First Isolation, Molecular Identification, and Phylogenetic Characterization of A3B5 Very Virulent Infectious Bursal Disease Virus in Pullets in Chile
Abstract
1. Introduction
2. Materials and Methods
2.1. Examination and Sampling of the Pullets
2.2. Viral Isolation and Propagation in Specific Pathogen-Free Embryonated Chicken Eggs (SPF-ECE)
2.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.4. VP1 and VP2 Sequence Analysis and Phylogenetic Analysis
3. Results
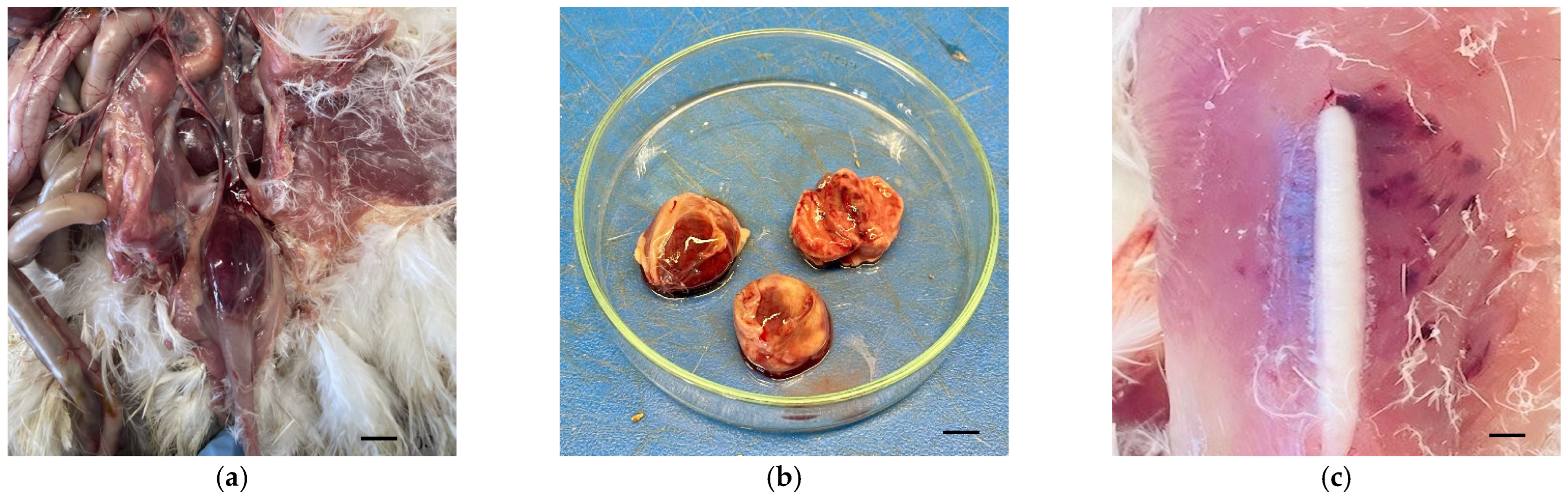
3.1. Examination of the Pullets
3.2. Viral Isolation
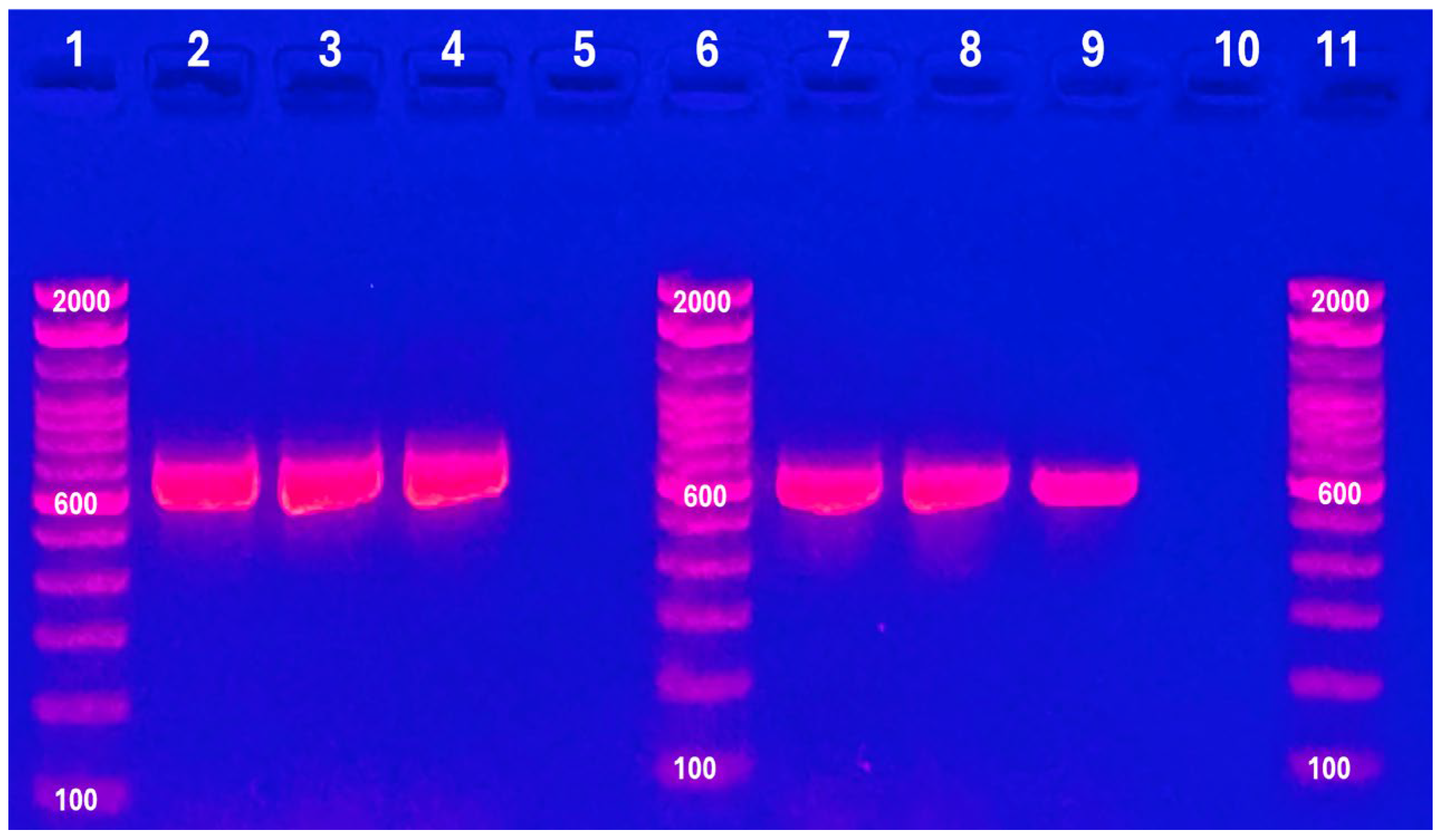
3.3. Identification of the IBDV
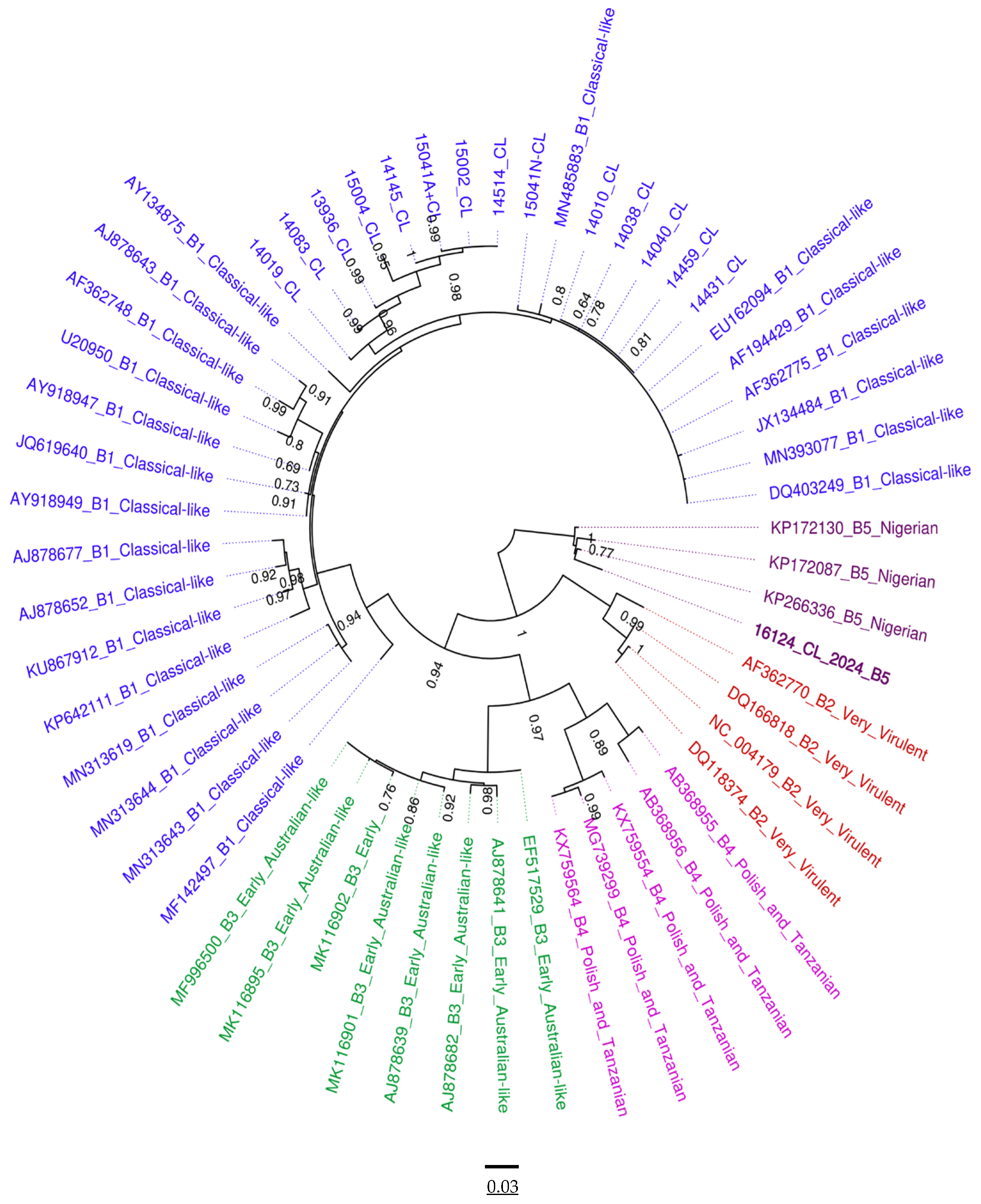
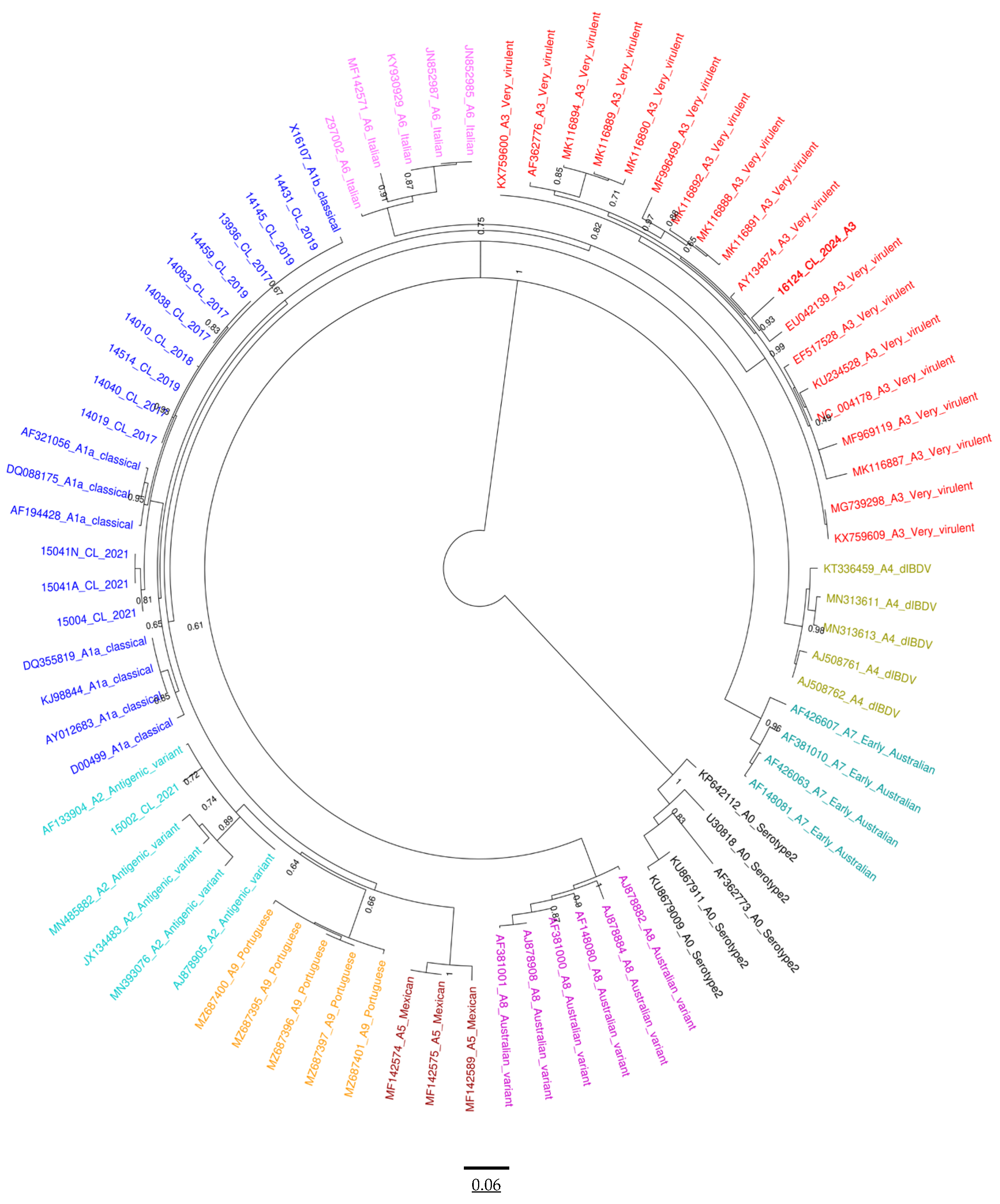
3.4. Phylogenetic Analysis of VP1 and VP2
3.5. Nucleotide Sequence Analysis of Segments A and B
3.6. Amino Acid Sequence Analysis for Segments A and B
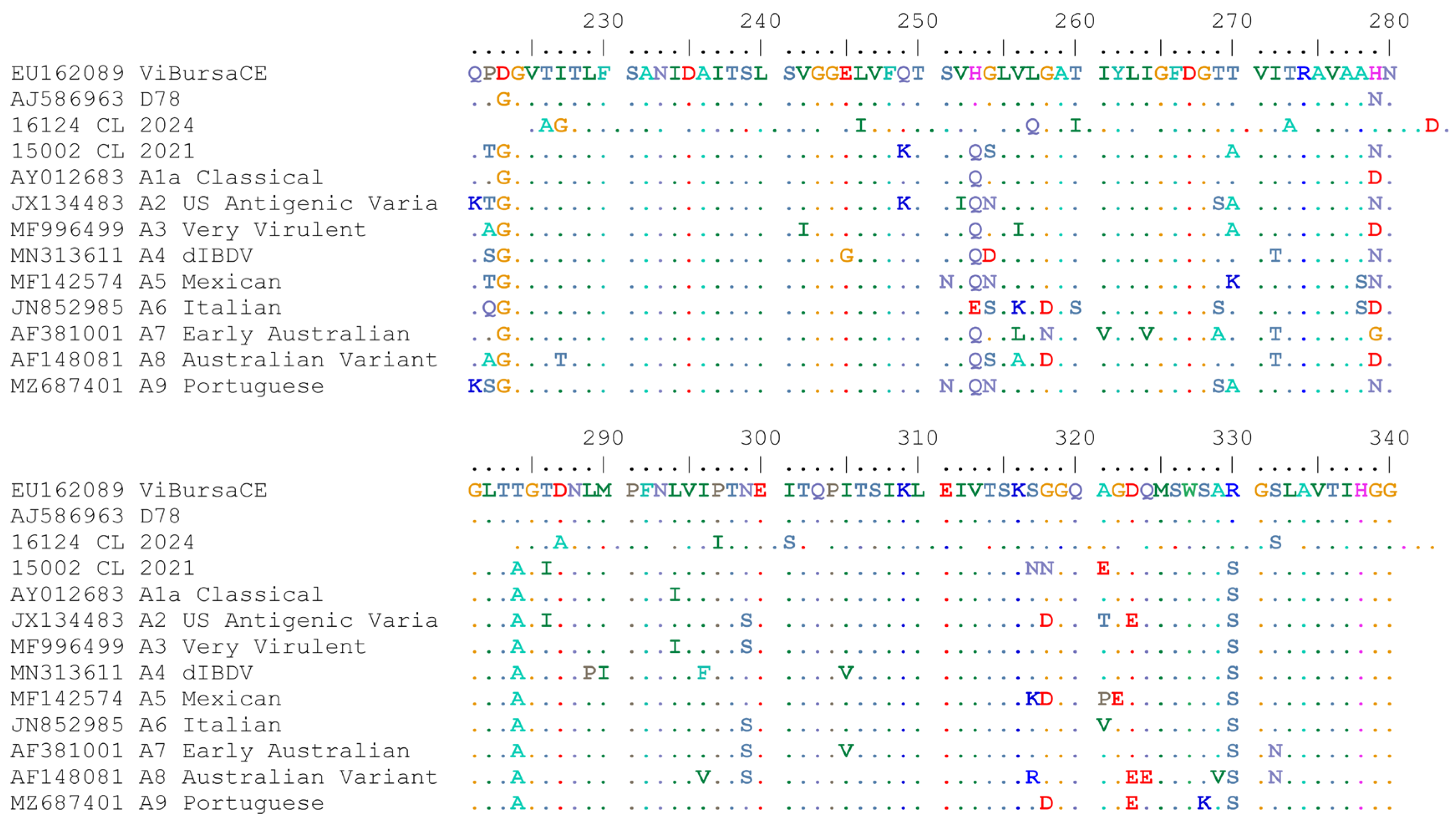
3.7. Analysis of VP2 Hypervariable Region (hvVP2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobos, P.; Hill, B.J.; Hallett, R.; Kells, D.T.; Becht, H.; Teninges, D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J. Virol. 1979, 32, 593–605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahgoub, H.A.; Bailey, M.; Kaiser, P. An overview of infectious bursal disease. Arch. Virol. 2012, 157, e47–e57, Erratum in Arch. Virol. 2012, 157, 2059. [Google Scholar] [CrossRef] [PubMed]
- Faragher, J.T.; Allan, W.H.; Wyeth, P.J. Immunosuppressive effect of infectious bursal agent on vaccination against Newcastle disease. Vet. Rec. 1974, 95, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Delmas, B.; Attoui, H.; Ghosh, S.; Malik, Y.S.; Mundt, E.; Vakharia, V.N.; ICTV Report Consortium. ICTV virus taxonomy profile: Birnaviridae. J. Gen. Virol. 2019, 100, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Ko, T.P.; Chou, C.C.; Yoshimura, M.; Doong, S.R.; Wang, M.Y.; Wang, A.H. Crystal structure of infectious bursal disease virus VP2 subviral particle at 2.6A resolution: Implications in virion assembly and immunogenicity. J. Struct. Biol. 2006, 155, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, C.D.; Spies, U.; Shaw, K.; Peters, R.W.; Papageorgiou, A.; Müller, H.; Boursnell, M.E. A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. J. Gen. Virol. 1990, 71 Pt 6, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Delmas, B. Birnaviruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Elsevier: Oxford, UK, 2008; Volume 1, pp. 321–328. [Google Scholar]
- Brandt, M.; Yao, K.; Liu, M.; Heckert, R.A.; Vakharia, V.N. Molecular determinants of virulence, cell tropism, and pathogenic phenotye of infectious bursal disease virus. J. Virol. 2001, 75, 11974–11982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coulibaly, F.; Chevalier, C.; Gutsche, I.; Pous, J.; Navaza, J.; Bressanelli, S.; Delmas, B.; Rey, F.A. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell 2005, 120, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J.; Saif, Y.M.; Moorhead, P.D. Immunogenicity and antigenicity of infectious bursal disease virus serotypes I and II in chickens. Avian Dis. 1985, 29, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Chettle, N.; Stuart, J.C.; Wyeth, P.J. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 1989, 125, 271–272. [Google Scholar] [CrossRef]
- Jackwood, D.H.; Saif, Y.M. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987, 31, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J.; Sommer-Wagner, S.E.; Crossley, B.M.; Stoute, S.T.; Woolcock, P.R.; Charlton, B.R. Identification and pathogenicity of a natural reassortant between a very virulent serotype 1 infectious bursal disease virus (IBDV) and a serotype 2 IBDV. Virology 2011, 420, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.P.; Morales, D.; Eterradossi, N.; Rivallan, G.; Toquin, D.; Raue, R.; Zierenberg, K.; Zhang, M.F.; Zhu, Y.P.; Wang, C.Q.; et al. Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathol. 2004, 33, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Saif, Y.M. Immunosuppression induced by infectious bursal disease virus. Vet. Immunol. Immunopathol. 1991, 30, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; Stephens, C.B.; Pantin-Jackwood, M.J. The Effect of Infectious Bursal Disease Virus-Induced Immunosuppression on Vaccination Against Highly Pathogenic Avian Influenza Virus. Avian Dis. 2018, 62, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Niu, X.; Jiang, N.; Zhang, W.; Wang, G.; Li, K.; Huang, M.; Gao, Y.; Qi, X.; Wang, X. Comparative Pathogenicity of Three Strains of Infectious Bursal Disease Virus Closely Related to Poultry Industry. Viruses 2023, 15, 1257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Firquet, S.; Beaujard, S.; Lobert, P.E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cosgrove, A.S. An Apparently New Disease of Chickens: Avian Nephrosis. Avian Dis. 1962, 6, 385. [Google Scholar] [CrossRef]
- Rosenberger, J.K.; Cloud, S.S. Isolation and characterization of variant infectious bursal disease viruses. J. Am. Vet. Med. Assoc. 1986, 189, 357. [Google Scholar]
- Berg, T.P.; Gonze, M.; Morales, D.; Meulemans, G. Acute infectious bursal disease in poultry: Immunological and molecular basis of antigenicity of a highly virulent strain. Avian Pathol. 1996, 25, 751–768. [Google Scholar] [CrossRef] [PubMed]
- Di Fabio, J.; Rossini, L.I.; Eterradossi, N.; Toquin, M.D.; Gardin, Y. European-like pathogenic infectious bursal disease viruses in Brazil. Vet. Rec. 1999, 145, 203–204. [Google Scholar] [PubMed]
- Stoute, S.T.; Jackwood, D.J.; Sommer-Wagner, S.E.; Cooper, G.L.; Anderson, M.L.; Woolcock, P.R.; Bickford, A.A.; Sentíes-Cué, C.G.; Charlton, B.R. The diagnosis of very virulent infectious bursal disease in California pullets. Avian Dis. 2009, 53, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.O.; Jackwood, D.J. Classification of infectious bursal disease virus into genogroups. Arch. Virol. 2017, 162, 3661–3670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escaffre, O.; Le Nouën, C.; Amelot, M.; Ambroggio, X.; Ogden, K.M.; Guionie, O.; Toquin, D.; Müller, H.; Islam, M.R.; Eterradossi, N. Both genome segments contribute to the pathogenicity of very virulent infectious bursal disease virus. J. Virol. 2013, 87, 2767–2780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Islam, M.R.; Nooruzzaman, M.; Rahman, T.; Mumu, T.T.; Rahman, M.M.; Chowdhury, E.H.; Eterradossi, N.; Müller, H. A unified genotypic classification of infectious bursal disease virus based on both genome segments. Avian Pathol. 2021, 50, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, L.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.; Liu, C.; Cui, H.; Pan, Q.; Zhang, Y.-P.; et al. An improved scheme for infectious bursal disease virus genotype classification based on both genome-segments A and B. J. Integr. Agric. 2021, 20, 1372–1381. [Google Scholar] [CrossRef]
- Legnardi, M.; Franzo, G.; Tucciarone, C.M.; Koutoulis, K.; Duarte, I.; Silva, M.; Le Tallec, B.; Cecchinato, M. Detection and molecular characterization of a new genotype of infectious bursal disease virus in Portugal. Avian Pathol. 2022, 51, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Pikuła, A.; Lisowska, A.; Domańska-Blicharz, K. Epidemiology of Infectious Bursal Disease Virus in Poland During 2016–2022. Viruses 2023, 15, 289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lian, J.; Wang, Z.; Xu, Z.; Pang, Y.; Leng, M.; Tang, S.; Zhang, X.; Qin, J.; Chen, F.; Lin, W. Pathogenicity and molecular characterization of infectious bursal disease virus in China. Poult. Sci. 2022, 101, 101502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tammiranta, N.; Ek-Kommonen, C.; Rossow, L.; Huovilainen, A. Circulation of very virulent avian infectious bursal disease virus in Finland. Avian Pathol. 2018, 47, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Arowolo, O.A.; George, U.E.; Luka, P.D.; Maurice, N.A.; Atuman, Y.J.; Shallmizhili, J.J.; Shittu, I.; Oluwayelu, D.O. Infectious bursal disease in Nigeria: Continuous circulation of reassortant viruses. Trop. Anim. Health Prod. 2021, 53, 271. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.; Courtillon, C.; Briand, F.X.; Khalifa, M.; Selim, A.; Arafa, A.E.S.; Hegazy, A.; Eterradossi, N.; Soubies, S.M. Continuous circulation of an antigenically modified very virulent infectious bursal disease virus for fifteen years in Egypt. Infect. Genet. Evol. 2020, 78, 104099. [Google Scholar] [CrossRef] [PubMed]
- Legnardi, M.; Franzo, G.; Tucciarone, C.M.; Koutoulis, K.; Cecchinato, M. Infectious bursal disease virus in Western Europe: The rise of reassortant strains as the dominant field threat. Avian Pathol. 2023, 52, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Banda, A.; Villegas, P. Genetic characterization of very virulent infectious bursal disease viruses from Latin America. Avian Dis. 2004, 48, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Remorini, P.; Calderón, M.G.; Aguirre, S.; Periolo, O.; La Torre, J.; Mattion, N. Characterization of infectious bursal disease viruses from Argentina. Avian Dis. 2006, 50, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Cádiz, L.; Guerrero-Moncayo, A.; Cáceres, F.; Vidal, S.; Lapierre, L.; Sáenz, L.; Hidalgo, H. Molecular characterization of Infectious Bursal Disease Virus isolated in Chile reveals several mutations in VP2 coding region and a reassortment in its genome. Vet. Res. Commun. 2022, 46, 1281–1289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Organization of Animal Health (WOAH). Chapter 3.3.12—Infect Bursal Disease (Gumboro Disease). In Terrestrial Manual; WOAH: Paris, France, 2024; pp. 1–23. [Google Scholar]
- Eterradossi, N.; Arnauld, C.; Toquin, D.; Rivallan, G. Critical amino acid changes in VP2 variable domain are associated with typical and atypical antigenicity in very virulent infectious bursal disease viruses. Arch. Virol. 1998, 143, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Nouën, C.L.; Rivallan, G.; Toquin, D.; Darlu, P.; Morin, Y.; Beven, V.; de Boisseson, C.; Cazaban, C.; Comte, S.; Gardin, Y.; et al. Very virulent infectious bursal disease virus: Reduced pathogenicity in a rare natural segment-B-reassorted isolate. J. Gen. Virol. 2006, 87 Pt 1, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, A. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Setta, A.; Yehia, N.; Shaheen, M.; Shami, A.; Al-Saeed, F.A.; Alsamghan, A.; Amin, R.; El-Saadony, M.T.; El-Tarabily, K.A.; Salem, H.M. Continuous clinicopathological and molecular recognition of very virulent infectious bursal disease virus in commercial broiler chickens. Poult. Sci. 2024, 103, 103306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosad, S.M.; Eladl, A.H.; El-Tholoth, M.; Ali, H.S.; Hamed, M.F. Molecular characterization and pathogenicity of very virulent infectious bursal disease virus isolated from naturally infected turkey poults in Egypt. Trop. Anim. Health Prod. 2020, 52, 3819–3831. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.K.; Do, R.T.; Doan, H.T.T.; Nguyen, K.T.; Pham, L.T.K.; Le, T.H. Phylogenotyping of infectious bursal disease virus in Vietnam according to the newly unified genotypic classification scheme. Arch. Virol. 2023, 168, 201. [Google Scholar] [CrossRef] [PubMed]
- De Fraga, A.P.; Gräf, T.; Coltro, V.P.; Ikuta, N.; Fonseca, A.S.K.; Majó, N.; Lunge, V.R. Phylodynamic analyses of Brazilian antigenic variants of infectious bursal disease virus. Infect. Genet. Evol. 2019, 73, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Banda, A.; Hernández, D.; Panzera, F.; Pérez, R. Detection of very virulent strains of infectious bursal disease virus (vvIBDV) in commercial broilers from Uruguay. Avian Dis. 2006, 50, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Lu, P.; Yan, Y.X.; Hua, X.G.; Jiang, J.; Zhao, Y. Sequence and analysis of genomic segment A and B of very virulent infectious bursal disease virus isolated from China. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Nwagbo, I.; Milani, A.; Salviato, A.; Zamperin, G.; Sulaiman, L.; Maurice, N.; Meseko, C.; Fusaro, A.; Shittu, I. Genomic Analysis of Infectious Bursal Disease Virus in Nigeria: Identification of Unique Mutations of Yet Unknown Biological Functions in Both Segments A and B. Vaccines 2023, 11, 867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, F.M.; Vidigal, P.M.; Myrrha, L.W.; Fietto, J.L.; Silva, A., Jr.; Almeida, M.R. Tracking the molecular epidemiology of Brazilian Infectious bursal disease virus (IBDV) isolates. Infect. Genet. Evol. 2013, 13, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mundt, E. Tissue culture infectivity of different strains of infectious bursal disease virus is determined by distinct amino acids in VP2. J. Gen. Virol. 1999, 80 Pt 8, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.Z.; Ali, M.; Abbas, M.; Chaudhry, U.N.; Zia-Ur-Rehman, M.M. Molecular characterization of infectious bursal disease viruses from Pakistan. Arch. Virol. 2016, 161, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Hassan, A.; Muneeb, J.M.; Akram, T.; Haq, E.; Shah, R.A.; Ganai, N.A.; Ahmad, S.M.; Chikan, N.A.; Shabir, N. A multiepitope vaccine candidate against infectious bursal disease virus using immunoinformatics-based reverse vaccinology approach. Front. Vet. Sci. 2023, 9, 1116400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Segment | Gen | Primer | Sequence (5′ to 3′) | PCR Product (bp) | Reference |
|---|---|---|---|---|---|
| A | VP2 | U3 | TGTAAAACGACGGCCAGTGCATGCGGTATGTGAGGCTTGGTGAC | 604 | [39] |
| L3 | CAGGAAACAGCTATGACCGAATTCGATCCTGTTGCCACTCTTTC | ||||
| B | VP1 | +290 | TGTAAAACGACGGCCAGTGAATTCAGATTCTGCAGCCACGGTCTCT | 642 | [40] |
| −861 | CAGGAAACAGCTATGACCCTGCAGTTGATGACTTGAGGTTGATTTTG |
| Segment | Gene | Accession Number | % Identity | Accession Number | Country | Year |
|---|---|---|---|---|---|---|
| A | VP2 | PP824544 | 95.31% | JN982259 | Brazil | 2013 |
| 95.31% | JF811919 | Brazil | 2016 | |||
| 95.31% | DQ286035 | Brazil | 2008 | |||
| B | VP1 | PP824543 | 97.64% | JN982250 | Brazil | 2013 |
| 97.30% | JN982249 | Brazil | 2013 | |||
| 97.13% | DQ679811 | USA | 2008 |
| A0 | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | CL1 | CL2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A0 | 0.0674 | 0.0779 | 0.0714 | 0.0715 | 0.0769 | 0.0733 | 0.0719 | 0.0641 | 0.0778 | 0.0701 | 0.0717 | |
| A1 | 0.4360 | 0.0254 | 0.0210 | 0.0243 | 0.0284 | 0.0270 | 0.0267 | 0.0339 | 0.0279 | 0.0124 | 0.0206 | |
| A2 | 0.4924 | 0.1110 | 0.0281 | 0.0315 | 0.0281 | 0.0331 | 0.0346 | 0.0373 | 0.0232 | 0.0283 | 0.0282 | |
| A3 | 0.4679 | 0.0794 | 0.1157 | 0.0299 | 0.0332 | 0.0261 | 0.0298 | 0.0307 | 0.0325 | 0.0252 | 0.0035 | |
| A4 | 0.4708 | 0.0957 | 0.1324 | 0.1207 | 0.0333 | 0.0329 | 0.0300 | 0.0358 | 0.0329 | 0.0268 | 0.0299 | |
| A5 | 0.4993 | 0.1106 | 0.1021 | 0.1314 | 0.1307 | 0.0338 | 0.0388 | 0.0409 | 0.0278 | 0.0305 | 0.0329 | |
| A6 | 0.4535 | 0.1115 | 0.1396 | 0.1004 | 0.1388 | 0.1308 | 0.0325 | 0.0335 | 0.0347 | 0.0299 | 0.0265 | |
| A7 | 0.4769 | 0.1130 | 0.1601 | 0.1223 | 0.1220 | 0.1695 | 0.1347 | 0.0321 | 0.0379 | 0.0288 | 0.0300 | |
| A8 | 0.3857 | 0.1569 | 0.1767 | 0.1340 | 0.1538 | 0.1875 | 0.1381 | 0.1366 | 0.0405 | 0.0366 | 0.0311 | |
| A9 | 0.4906 | 0.1071 | 0.0708 | 0.1238 | 0.1210 | 0.0872 | 0.1368 | 0.1610 | 0.1823 | 0.0311 | 0.0321 | |
| CL1 | 0.4479 | 0.0481 | 0.1150 | 0.0921 | 0.1009 | 0.1133 | 0.1146 | 0.1202 | 0.1620 | 0.1146 | 0.0252 | |
| CL2 | 0.4643 | 0.0716 | 0.1078 | 0.0102 | 0.1153 | 0.1249 | 0.0968 | 0.1169 | 0.1297 | 0.1153 | 0.0860 |
| B1 | B2 | B3 | B4 | B5 | CL2 | CL1 | |
|---|---|---|---|---|---|---|---|
| B1 | 0.0220 | 0.0199 | 0.0199 | 0.0244 | 0.0267 | 0.0075 | |
| B2 | 0.1273 | 0.0221 | 0.0238 | 0.0231 | 0.0244 | 0.0227 | |
| B3 | 0.1241 | 0.1361 | 0.0165 | 0.0271 | 0.0295 | 0.0205 | |
| B4 | 0.1310 | 0.1492 | 0.1036 | 0.0249 | 0.0287 | 0.0204 | |
| B5 | 0.1484 | 0.1385 | 0.1633 | 0.1498 | 0.0090 | 0.0249 | |
| CL2 | 0.1625 | 0.1466 | 0.1804 | 0.1699 | 0.0365 | 0.0272 | |
| CL1 | 0.0470 | 0.1305 | 0.1320 | 0.1369 | 0.1540 | 0.1642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cádiz, L.; Guzmán, M.; Rivera, P.; Navarrete, F.; Torres, P.; Hidalgo, H. First Isolation, Molecular Identification, and Phylogenetic Characterization of A3B5 Very Virulent Infectious Bursal Disease Virus in Pullets in Chile. Microbiol. Res. 2025, 16, 31. https://doi.org/10.3390/microbiolres16020031
Cádiz L, Guzmán M, Rivera P, Navarrete F, Torres P, Hidalgo H. First Isolation, Molecular Identification, and Phylogenetic Characterization of A3B5 Very Virulent Infectious Bursal Disease Virus in Pullets in Chile. Microbiology Research. 2025; 16(2):31. https://doi.org/10.3390/microbiolres16020031
Chicago/Turabian StyleCádiz, Leandro, Miguel Guzmán, Paola Rivera, Fernando Navarrete, Paulina Torres, and Héctor Hidalgo. 2025. "First Isolation, Molecular Identification, and Phylogenetic Characterization of A3B5 Very Virulent Infectious Bursal Disease Virus in Pullets in Chile" Microbiology Research 16, no. 2: 31. https://doi.org/10.3390/microbiolres16020031
APA StyleCádiz, L., Guzmán, M., Rivera, P., Navarrete, F., Torres, P., & Hidalgo, H. (2025). First Isolation, Molecular Identification, and Phylogenetic Characterization of A3B5 Very Virulent Infectious Bursal Disease Virus in Pullets in Chile. Microbiology Research, 16(2), 31. https://doi.org/10.3390/microbiolres16020031






