilization capacity [21].
2.5.3. Screening of Nitrogen-Fixing Bacteria
The isolated strains were inoculated onto Ashby medium using sterile toothpicks and subsequently incubated at a temperature of 28 °C for seven days. Bacterial colonies that exhibited viscous translucent growth with white, brown, or dark brown pigmentation indicated their nitrogen-fixing capability, which was assessed based on the measurement of their growth diameters [22].
2.5.4. Screening of Strains with Iron Chelating Ability
The isolated strains were streaked onto a CAS detection medium, and each pure culture strain was incubated at 28 °C for 7 days with three replicates. Following this incubation period, the nitrogen fixation zone was examined, and strains that exhibited a pronounced orange chelating ring were selected. The iron-chelating capacity of the strains was assessed based on the diameter of the orange chelate ring [23].
2.5.5. Revealing the Potential of Potassium Solubilizing Bacteria Through Screening
The strains were subsequently inoculated onto a silicate bacterial medium using the dot method and incubated at 28 °C for a duration of 7 days. The yellow halo surrounding each colony was monitored at regular intervals of 24 h. Upon completion of the 7-day incubation period, the potassium solubility of the strain was preliminarily assessed based on the size of the halo [24].
2.6. Identification of Growth-Promoting Bacteria Using Molecular Biology Techniques
The selected growth-promoting bacteria underwent DNA extraction and amplification of their 16S rRNA region, the bacterial universal primers was as shown in Table 1, the PCR system was as shown in Table 2, and with PCR conditions (Table 3). Strain identification and related primer sequences. The resulting amplification products were analyzed through electrophoresis and subsequently sent for sequencing to the Shanghai Shenggong Sequencing Company. Upon the completion of the sequencing process, alignment analysis was conducted on the NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 13 August 2024). The NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 22 March 2025) was utilized to submit the obtained DNA sequences to the GenBank database, where they were compared with known sequences. Sequences exhibiting high homology were selected for further analysis. A phylogenetic tree was constructed using MEGA 4.1 software, and we employed the Complete Deletion mode of the neighbor-joining method to build the tree.

Table 1.
Strain identification and related primer sequences.

Table 2.
PCR reaction system.

Table 3.
PCR reaction conditions.
3. Results
3.1. Nutrients in the Rhizosphere Soil
The soil at the sample site of H. arenarium (L.) Moench was primarily composed of gravel, with a content of approximately 13.14%. The soil water content ranged from 5.12% to 6.21%, while pH levels varied between neutral and slightly acidic, measuring between 6.68 and 7.06. Nutrient elements and organic matter content in the soil were relatively low; alkaline-dissolved nitrogen was measured at around 68.6 mg/kg, organic matter was approximately 24.1 g/kg, effective phosphorus was about 8.3 mg/kg, quick-acting potassium was recorded at 129 mg/kg, and total nitrogen was estimated to be 1.26 g/kg (Table 4). The sandy gravel soil provided optimal growth conditions for H. arenarium (L.) Moench, which demonstrated lower nutrient requirements for nitrogen and phosphorus along with reduced organic matter content due to its well-drained characteristics.

Table 4.
Results of rhizosphere soil testing at sampling sites of H. arenarium (L.) Moench.
3.2. The Assessment of Sequencing Data
After splicing the high-throughput sequencing data obtained from rhizosphere soil samples, quality control measures were implemented, including the removal of chimeras [25,26]. It was observed that over 90% of bases exhibited values greater than or equal to 20 (Q20) and 30 (Q30) (Table 5), while more than 90% of cases surpassed the total number of bases. Furthermore, the GC content ranged between 50 and 60%, indicating that the processed data met the requisite criteria for the subsequent analysis.

Table 5.
Results from the analysis of sequencing samples of H. arenarium (L.) Moench.
3.3. Sequencing Data Accuracy and Assessment of Sequencing Volume
The dilution curve was generated by randomly subsampling sequences from the samples, considering both the number of obtained sequences and the number of operational taxonomic units (OTUs) that can be represented based on the sequencing depth [15,16]. As illustrated in Figure 2, the curve reaches a plateau as sequencing depth increases, indicating that OTU richness, Shannon diversity index, and Simpson evenness index attain saturation at the current sequencing depth. Consequently, this level of sequencing is adequate to capture most bacterial diversity present in the tested samples and can be utilized for subsequent data analysis.
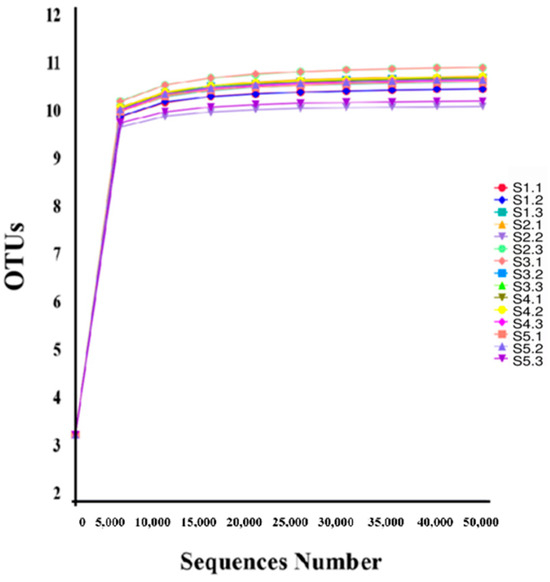
Figure 2.
Species dilution curve.
3.4. Enhanced Alpha Diversity of Bacterial Microbial Community in the Rhizosphere
The diversity and richness indices (Shannon, Simpson, and Chao1) (Table 6) of bacteria and fungi in the rhizosphere soil of plants exhibited significant differences compared to those in the corresponding bulk soil samples (p > 0.05), with notable variations observed (p < 0.05). Furthermore, there were marked discrepancies in the microbial community’s diversity and richness within the rhizosphere soil of H. arenarium (L.) Moench.

Table 6.
α diversity of the microbial community in the rhizosphere soil of H. arenarium (L.) Moench.
3.5. Comparisons and Distinctions in the Composition of Soil Microbial Communities
Principal component analysis (PCA) effectively captures the variations and similarities in the composition of rhizosphere communities across different samples. Each point, represented by distinct colors or shapes, corresponds to a specific sample, with closer proximity indicating greater similarity in species composition [27]. When PC1 accounted for 10.49% (Figure 3A), two rhizosphere bacterial community samples from H. arenarium (L.) Moench exhibited significant dissimilarity to other samples, suggesting distinct species compositions. In contrast, the remaining samples displayed close proximity and strong correlations among one another. Repeated samples within each group showed relatively small distances, indicating a consistent spatial distribution pattern of floral complexity. When PC1 accounted for 9.58% (Figure 3C), from the perspective of sample species, the distances among most samples of rhizosphere fungal communities were remarkably close. This indicates a high degree of similarity in species composition across the samples and a strong correlation among them. However, one sample exhibited a significant deviation in distance. The distances between repeated samples within each group were relatively consistent, suggesting that the spatial distribution complexity of the flora was fairly uniform.
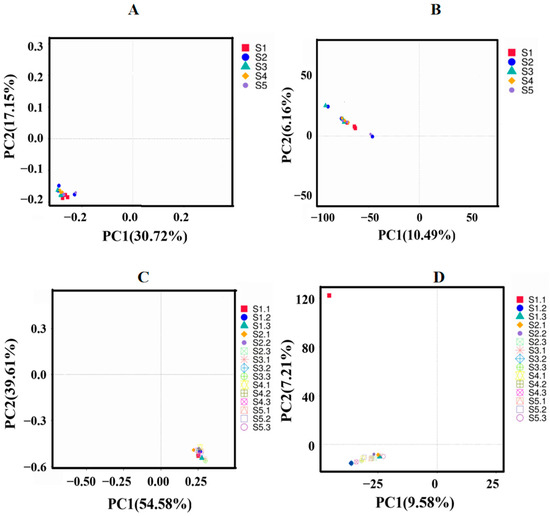
Figure 3.
(A) refers to H. arenarium (L.) Moench bacterial PCA distribution; (B) refers to H. arenarium (L.) Moench bacterial PCoA distribution; (C) refers to H. arenarium (L.) Moench fungal PCA distribution; (D) refers to H. arenarium (L.) Moench fungal PCoA distribution.
Principal component analysis (PCoA) reveals dissimilarities in community species composition among samples based on the similarity of distances. Different colors or shapes of points represent distinct samples, with closer distances indicating more similar species compositions between them. When PC1 accounted for 30.72% and PC2 accounted for 17.15% (Figure 3B), the bacterial communities from each sample of H. arenarium (L.) Moench exhibited significant aggregation, demonstrating extremely close distances to one another, which suggests a lack of apparent differences in microbial bacterial flora. Each data point in Figure 3C corresponds to a sample, and the distribution of these points indicates that the bacterial community composition within each rhizosphere sample is centralized; however, a few samples displayed substantial variations. When PC1 was at 54.58% (Figure 3D), certain distances were observed among rhizosphere soil fungal communities within each sample, indicating some degree of variability in species compositions across samples; when PC2 reached 39.61%, three rhizosphere fungal communities exhibited considerable differences compared to other fungal communities.
Each data point in the figure represents an individual sample, and the distribution of these points suggests that the microbial community composition within each rhizosphere sample of H. arenarium (L.) Moench is relatively decentralized. However, significant variations are observed among a select few samples.
3.6. Numerical Analysis of Operational Taxonomic Units (OTUs)
The findings from the analysis of variations in rhizosphere microbial communities across the samples are illustrated in Figure 4. The rhizosphere microbial communities demonstrated high levels of richness and distinctiveness, as evidenced by the unique operational taxonomic unit (OTU) values associated with each sample.
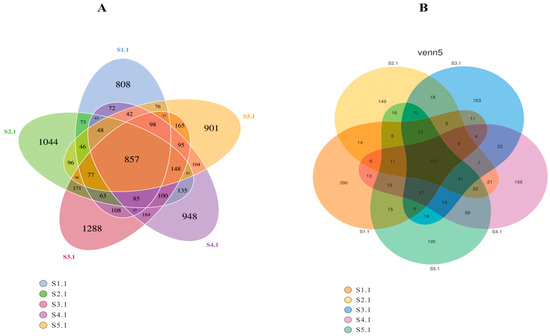
Figure 4.
Venn diagram illustrating the distribution of H. arenarium (L.) Moench in OUT groups. Note: (A) refers to bacteria, (B) refers to fungi.
The rhizosphere soil bacteria comprised a total of 8261 operational taxonomic units (OTUs), with each soil sample displaying both shared and unique OTUs. Specifically, sample S1.1 exhibited 808 unique OTUs, accounting for 9.78% of the overall total; sample S2.1 contained 1044 unique OTUs, representing 12.64% of the total; sample S3.1 possessed 1288 unique OTUs, amounting to 15.59% of the toral; sample S4.1 included 948 specific OTUs, contributing to 11.48% of the total of the total; and finally, sample S5.1 harbored a distinct set of 901 OTUs, comprising approximately 10.91% of the total.
The rhizosphere soil fungi associated with H. arenarium (L.) Moench comprised a total of 1465 operational taxonomic units (OTUs). Each soil sample displayed both common and unique OTU counts. Specifically, sample S1.1 contained 390 OTUs, accounting for 26.62% of the overall total; sample S2.1 included 149 OTUs, representing 10.17% of the total number of OTUs; sample S3.1 had 163 OTUs, which constituted 11.13%; sample S4.1 recorded 166 OTUs, equivalent to 11.33%; and finally, sample S5.1 exhibited a count of 195 OTUs, comprising 13.31% of the total.
3.7. Revealing the Distribution Patterns of Bacterial Species Abundance Through Comprehensive Analysis
The high-throughput amplification sequencing results indicated that the soil bacteria in the rhizosphere of H. arenarium (L.) Moench were classified into 35 phyla, 93 classes, 215 orders, 324 families, 521 genera, and 231 species (Table 7). Several bacterial communities within the rhizosphere of H. arenarium (L.) Moench exhibited a relative abundance exceeding 1% at the phylum level: Actinobacteriota constituted 41.30%, Proteobacteria comprised 14.08%, Acidobacteriota represented 8.32%, Verrucomicrobiota accounted for 7.86%, Gemmatimonadota made up 9.21%, Bacteroidota contributed to 3.02%, Chloroflexi encompassed 7.47%, and Planctomycetota formed approximately 1.45% of the total. At the genus level, several bacterial communities also demonstrated a relative abundance greater than 1%. These included Candidatus Udaeobacter at a proportion of 6.11%, followed by strain 67-14 with an abundance of 4.48%. Gemmatimonas accounted for 3.26%, MB-A2-108 represented 3.26%, and RB41 contributed 2.67% (Figure 5). Notably, Actinobacteria predominated at the phylum level.

Table 7.
Taxonomic-level analysis of habitat and rhizosphere soil microorganisms associated with H. arenarium (L.) Moench for species distribution assessment.
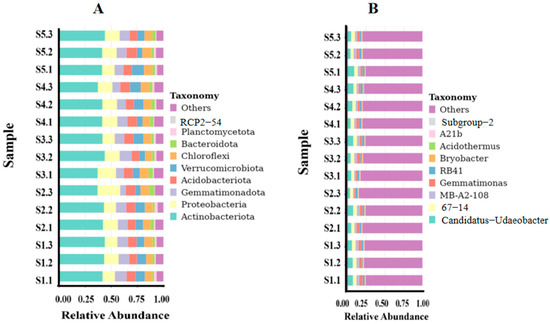
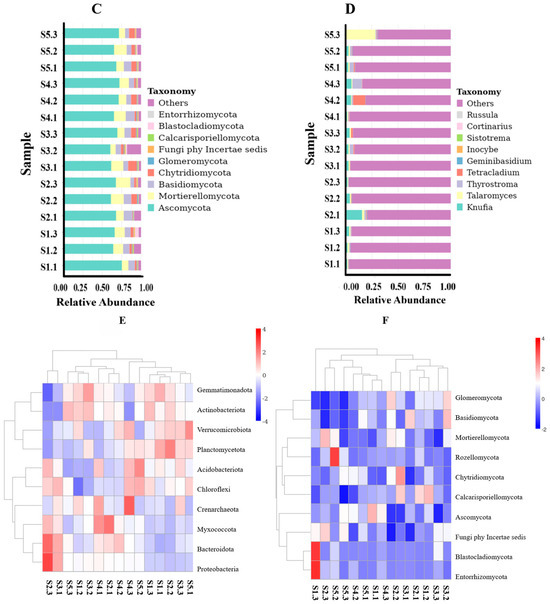
Figure 5.
Histogram depicting relative abundance of species at both phylum and genus levels. Note: (A) refers to histogram of relative abundance of species at H. arenarium (L.) Moench bacterial phylum level, (B) refers to histogram of relative abundance of species at H. arenariu (L.) Moench bacterial genus level; (C) refers to histogram of relative abundance of species at H. arenarium (L.) Moench fungal phylum level, (D) refers to histogram of relative abundance of species at H. arenarium (L.) Moench fungal genus level; (E) refers to heat map analysis of clustering of fungal species abundance; (F) refers to species evolutionary tree at fungal genus level.
The high-throughput amplification sequencing results indicated that the rhizosphere soil fungi associated with H. arenarium (L.) Moench comprised 14 phyla, 47 classes, 96 orders, 204 families, 378 genera, and 371 species (Table 7). Several fungal communities linked to H. arenarium (L.) Moench at the phylum level demonstrated a relative abundance exceeding 1%. Notably, Basidiomycota accounted for 7.80%, Ascomycota represented 67.21%, Mortierellomycota constituted 12.05%, and Chytridiomycota made up 4.61%. At the genus level, several fungal communities associated with H. arenarium (L.) Moench were also found to have a relative abundance surpassing 1%. These included Talaromyces at 2.94%, Knufia at 3.80%, Tetracladium at 1.28%, and Thyrostroma at 1.73% (Figure 5). Ascomycota was identified as the dominant phylum in this analysis.
3.8. Species LEfSe Analysis
LEfSe analysis identified a total of 1 order, 3 families, 5 genera, and 1 species of bacterial differential groups, as well as 1 class, 1 order, 2 families, 7 genera, and 7 species of fungal differential groups(Figure 6). No significant differences were observed at the gate level. At the class level, while no bacteria exhibited significant differences, Geminibasidiomycetes showed notable distinctions. At the order level, significant differences were found in the bacterial order Dongiales and the fungal order Geminibasidiales. At the family level, significant variations were noted in Acidothermaceae, Dongiaceae, and Verrucomicrobiaceae for bacteria; for fungi, significant differences were observed in Geminibasidiaceae and Chaetomiaceae. Among all families analyzed, Chaetomiaceae recorded the highest LDA score. At the genus level, specifically bacteria, examples with significant differences included Acidothermus, Dongia, Psychrobacillus, Aridibacter, and Polaromonas; fungi with notable distinctions included Arxotrichum (which had the highest LDA score among all genera), along with Geminibasidium, Fimicolochytrium, Calcarisporiella, Parachaetomium, Calycina, and Lophiostoma.
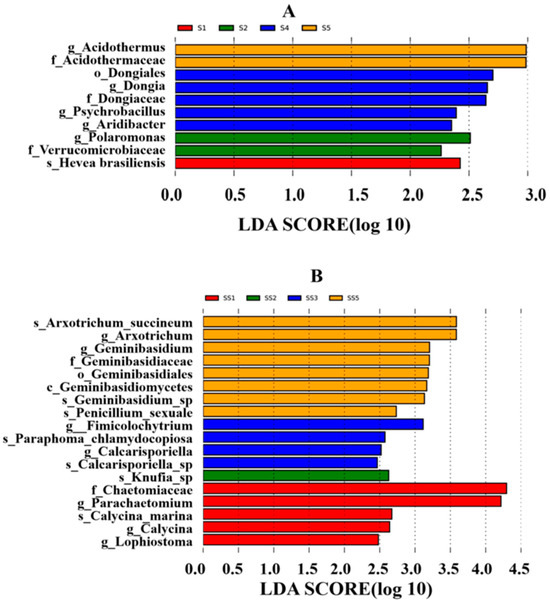
Figure 6.
Histogram of LDA value distribution of rhizosphere soil of H. arenarium (L.) Moench. Note: (A) refers to bacteria; (B) refers to fungi.
3.9. Screening of Rhizosphere Growth-Promoting Bacteria
3.9.1. Screening for Phosphorus Solubilization Ability
A total of 43 strains of rhizosphere soil bacteria were isolated through morphological screening. All bacterial strains were inoculated onto the selected medium, revealing that S31, S29, and S16 exhibited the ability to dissolve inorganic phosphorus (Table 8). Additionally, strain S31 demonstrated the capacity to dissolve organophosphorus compounds, with distinct phosphorus dissolution zones observed on the selected medium.

Table 8.
The strain’s capacity to solubilize inorganic phosphorus.
3.9.2. Screening of Nitrogen Fixation Capacity of Strains
The nitrogen fixation capabilities of 43 rhizosphere bacteria were evaluated. The experimental results indicated that two strains, designated as S7 and S10, demonstrated the ability to fix nitrogen, as evidenced by the distinct transparent zones observed on the Ashu shellfish medium. The soluble indices were recorded at 1.10 and 1.23 (Table 9).

Table 9.
The capacity of the strain to dissolve inorganic phosphorus.
3.9.3. Screening of Siderophore Production Capacity of Strains
The iron-binding capacity of 43 rhizosphere bacteria was evaluated, leading to the identification of ten strains (S2-1, S3, S10, S13, S14, S16, S18, S31, and S29) that exhibited pronounced orange chelation zones on the CAS medium due to their exceptional iron-binding abilities. Notably, strain S16 displayed the highest D/d value of 2.96 among all tested strains (Table 10).

Table 10.
The capacity of a strain to generate iron carriers.
3.9.4. Screening of Potassium-Solubilizing Capacity of Strains
The potassium-solubilizing capabilities of 43 rhizosphere bacteria were evaluated; however, strains exhibiting potassium-sowing abilities were excluded from the screening process.
3.10. Construct a Phylogenetic Tree of the Strains
Genomic DNA was extracted from nine growth-promoting strains, and the 16S rRNA sequences were determined and compared using the NCBI database. The phylogenetic tree was constructed utilizing MEGA11 software. The sequencing results indicated that all nine growth-promoting strains exhibited a degree of similarity greater than 99%. These strains were classified into three genera: Bacillus within Firmicutes, Alcaligenes within Proteobacteria, and Kocuria within Actinomyces. Among these, five strains belonged to the genus Bacillus, three strains belonged to the genus Alcaligenes, and one strain belonged to the genus Kocuria. Within the genus Bacillus, strain S2-1 was tentatively identified as Priestia megaterium. We identified strain S3 as B. mobilis, strain S31 as B. sonorensis, and strain S10 as B. bataviensis. Strain S14 was identified as B. mycoides, while strain S29 was classified as B. paramobilis. Strains S18, S13, and S16 of the genus Alcaligenes were identified as A. faecalis. Lastly, strain S7 within the genus Kocuria was tentatively identified as Kocuria rosea (Figure 7).
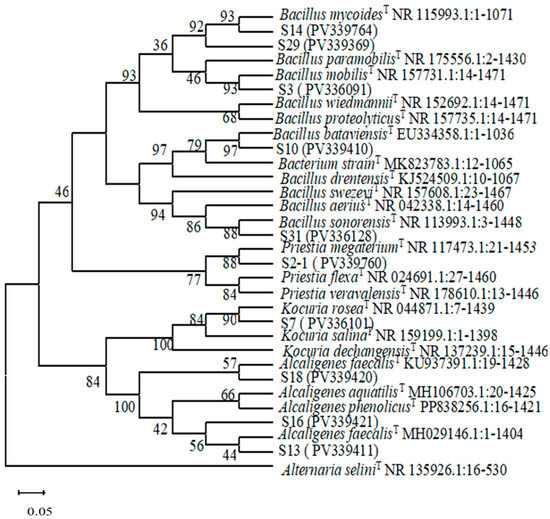
Figure 7.
Phylogenetic tree constructed based on 16S rRNA gene sequence analysis.
4. Discussion
The microbial community structure in the rhizosphere soil plays a pivotal role in nutrient acquisition by H. arenarium (L.) Moench. Consequently, we undertook a comprehensive study and analysis of the microbial community structure within the rhizosphere soil, alongside screening for growth-promoting and antagonistic bacteria. Our findings indicated that bacteria exhibited greater species richness, community diversity, and abundance compared to fungi in the rhizosphere soil environment. This observation underscores a predominant bacterial influence on root ecosystems, corroborated by previous studies highlighting bacterial dominance among soil microorganisms [26,27]. Furthermore, increased bacterial diversity is indicative of enhanced soil resilience [28]. Based on OTU analysis results, eight major bacterial phyla were identified within the rhizosphere microbial community (with relative abundances exceeding 1%), among which Actinobacteriota and Proteobacteria emerged as the dominant phyla. Notably, Proteobacteria accounted for over 40% of all identified phyla and were consistently enriched in the rhizosphere soils of various plants such as alfalfa [29], strawberry [30], potato [31], and asparagus [32]. This suggests that Proteobacteria possess adaptability to diverse plant-specific rhizospheric environments while serving as key constituents of these communities. However, it is important to note that different plant species exhibit distinct characteristics regarding microbial diversity within their respective rhizosphere soils. In our study area—which encompasses mounds, semi-dunes, and slopes with wet saline or gravelly soils at altitudes ranging from 900 m to 2400 m in pine forests or grasslands—pH levels ranged between 6.68 and 7.06, predominantly representing neutral to slightly acidic conditions.
Based on the unique growth environment, Actinobacteriota, Proteobacteria, Acidobacteriota, Verrucomicrobiota, and Gemmatimonadota are the predominant phyla in H. arenarium (L.) Moench. However, while soil fungi are less abundant than bacteria, they play a critical role in maintaining ecosystems [33]. In the rhizosphere microbial community of H. arenarium (L.) Moench, four major fungal phyla exhibit relative abundances greater than 1%, with Ascomycota and Mortierellomycota being the dominant groups alongside Basidiomycota.
At the genus level, the relative abundance of Candidatus Udaeobacter, 67-14, RB41, Gemmatimonas, and MB-A2-108 exceeds 1%. The Gemmatimonas belong to Gemmatimonadota; 67-14 and MB-A2-108 belong to Actinobacteriota. The results are consistent with those of dominant bacteria. Gemmatimonas is a beneficial bacterium found in the rhizosphere that colonizes a majority of plants within this environment. RB41 was found to be colonized in the rhizosphere of winter wheat [34]. Candidatus Udaeobacter is a dominant genus of bacteria and a key core group in rhizosphere soil [32]; 67-14 is a bacterium that predominates in the rhizosphere of olive trees [35]. MB-A2-108 has established itself in the rhizosphere of wheat [36] and is also a predominant bacterium within the rhizosphere of Chinese cabbage [37].
Notably, Ascomycota is the most prevalent phylum, accounting for over 70% of all identified taxa; this finding aligns with previous studies conducted by Li Qingshan et al. [38]. Ascomycota primarily decomposes recalcitrant organic matter such as plant litter and the lignin present in soil. Ren Neifan et al. isolated a strain of Penicillium cinerea BIBA-G563 from healthy Ruthenica roots belonging to Ascomycota, demonstrating a control efficacy of 75.4% against root rot [39]. Given that H. arenarium (L.) Moench is a root-breeding plant, it provides numerous attachment points for rhizosphere microorganisms, thereby enhancing their abundance and diversity while facilitating the enrichment of dominant flora.
At the genus level, the relative abundance of Talaromyces, Knufia, Tetracladium, and Thyrostroma is greater than 1%. Talaromyces, Knufia, and Tetracladium are classified within the Ascomycota phylum, while Thyrostroma is categorized under Basidiomycota. These classifications align with the findings regarding the dominant flora at the phylum level. Talaromyces was identified in the blood-red mushroom pond [40]. Xu Yunlong successfully isolated a strain of Talaromyces purpureogenus, designated XZY3PSF, from corn rhizosphere soil. This strain has demonstrated a significant ability to enhance the primary nutritional indices of crops [41]. Knufia was the predominant genus of fungi found in both herbaceous litter soil [42] and Pinus tabulaeformis sand-fixing forests [43] within desert regions. Tetracladium was predominantly found in the roots of P. muris [44], while Thyrostroma serves as the dominant fungal species within the stems of P. pseudoephedra [45].
Rhizosphere growth-promoting bacteria enhance plant nutrient absorption through various mechanisms, including biological nitrogen fixation, phosphorus solubilization, potassium solubilization, the secretion of plant hormones, and the production of iron carriers. In this study, we screened nine strains of growth-promoting bacteria: five strains belonging to the genus Bacillus within the phylum Firmicutes; three strains from the genus Alcaligenes under Proteobacteria; and one strain from the genus Kocuria in the Actinobacteria phylum. Firmicutes and Proteobacteria were identified as rhizosphere growth-promoting bacteria and dominant taxa in high-throughput sequencing analyses. Additionally, Bacillus emerged as a predominant growth-promoting bacterium within the rhizosphere, aligning with the findings from high-throughput sequencing results.
Previous studies have predominantly identified Bacillus and Pseudomonas as the primary growth-promoting bacteria. Among these, strain S16, which belongs to Alcaligenes, exhibits the highest capacity for phosphorus detoxification and iron carrier production. In contrast, strain S10 from Bacillus demonstrates superior nitrogen fixation abilities. Nitrogen-fixing bacteria convert atmospheric nitrogen into ammonia, thereby enhancing soil nitrogen content. Meanwhile, phosphorus-solubilizing bacteria transform insoluble phosphorus into forms that are accessible to plants, increasing effective phosphorus levels in the soil. Additionally, bacterial strains that produce iron carriers facilitate iron nutrition in plants through the synthesis and utilization of these carriers [46,47,48]. In this study, the nitrogen-fixing medium was employed to assess the nitrogen-fixing capabilities of various strains. However, the nitrogen-fixing medium alone proved to be somewhat inadequate for a comprehensive evaluation of these abilities. Over 90% of rhizosphere microorganisms remain unculturable using traditional media, leading to the identification of only a limited number of culturable strains (such as Bacillus and Pseudomonas). This limitation results in the underestimation of the diversity present among rhizosphere nitrogen-fixing bacteria. Furthermore, certain azotobacter species within the rhizosphere require plant signaling molecules (such as nodulation factors) or collaboration with other microorganisms for their activation. When the strain is cultured in isolation, its nitrogen-fixing genes may become silenced, resulting in the inaccurate assessment of the strain’s functional capabilities. Furthermore, different strains may only exhibit nitrogen fixation abilities under specific plant host or soil conditions. The homogenization condition of the medium fails to adequately reflect niche differentiation, leading to the exclusion of certain functional strains. Furthermore, bacteria within the medium may experience survival stress (such as carbon limitation), which can inhibit nitrogenase expression. In contrast, carbon sources secreted by plant roots in natural environments (such as organic acids) have the potential to enhance nitrogen fixation activity. In the subsequent phase, quantifiable techniques such as the acetylene reduction assay (ARA) can be employed to assess the nitrogenase activity of various strains, thereby facilitating the identification of superior nitrogen-fixing bacteria. This study focused exclusively on isolating and identifying rhizosphere growth-promoting bacteria. It is important to note that the strains isolated through culturable methods only represent a fraction of the culturable component within the rhizosphere microbial community; consequently, there may be an underestimation of the diversity present among actual functional strains. In subsequent phases of this study, optimal growth-promoting bacteria will be selected based on strain combination experiments. This approach aims to establish an experimental foundation for developing and promoting microbial fertilizers specific to H. arenarium (L.) Moench, while also laying a research groundwork for conserving wild resources of H. arenarium (L.) Moench, exploring rhizosphere microorganism development and utilization, and providing robust support for future applications of H. arenarium (L.) Moench in food and medicine.
5. Conclusions
The high-throughput sequencing results identified Actinomycetes, Proteobacteria, and Blastomonas as the predominant bacterial phyla in the rhizosphere soil of H. arenarium (L.) Moench, while Ascomycetes and Mortieromycetes were recognized as the primary fungal phyla. The assessment of efficiency promotion for 43 bacterial species isolated from the rhizosphere soil of H. arenarium (L.) Moench demonstrated that three strains—namely S31, S16, and S29—exhibited phosphorus solubilization capabilities. Notably, strain S16 exhibited the highest inorganic phosphorus solubility index at 1.23. Furthermore, strain S31 displayed organophosphorus solubilization abilities. Additionally, strains S7 and S10 possessed nitrogen fixation capacity; however, none of the isolated strains demonstrated proficiency in dissolving potassium compounds.
A total of ten strains with exceptional iron-bearing capacity were identified, among which strain S16 demonstrated both the highest D/d value and the most robust iron-bearing capability. These ten growth-promoting strains belong to the genera Bacillus, Alcaligenes, and Kocuria. In particular, this is Priestia megaterium for strain S2-1; B. mobilis for strain S3; Kocuria rosea for strain S7; B. bataviensis for strain S10; B. mycoides for strain S14; B. paramobilis for strain S29; B. sonorensis for strain S31; and A. faecalis for strains S18, S13, and S16.
In this study, we focused exclusively on analyzing the rhizosphere community structure of wild H. arenarium (L.) Moench and conducted an initial screening of growth-promoting bacteria. However, there remains significant potential for further research in this area. In subsequent phases, we will quantitatively assess the growth-promoting bacteria for their capabilities in nitrogen fixation, phosphorus solubilization, and indole-3-acetic acid (IAA) production. Additionally, we aim to identify optimal strains of growth-promoting bacteria through experiments designed to evaluate their efficacy in promoting the growth of H. arenarium (L.) Moench. Our study provides a foundational experimental basis for the development and dissemination of microbiological fertilizers derived from H. arenarium (L.) Moench.
Author Contributions
S.S. and W.H. designed this study. J.Y. and Y.C. collect plant materials. J.Z. isolated and identified the rhizosphere growth-promoting bacteria of plants. X.X. and S.S. interpret data and write articles. J.X. reviewed and edited the manuscript. X.H. software. All authors have read and agreed to the published version of the manuscript.
Funding
This research was found by the Research and application of high quality and efficient production technology and equipment of Vaccinium myrtillus L. and Helichrysum arenarium (2023B02023-2-02).
Data Availability Statement
The original data presented in the study are included in the article.
Acknowledgments
This study was supported by the Research and application of high quality and efficient production technology and equipment of Vaccinium myrtillus L. and Helichrysum arenarium (2023B02023-2-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xinjiang Flora Editorial Committee. Flora of Xinjiang (Volume IV); Xinjiang Science, Technology and Health Press: Urumqi, China, 2004; p. 10. [Google Scholar]
- Ma, Z.; Yang, C. Survey of Chinese herbal medicines in Altay region of Xinjiang. China J. Chin. Mater. Medica 2002, 27, 70–71. [Google Scholar]
- Liu, R. Research on Synergistic Development of Ethnic Economy in Burzin County Under the Domination of Tourism. Master’s Thesis, Shihezi University, Shihezi, China, 2020. [Google Scholar]
- Czinner, E.; Hagymási, K.; Blázovics, A.; Kéry, A.; Szoke, E.; Lemberkovics, E. In Vitro antioxidant properties of H. arenarium (L.) Moench. J. Ethnopharmacol. 2000, 73, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Wang, C.; Sun, S.; Xu, B.; Wu, L. Chemical composition of the lipid-lowering active site of the sandy wax chrysanthemum flower (III). Chin. J. Med. Chem. 2012, 22, 220–222, 226. [Google Scholar]
- Li, Y.; Wang, Y.; Lan, W. Study on the optimal extraction process of total flavonoids from Bupleurum officinale. J. Xinjiang Med. Univ. 2019, 42, 805–807, 812. [Google Scholar]
- Zhang, Y. Studies on the Chemical Composition and Bioactivity of the Aboveground Parts of Corydalis alba and Corydalis alba. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2009. [Google Scholar]
- Kihyuck, C.; Raees, K.; Woo, S.L. Dissection of plant microbiota and plant-microbiome interactions. J. Microbiol. 2021, 59, 281–291. [Google Scholar]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Crit. Rev. Plant Sci. 2010, 22, 107–149. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.C. Research progress on main ecological functions of plant root exudates. Chin. Bull. Bot. 2020, 55, 788–796. [Google Scholar]
- Huang, L.; Bao, W.K.; Li, F.L.; Hu, H. Effects of soil structure and vegetation on microbial communities. Chin. J. Appl. Environ. Biol. 2021, 27, 1725–1731. [Google Scholar]
- Huang, Y.Q.; Han, X.R.; Yang, J.F.; Han, M.; Bai, H.Z. Effect of peanut root exudates on soil microbial characteristics and community functional diversity. J. Shenyang Agric. Univ. 2015, 46, 48–54. [Google Scholar]
- Chen, H.; Tang, H.Y.; Guo, J.H.; Lin, W.X.; Li, Y.J.; Wang, Y.Q. Root exudates’ roles and analytical techniques progress. Soils 2023, 55, 225–233. [Google Scholar]
- Wu, C.-H.; Liu, J.-Z. Progress in the study of rhizosphere microbial influences and their interactions with plants. J. Hebei Norm. Univ. Nat. Sci. Ed. 2022, 46, 603–613. [Google Scholar]
- Garima, Y.; Mukesh, M. Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnol. Rep. 2021, 345, 89–92. [Google Scholar]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 128, 92–99. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, L. Progress on the mechanism of endophytic bacteria of medicinal plants on host plants. Microbiol. Bull. 2023, 50, 1653–1665. [Google Scholar]
- Lu, R. Methods for Agricultural Chemical Analysis of Soil; China Agricultural Science and Technology Press House: Beijing, China, 2000. [Google Scholar]
- Gu, M.; Tang, G.; Zhang, Y.; Li, X.; Wang, J.; Chen, L.; Liu, H. Effects of Organic Fertilizers and Biochar on Microorganism Community Characteristics in Saline-alkali Sandy Soil of Xinjiang. Ecol. Environ. Sci. 2023, 32, 1392–1404. [Google Scholar]
- Wang, Y.M.; Fu, J.H.; Qin, X.Z.; Wang, J.; Ruan, W.W.; Cui, F.Z.; Nie, H.L. Isolation, screening and growth promoting characteristics of growth-promoting bacteria from mossy crusty soil in Gurbantunggut Desert. J. Microbiol. 2019, 43, 47–57. [Google Scholar]
- Zhang, L.; Yu, H.; Bi, Y.; Wang, Z.G.; Xu, W.H.; Liu, Z.L. Screening of Rhizosphere Growth-promoting Bacteria of Pepper (Capsicum annuum) and Analysis of Their Beneficial Effects. J. Agric. Biotechnol. 2024, 32, 2124–2136. [Google Scholar]
- Wang, X.X.; Chen, J.Y.; Fan, C.; Cao, X.; Meng, L.Q.; Liu, Z.T.; Zhao, X.Y.; Zhang, S.; Song, Y. Screening, identification, salt tolerance and growth promotion effect of two strains of phosphorus solubilizing fungi. Jiangsu Agric. Sci. 2024, 52, 219–226. [Google Scholar]
- Du, L.; Wang, S.P.; Chen, G.; Hong, J.; Huang, X.; Zhang, L.H.; Ye, L.X.; Lian, Z.C.; Zhang, G.Y. Screening, identification and phosphorus solubilization capacity of a highly efficient phosphorus solubilizing bacterium. China Soil Fertil. 2017, 23, 136–141. [Google Scholar]
- Wang, J.; Tan, A.; Liu, J.; Tang, W.P.; Yi, J.M.; Guo, M.; Sun, B.W.; Sun, Z.R. Screening of rhizosphere growth-promoting bacteria of desertplants and its promoting effects on silage maize seedlings. Agric. Res. Arid. Areas 2024, 42, 210–221. [Google Scholar]
- Li, J.; Wang, F.; Xu, L. High-throughput analysis of soil bacterial community structure in the rhizosphere zone of Taishan white foxglove. Guangdong Agric. Sci. 2022, 49, 75–84. [Google Scholar]
- Zhang, C. Structural Analysis of Endophytic and Rhizosphere Microflora and Saponin Biotransformation in American Ginseng. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2021. [Google Scholar]
- Chen, K.; Chen, M.; Lin, L.; Zou, H.L. Characterization of microbial communities contaminated by nine traditional Chinese medicine tablets based on 16S rRNA high-throughput sequencing. J. Tradit. Chin. Med. 2022, 28, 53–56, 61. [Google Scholar]
- Wang, F.; Li, W.; Liu, X.; Li, W.C.; Zhao, J.B.; Zhang, Z.H.; Yang, Z. Response of rhizosphere soil bacterial community of alfalfa to decomposed cow manure. Acta Acreologica Sin. 2022, 30, 603–611. [Google Scholar]
- Wang, Q.-F.; Zhou, D.-P.; Chu, C.-B.; Zhao, Z.; Yang, Q.G.; Wu, S.H. Difference of rhizosphere bacterial community structure and function between healthy and anthracnose infected strawberries. Chin. J. Soil Sci. 2022, 53, 1404–1412. [Google Scholar]
- Ge, Y.; Sun, T. Microbial community structure and diversity in potato rhizosphere and non-rhizosphere soil. Chin. J. Ecol. Environ. 2020, 29, 141–148. [Google Scholar]
- Sheng, Y.; Huang, L.; Ye, P.; Lai, J.; Liu, Y.; Zhang, Q.F.; Liu, J.; Li, F.S.; Wei, S.G. Microbial community structure and diversity in rhizosphere and non-rhizosphere soil of asparagus. J. Sichuan Agric. Univ. 2024, 42, 330–338. [Google Scholar]
- Zhao, J.; Liao, Y. Characteristics and effects of decomposer subsystem in Leymus chinensis grassland. Acta Ecol. Sin. 1995, 15, 359–364. [Google Scholar]
- Zhou, X.; Li, C.; Chen, X.; Yang, Y.Z.; Gao, W.H. Study on microbial community diversity in rhizosphere soil of winter wheat in Pingliang region based on 16SrRNA amplicon sequence. Agric. Technol. 2018, 44, 20–26. [Google Scholar]
- Lu, Y.-H.; Geng, G.G.; Wang, L.-H.; Qiao, F. Physicochemical properties and microbial communities characteristics of rhizosphere soils in different distribution areas of Lamiophlomis rotata on the Winghai-Tibetan Plateau. Pratacultural Sci. 2024, 1–20. [Google Scholar] [CrossRef]
- Du, Y.; Hu, Q.; Xin, P.; Lu, B.; Guo, Y.Q.; Zhao, M.; Hao, J.B.; Zhao, C.; Xu, L. Microbial diversity in olive soil treated with slow release fertilizer. West. For. Sci. 2019, 53, 145–155, 162. [Google Scholar]
- Sun, H.; Chen, M.; Wei, L.; Xue, P.Y.; Zhao, Q.L.; Gao, P.P.; Geng, L.P.; Wen, Q.X.; Liu, W.J. Roots recruited distinct rhizo-microbial communities to adapt to long-term Cd and as co-contaminated soil in wheat-maize rotation. Environ. Pollut. 2024, 342, 123053. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Effects of Fertilization Methods on Soil Nutrients, Resistance Genes and Microbial Communities of Chinese Cabbage. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2023. [Google Scholar]
- Li, Q.; Wang, M.; Liu, J. Diversity and differences of rhizosphere soil microbial community structure in different periods of rapeseed. North. Hortic. 2023, 24, 72–80. [Google Scholar]
- Ren, N.; Liu, X.; Tang, J.; Dong, C.; Bai, Z.; Xuan, Y.; Li, M. Effect of Penicillium griseus BIBA-G563 on Fusarium root rot of alfalfa. Chin. J. Grassl. Sci. 2023, 45, 95–103. [Google Scholar]
- Wang, X.; Bian, L.; Liu, H.; Ma, Q.; Chen, L.; Miao, S. Diversity of soil microbial communities in the red mushroom pond. Chin. J. Microbiota 2025, 1–16. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Guo, L.; Wang, J.; Liang, C.C.; Yang, Y.; Ta, Y.Q.; Han, B.J.; Yang, L.Y. Optimization of phosphorous solubilization conditions of purple cyanobacteria and its effect on the growth and quality of capsicum. J. Microbiol. 2025, 1–17. [Google Scholar] [CrossRef]
- Kong, J.; Zhai, P.; Wang, R.-X.; Wang, J.; Liu, H.L.; Ma, R.S.; Kang, P.; Liu, B.R. Effects of herbaceous litter thickness on community structure and physicochemical properties of fungi in aeolian sand soil. Res. Soil Water Conserv. 2025, 32, 119–129. [Google Scholar]
- Gao, H.; Zhang, S.; Yang, Z.; Zhang, L.; Hang, H.G.; Yan, D.R. Soil fungal community structure and function in the sand-fixing forest of Pinus tabulatus in Horqin Sandy Land. Arid Land Res. 2019, 42, 118–126. [Google Scholar]
- Qiu, X.D. Study on Rhizosphere and Rhizosphere Microbial Diversity of Sausselia tianshanensis and Trexonella muris. Master’s Thesis, Peking Union Medical College, Beijing, China, 2024. [Google Scholar]
- Han, B. Study on Niche Selection and Microevolution of Fungi from Rhizosphere to Ephedra Herba. Master’s Thesis, Shanxi University, Taiyuan, China, 2024. [Google Scholar]
- Persello-Cartieaux, F.; Nussaume, L.; Robaglia, C. Tales from the underground: Molecular plant–rhizobacteria interactions. Plant Cell Environ. 2003, 26, 189–199. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, K. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Ren, X.-P.; Li, S.; Qi, Y.-L.; Ran, S.; Zhang, C.; Zeng, X.X. Screening, identification and application of iron-producing carrier fungus Ti-11. J. Hunan Univ. Technol. 2023, 37, 15–21. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).