Abstract
Introduction: Antimicrobial resistance (AMR) is a global public health issue, particularly in resource-limited, low- to middle-income countries like Bangladesh. In this study, we analyze and present four years of data on AMR from a tertiary care hospital in Bangladesh to inform policymakers and the wider community. Methods: In a retrospective cross-sectional study, we collected data for 4403 bacterial isolates reported between January 2017 and February 2020 at Mymensingh Medical College Hospital (MMCH), Bangladesh. All data were entered, cleaned, and analyzed using the software Stata Version-16.0, WHONET, a microbiology laboratory data management solution, and Quick Analysis of Antimicrobial Patterns and Trends (QAAPT), an AMR data visualization platform. Results: The bacteria were most commonly isolated from urine (71.66%, n = 3155), followed by pus (11.63%, n = 512), sputum (6.70%, n = 295), wound swabs (6.70%, n = 295), stool (1.91%, n = 84), endotracheal aspirate (1.20%, n = 53), and blood (0.20%, n = 9). Gram-negative bacteria predominated in all samples. Escherichia coli was the most common Gram-negative bacterium (31.30%, n = 1378), while Staphylococcus aureus was the most common Gram-positive bacterium (4.38%, n = 193). Antimicrobial susceptibility testing (AST) showed that multidrug resistance in Gram-negative bacteria such as E. coli, Klebsiella sp., and Acinetobacter sp. was common. S. aureus exhibited high resistance rates for beta-lactams, macrolides, and quinolones. In the urine samples, E. coli demonstrated high resistance to antibiotics like amoxicillin/clavulanic acid, ciprofloxacin, ceftriaxone, and cefuroxime (60–100%). Critical and high-priority pathogens as listed by the WHO constituted approximately 60% of the isolates. The AMR trends over three months showed increased resistance to amoxicillin/clavulanic acid for E. coli and to cefuroxime for Klebsiella sp. For S. aureus, the resistance to ciprofloxacin increased over three years, while the resistance to azithromycin decreased. Conclusions: There is a rise in bacterial resistance to the available antibiotics, with a significant prevalence of critical and high-priority pathogens in Bangladesh. We recommend vigilant AMR surveillance and stewardship programs to control the AMR in this country.
1. Introduction
Antimicrobial resistance (AMR) is currently considered an alarming issue in the world, and Bangladesh is at risk of AMR due to the poor healthcare facilities, the absence of proper guidelines, the overuse of antibiotics, and the inadequate sanitation and hygiene for both humans and animals [1,2]. At present, the rate of AMR is increasing rapidly in Bangladesh owing to the inadequate diagnosis of diseases, a human knowledge gap, improper use, inappropriate prescriptions, and the random application of antimicrobials to human and animal health sectors [3]. There has recently been an upsurge in AMR, making the treatment of common pathogens challenging, consequently resulting in long-term illness [4]. Besides this, the development of newer types of antibiotics has also declined [5]. Drug-resistant microorganisms cause several severe health complications, resulting in serious morbidity and death, increasing the overall treatment costs [6,7]. The proportion of hospital-acquired infections and other health complications is rising dreadfully due to the increasing number of antibiotic-resistant microorganisms; however, the importance of this matter often gets little attention from primary healthcare providers [8].
Ample antibiotics are frequently used in hospitals for immunodeficient or diseased individuals. Hence, a hospital is considered a central point for the emergence of AMR by providing a suitable environment for acquiring resistant pathogens from different sources, such as other patients, shared apparatus, and hospital employees [9]. One of the most economical measures to prevent AMR is properly implementing infection and prevention control (IPC) [10]. Considering this, the WHO has launched a tool titled the “Infection Prevention and Control Assessment Framework” (IPCAF) to assess, analyze, and enhance healthcare facilities’ IPC measures and activities [11]. Using this tool, a study demonstrated that an audit system and the monitoring of IPC were not available in about 90% of the selected hospitals in Bangladesh and that IPC surveillance systems were completely absent [12]. Despite their great importance, IPC measures have still not been appropriately considered in Bangladesh’s hospital settings, which is one of the solid reasons for the rising AMR incidence.
Additionally, to better understand and respond to AMR patterns and critical drivers, the information about the incidence and prevalence of AMR must be comprehensible. Other predominant reasons for the emergence of AMR are the absence of a stringent action plan and the lack of standard AMR surveillance systems [13]. A global emphasis on surveillance and evidence-based research is necessary to address the growing health security challenges of AMR. Therefore, government and non-government agencies should also pursue innovative research and promote surveillance systems to establish the causes and impacts of AMR [14].
Furthermore, policies and strategies that support the rational and proper use of antimicrobials are necessary for fruitful interventions to suppress the development and extent of AMR in Bangladesh. However, to contain AMR, Bangladesh has developed a national action plan (NAP) per the guidelines of the WHO global action plan [15]. The NAP (2017–2022) is based on national strategies covering human health, animal health, and environmental areas developed by national stakeholders for AMR containment in Bangladesh [16]. However, a detailed study of the current AMR situation in Bangladesh is required before the NAP can be implemented successfully. Although small-scale studies have been carried out on antibiotic usage, consumption and antibiogram patterns, and actions to contain them in the human health sectors of Bangladesh, very few studies have examined the issue considerably [17,18,19]. Due to the establishment of AMR surveillance, emphasized in the NAP, a five-year nationwide AMR surveillance program was started for the first time in Bangladesh in 2016. The surveillance began with case-based surveillance conducted by the Institute of Epidemiology, Disease Control and Research (IEDCR) in 11 sentinel sites nationwide, including 10 public and 1 private hospital [17].
Moreover, to tackle this horrifying upsurge in the AMR incidence, the WHO has prompted healthcare providers to implement antimicrobial stewardship programs to restrain the inappropriate use of antimicrobials [12]. However, before implementing antimicrobial stewardship programs, it is obligatory to understand and gain knowledge of the AMR profiles and diverse related issues [20].
Despite different studies in Bangladesh on AMR patterns, no significant research has been conducted on a large scale at the Mymensingh Medical College Hospital (MMCH), a government-run institution providing clinical services to more than 1000 outpatients per day. Therefore, this first-ever large-scale study aims to depict the current patterns and trends of resistance shown by the most clinically significant pathogens and to provide a comprehensive idea of their resistance patterns in the MMCH, and further intends to focus on the gaps in laboratory diagnosis, providing a reference work to guide our policymakers and clinicians towards the implementation of the best strategy to lower the AMR rate in Bangladesh.
2. Methods
2.1. Study Design and Setting
The CAPTURA project activities at the MMCH took place between May 2020 and December 2022. Retrospective data were collected from January 2017 to February 2020 in the Department of Microbiology at the Mymensingh Medical College Hospital. The clinical profiles of the patients, including age, sex, date of specimen collection, specimen type, and location from both inpatient and outpatient departments, were recorded in handwritten register books. All specimens were sent to the laboratory for routine microbiological investigations and antimicrobial susceptibility testing (AST).
2.2. Isolation and Identification of Bacteria
A total of 21,523 clinical specimens were received during the study period. The data on the microorganisms’ culture were collected from different specimens such as urine, blood, wound swabs, pus, stool, sputum, and tracheal aspirations. For the primary isolation, all samples were inoculated in three culture media—blood agar, MacConkey agar, and nutrient agar—followed by aerobic incubation at 37 °C for 24 h. For the blood samples, the traditional culture medium trypticase soy broth was used for the primary inoculation, followed by aerobic incubation at 37 °C for 24 h. The cultures were examined for growth for five successive days. The isolation and identification of the microorganisms were carried out using the standard operating procedures (SOPs). For the Gram-positive bacterial identification, we used catalase, coagulase, bile esculin, and mannitol salt agar test for the isolation. For the Gram-negative bacterial isolation, we used the oxidase test, triple sugar iron (TSI), motility indole urease (MIU), citrate agar test, etc.
2.3. Antimicrobial Susceptibility Testing
The antimicrobial susceptibility testing was performed using a disk diffusion method following the updated version of the Clinical and Laboratory Standards Institute (CLSI) guidelines. All the susceptibility results of the isolates were recorded in a logbook.
2.4. Data Collection and Management
The laboratory kept records in manual registers with patient identifiers, demographic information, and laboratory test results in the specified periods. The WHONET software, developed by the WHO Collaboration Centre for Surveillance of AMR, was installed and configured at this laboratory, and trained laboratory technologists entered the data between November 2020 and March 2021. WHONET is free software that is currently used in over 2300 microbiology laboratories all over the world for the purpose of data management and analysis [21].
2.5. Data Management and Analysis
A standard WHONET data quality report was used to find the records missing key data elements or with outlier or unlikely values. The data were curated using the DB Browser for SQLite version 1 software [22]. Then, the WHONET raw data were extracted and uploaded into the Quick Analysis of Antimicrobial Patterns and Trends (QAAPT) software [23]. The statistical data analysis software Stata version 16.0 was used to measure the demographics and isolate the distributions. The QAAPT application was used to determine the trends and patterns [24].
2.6. Inclusion and Exclusion Criteria
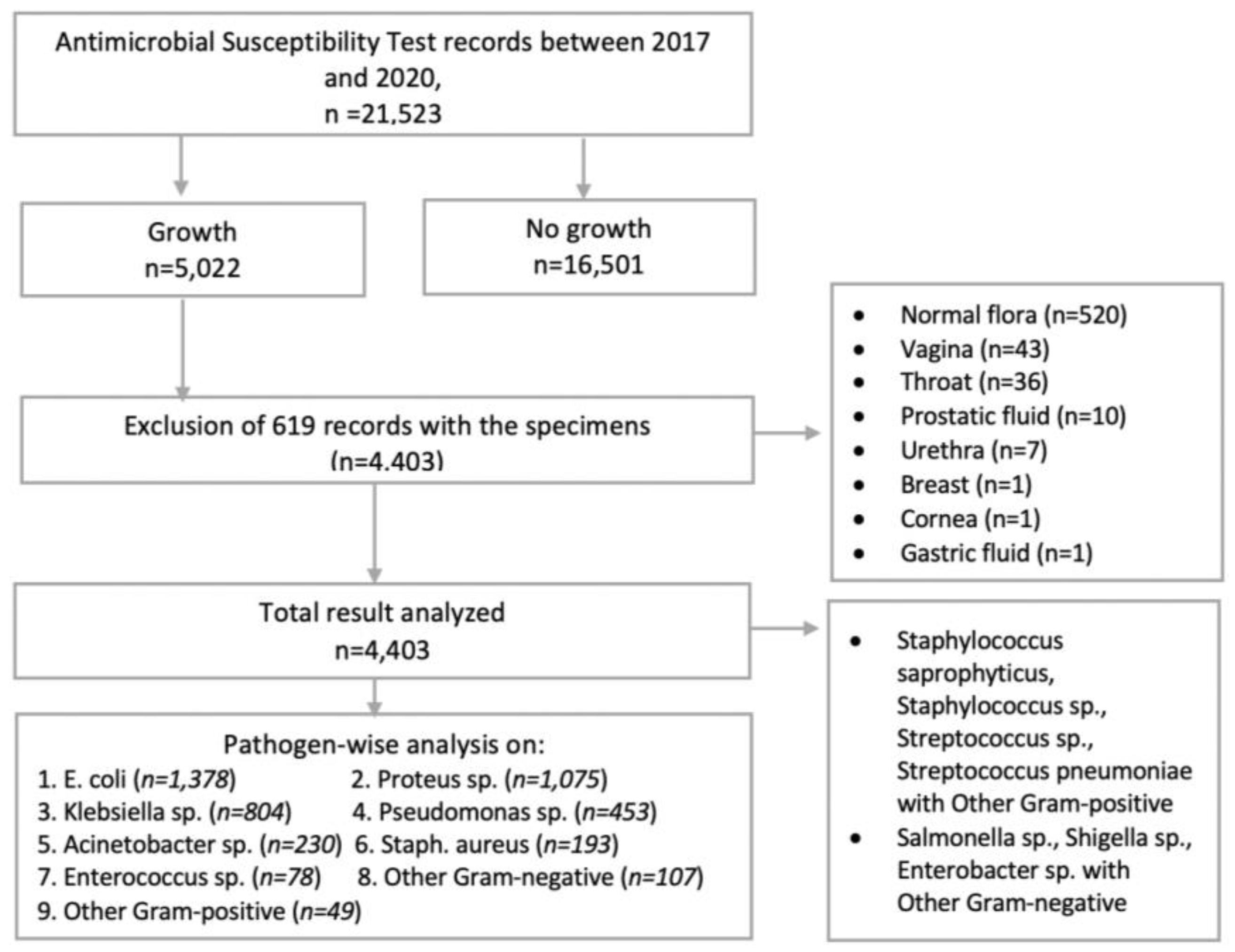
All positive isolates from samples of the inpatient and outpatient departments of hospitals over the study period were included in this study. The incomplete records (e.g., missing age and sex) and the records with normal flora were excluded. The positive results from vaginal fluid, throat swabs, prostatic fluid, urethral swab, breast swabs, corneal fluid, and gastric fluid were also excluded due to the low number of samples and the incomplete data records (Figure 1).
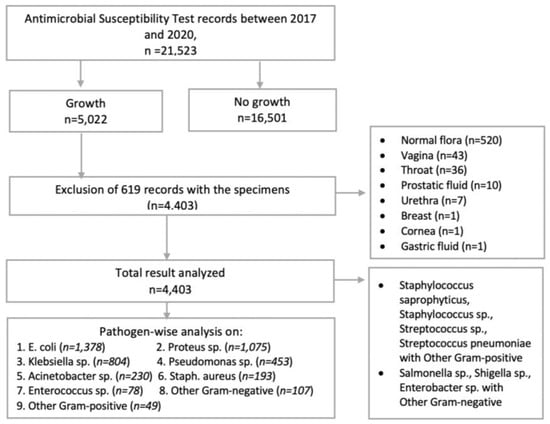
Figure 1.
Flowchart showing the total number of clinical specimens received for microbiological culture and the inclusion of isolates for analysis.
2.7. Quality Control
The American Type Culture Collection (ATCC) reference strains were used for the quality control for the bacterial culture and drug susceptibility testing. The data were thoroughly reviewed by experienced microbiologists and data management specialists using WHONET standard reports.
2.8. Ethical Consideration
The CAPTURA consortium project received official approval in Bangladesh from the Communicable Disease Control (CDC) Programme, the Directorate General of Health Services (DGHS), and the Ministry of Health and Family Welfare (MoHFW) on 17 May 2020 under the reference number DGHS/DC/ARC/2020/1708. This approval was granted for CAPTURA and its project activities in the country as part of the Fleming Fund Regional Grant. Before the data collection commenced, a tri-party collaborative agreement was established on 24 November 2020 among the DGHS, the MMCH, and the International Vaccine Institute (IVI)–CAPTURA.
The CAPTURA project was exempt from ethical review by the Institutional Review Board (IRB) of the IVI because the project did not involve intervention or interaction with individuals and the information collected was not individually identifiable. This exemption is per the IVI IRB SOP D-RB-4-003. The CAPTURA project undertook the retrospective data collection and curation, and the authors used the digitized data to prepare this manuscript.
3. Results
3.1. Demographic Characteristics
A total of 4403 positive isolates were included in this study (Figure 1). Of these, 61.14% (n = 2692) were female and 38.86% (n = 1711) were male. Most isolates were obtained from patients aged 5–14 years (17.49%, n = 770), followed by 15–24 years (14.97%, n = 659), 25–34 years (14.97%, n = 659), and 35–44 years (12.22%, n = 538). The overall mean age and standard deviation was 29.27 ± 20.87 years. The highest number of isolates was recorded in 2019 (37.34%, n = 1644). Due to the COVID-19 pandemic, the lowest number of tests was conducted in 2020 (Table 1).

Table 1.
Demographic characteristics of patients and clinical–microbiological profile of bacterial isolates.
3.2. Frequency of Bacterial Isolates
More than half of the microorganisms were isolated from inpatients (53.96%, n = 2376), while outpatients contributed 46.04% (n = 2027). Among the seven types of specimens, 71.66% (n = 3155) isolates were from urine, pus (11.63%, n = 512), sputum (6.70%, n = 295), wound swabs (6.70%, n = 295), stool (1.91%, n = 84), endotracheal aspirate (1.20%, n = 53), and blood (0.20%, n = 9). Of the growth results, the most common bacteria isolated were E. coli (31.30%, n = 1378), followed by Proteus sp. (24.42%, n = 1075), Klebsiella sp. (19.08%, n = 840), Pseudomonas sp. (10.29%, n = 453), Acinetobacter sp. (5.22%, n = 230), S. aureus (4.38%, n = 193), and Enterococcus sp. (1.77%, n = 78) (Table 1). Similarly, Table 2. shows the overall distribution of the identified bacterial pathogens from the different clinical samples at the MMCH. In this study, most isolates were from Gram-negative bacteria (92.74%, n = 4083), while the remaining were from Gram-positive bacteria (7.26%, n = 320). From urine culture, E. coli was the predominant pathogen (40.48%, n = 1277). Most of the pus-producing microorganisms were S. aureus (20.51%, n = 105). Klebsiella sp. was frequently isolated in both sputum (56.27%, n = 166) and endotracheal aspirates (41.51%, n = 22).

Table 2.
Distribution of bacterial isolates with the specimens, 2017–2020 (n = 4403).
3.3. Antimicrobial Resistance Patterns
All the isolates were routinely tested for susceptibility against amoxicillin/clavulanic acid, ampicillin, cefuroxime, ceftazidime, cefixime, trimethoprim/sulfamethoxazole, azithromycin, ceftriaxone, aztreonam, ciprofloxacin, and others. The majority of the pathogens showed high levels of resistance to the routinely used antimicrobials. E. coli was resistant to ampicillin (95.05%), cefuroxime (90.28%), amoxicillin/clavulanic acid (80.66%), trimethoprim/sulfamethoxazole (72.12%), ceftazidime (68.23%), azithromycin (53.69%), and ciprofloxacin (46.31%). The resistance was lowest for imipenem (14.29%) and meropenem (14.17%). Likewise, Klebsiella sp. showed a high resistance rate to the major drugs ampicillin, amoxicillin/clavulanic acid, azithromycin, cefuroxime, cefixime, ceftazidime, trimethoprim/sulfamethoxazole, and aztreonam at 100%, 82.34%, 47.69, 92.21%, 77.12%, 80.56%, 73.00%, and 72.73%, respectively. Proteus sp. was resistant to the valuable drugs ampicillin (96.23%), amoxicillin/clavulanic acid (81.14%), cefuroxime (91.45%), trimethoprim/sulfamethoxazole (72.57%), cefixime (71.96%), azithromycin (60.84%), and aztreonam (63.30%) over the period. For pseudomonal infection, the resistance rates of anti-pseudomonal antibiotics like ceftazidime, piperacillin–tazobactam, and cefepime were alarmingly high at 80.80%, 66.86%, and 81.25%, respectively. The methicillin-resistant S. aureus (MRSA) rate was alarmingly high at 85.70%. The resistance to the WHO-defined “reserve” agent linezolid was 24.70% in S. aureus and 48.21% in Enterococcus sp. (Table 3).

Table 3.
Average trend of antimicrobial resistance among bacteria reported during 2017–2020.
3.4. Antimicrobial Resistance Trends
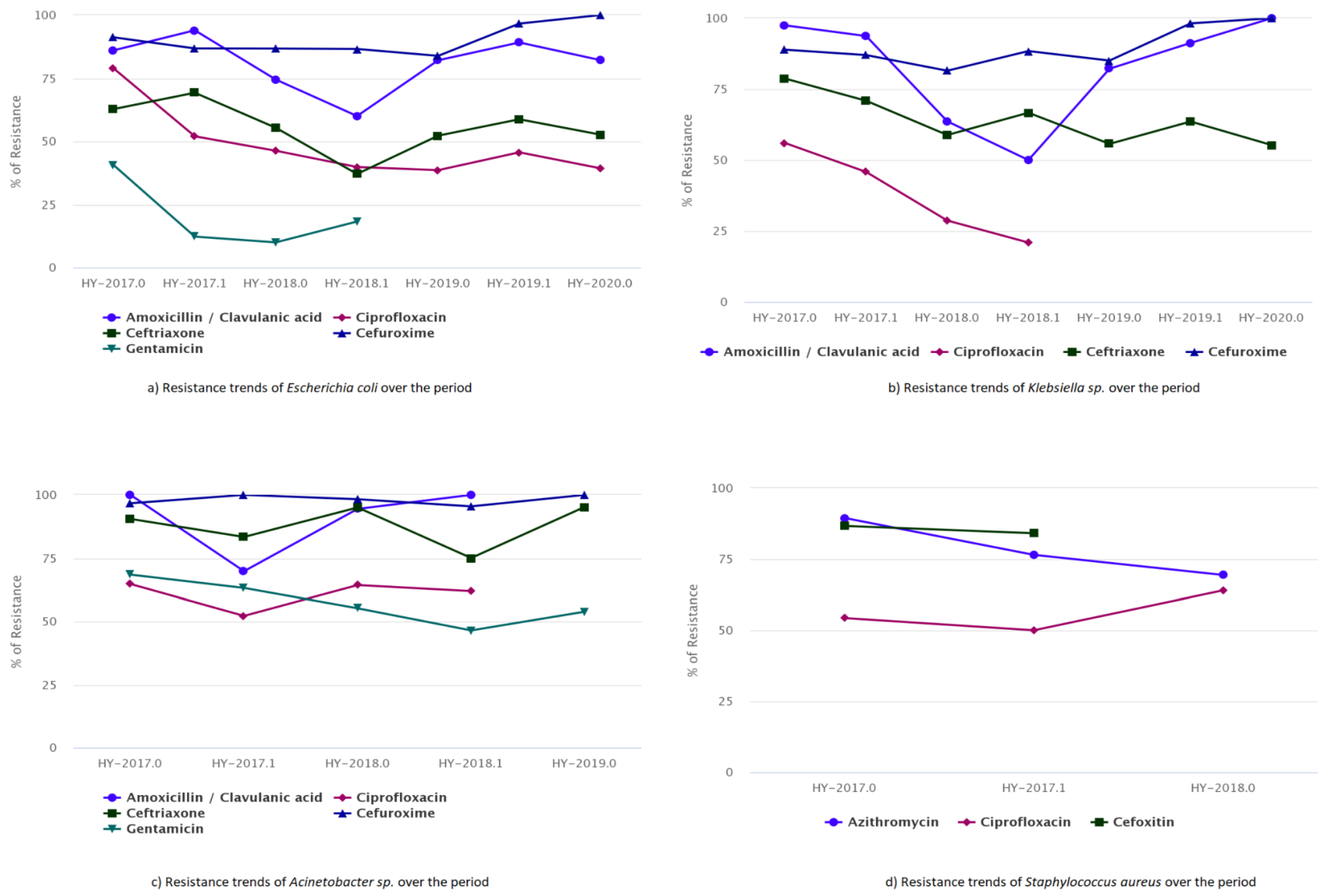
The trends of resistance in five antimicrobials (amoxicillin/clavulanic acid, ciprofloxacin, ceftriaxone, cefuroxime, and gentamicin) for E. coli is presented in Figure 2a. The resistance patterns for ciprofloxacin, cefuroxime, and ceftriaxone did not change significantly over the year. However, the resistance to most drugs used in hospitals, such as amoxicillin/clavulanic acid, appeared to rise over time (Figure 2a). Similarly, the increasing trends in Klebsiella sp. show resistance of up to 100% for cefuroxime. The resistance trend fluctuated for ceftriaxone, while ciprofloxacin showed a decreasing resistance rate for two consecutive years (Figure 2b). The drug amoxicillin/clavulanic acid showed high resistance at the beginning of the study period, which decreased in successive years before again reaching up to 100% resistance. It should be noted that the data for 2020 covers only two months, making it difficult to draw definitive conclusions.
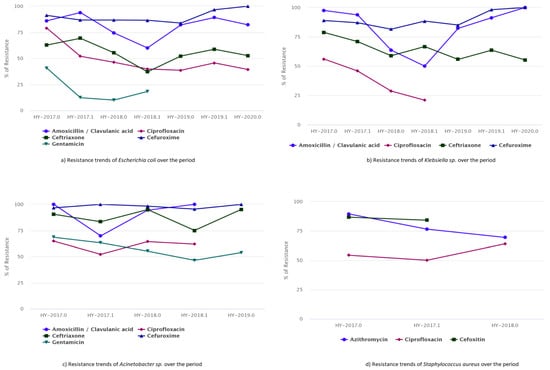
Figure 2.
Trends of antibiotic susceptibility to the five most common bacteria, (a) Resistance trends of E. coli, (b) Resistance trends of Klebsiella sp., (c) Resistance trends of Acinetobacter sp., and (d) Resistance trends of S. aureus, over the period of 2017–2020.
Similarly, the drug resistance curve for Acinetobacter sp. was relatively high, increasing from approximately 60.00% to 100% over the study period for the drugs amoxicillin/clavulanic acid, ciprofloxacin, ceftriaxone, cefuroxime, and gentamicin (Figure 2c). S. aureus exhibited minimal drug resistance (Figure 2d). The antibiogram data analysis showed that azithromycin drug resistance decreased from 89.00% to 69.00%, whereas ciprofloxacin resistance showed increasing trends from 54.00% to 64.00%. MRSA was quite common in hospital-admitted patients, and its prevalence was in part due to poor infection control in the hospital area. Healthcare-associated MRSA was found to be more drug resistant.
4. Discussion
AMR is considered a top 10 threat to human as well as animal health, and it has been estimated that it may cause up to 10 million deaths globally per year by 2050 in the absence of action [25]. Therefore, continual surveillance is recommended by the World Health Organization (WHO) to control the emergence of resistance to different pathogens [25]. Despite the urgency of investigating the trends and patterns of AMR, very limited studies have been reported on the resistance trends in pathogens in Mymensingh, Bangladesh. However, the lack of sufficient data on antibiotic resistance makes it difficult to clearly and precisely understand the magnitude of the problem. In this study, we aimed to explain the AMR patterns and trends of the most common microorganisms at the MMCH, hoping that the knowledge and evidence of the AMR pattern might assist physicians and local administrations to seek solutions to the AMR problems in Bangladesh. In addition, this study allowed us to identify errors in the data and to assess the gaps in data quality in the hospital. These findings could be used to periodically monitor and investigate the hospital’s AMR trends and patterns. Our analysis was based on retrospective data; however, due to the COVID-19 pandemic, the AMR data were limited to 2020.
Similar findings for uropathogens were reported by Afroz et al. [18] and Haque et al. [26]. Amoxicillin/clavulanic acid, the commonly used antibiotic for E. coli, was 80.50% resistant, which was consistent with the 89.40% resistance reported by Nobel et al. in the Mymensingh district, Bangladesh [19]. Considering the cephalosporin group, the resistance patterns of cefuroxime, cefixime, ceftazidime, and ceftriaxone were 90.28%, 71.24%, 68.23%, and 57.46%, respectively, according to this study. Second- and third-generation cephalosporin groups have been used for prolonged periods in hospital areas. Due to the indiscriminate and overuse of these groups over time, the microorganisms have developed significant resistance against these antibiotics [27].
Proteus sp. was the second most commonly found microorganism among the isolates in the hospital samples. It was seen that cefuroxime and trimethoprim/sulfamethoxazole were ineffective against 91.45% and 72.57% of the tested Proteus sp. isolates, respectively. These results were similar to those reported by Ahmed et al. [1].
Another Gram-negative bacterium, Klebsiella sp., was mainly found as a bacterial pathogen from sputum (56.27%), which was consistent with another study conducted by Rahman et al. [28], where Klebsiella sp. was the most prevalent (32.00%) among the bacterial pathogens in adult patients. These data suggest that most isolates of Klebsiella sp. are resistant to many antibiotics, specifically 50–90% resistance to amoxicillin/clavulanic acid, cefuroxime, ceftazidime, trimethoprim/sulfamethoxazole, aztreonam, and ceftriaxone. This result was very similar to a study by Tanni et al. [29], where Klebsiella sp. had shown 40–80% resistance to the cephalosporin agents cefuroxime, ceftriaxone, and ceftazidime from different samples. A lower resistance (20–30%) to gentamicin, imipenem, and ciprofloxacin was observed. However, most respiratory infections were caused by Klebsiella sp., especially in immunocompromised patients and in the intensive care unit (ICU), which has higher resistance rates.
In this study, S. aureus exhibited high resistance to penicillin G (95.24%), azithromycin (78.07%), and ciprofloxacin (54.78%). MRSA was detected by susceptibility testing to cefoxitin (85.70%). Similarly, a study by Ahmed et al. [1] showed that S. aureus exhibited high resistance to penicillin (89.70%) and an MRSA proportion of 46.70%—also, a study by F.-P. Hu et al. mentioned that from 2005 to 2014, the rate of MRSA decreased from 69.00% to 44.60%, while the rate of MRSA that we observed was higher and needed more analysis for an extended period (retrospective study) to represent the actual MRSA rate [30].
The multidrug-resistant Acinetobacter sp. was the most prevalent isolate in the hospital, especially in the ICU. In this study, we found that about 60% of the Acinetobacter sp. were significantly resistant to all groups of antibiotics except imipenem, which had a resistance rate of 36.70%, comparable to the study by Ahmed et al. [1]. A study from the MMCH in 2018 by Ifa et al. found the resistance to Acinetobacter sp. was 91.30%, 71.70%, and 60.90% for piperacillin–tazobactam, doxycycline, and colistin, respectively. The imipenem resistance was about 43.50%, showing a similar pattern to our study [31]. Therefore, Acinetobacter sp., a threat for hospital-acquired infections, became increasingly resistant to all usable antibiotics in hospital areas.
For the isolates, Pseudomonas sp. was most frequently found in wound swabs, followed by pus and urine. Our research demonstrated that ciprofloxacin and gentamicin showed 31.03% and 27.78% resistance to Pseudomonas sp., which was in agreement with the study by Ahmed et al., and for gentamicin, we found lower resistance [1].
Regarding the Gram-positive isolates other than S. aureus, a total of 2.19% (n = 69) of the Enterococcus sp. was found in urine samples. Ciprofloxacin is the most commonly prescribed drug for urinary tract infection (UTI) patients by clinicians in Bangladesh, and it exhibited 55.77% resistance [32]. However, nitrofurantoin showed significantly less resistance (16.67%) for Enterococcus sp., suggesting this may be a suitable drug for uropathogens. The previous AMR surveillance study by the IEDCR found a 59.00% and 25.00% resistance to ciprofloxacin and nitrofurantoin, respectively, which was similar to our findings. The resistance of Enterococcus sp. to linezolid was 20.00%, whereas our results demonstrated about 48.21% resistance, which is a matter of concern [17].
The threat of AMR will have a highly negative impact in the future because certain drugs exhibit different resistances. Eventually, there will not be any drugs available for the treatment of infection. Most antibiotics are relatively resistant to specific pathogens, which will pose significant obstacles and challenges in the future when treating people with these medicines for those particular infections. This regional study on AMR trends and patterns is of great importance to policymakers in guiding the treatment plan for bacterial infections and developing suitable antibiotic prescription guidelines to plummet the improper use of antibiotics, thereby addressing the rapidly increasing trend of AMR.
5. Limitations
Our study faced several limitations. First, the number of blood samples was limited, and although the data were included, they were not analyzed due to the small sample size. Additionally, the inconsistent application of the standard guidelines and the variability in the quality of the test reagents compromised the data quality, leading to biases in patient presentation, sample collection, and testing. Routine data collection often underestimates disease rates while overestimating resistance rates. Furthermore, the data were recorded manually in laboratory register books, introducing the potential for human error and affecting data accuracy. The patients’ history and clinical profiles were also missing from the recorded data. Finally, the scope of this study was confined to a specific area, limiting the generalizability of the findings to the entire nation.
6. Conclusions
This study demonstrates that antibiotic resistance among the predominant microorganisms to commonly used antibiotics has increased over the study period in a tertiary care hospital in the Mymensingh area. The extent of this study was limited to a particular area, and the impact of the findings cannot represent the whole nation. Hence, further studies should focus on the judicious use of the available antibiotics and the implementation of proper infection control measures to avoid these strains’ rapid spread or clonal dissemination. Therefore, prompt antimicrobial surveillance needs to be conducted to develop local antibiograms in the hospital so that efficient antimicrobial therapy can be chosen.
Based on our study, we highly recommend replacing the manual recording system with an electronic recording procedure to achieve a sustainable and quality laboratory practice. In this regard, developing electronic data-recording systems is crucial to improve and strengthen the AMR data-sharing practices in the facility in day-to-day practice. Therefore, we strongly suggest that WHONET could be used as a primary tool in the MMCH microbiology laboratory to input their data, and that QAAPT could be used for advanced data visualization and the periodic development of antibiograms.
Author Contributions
M.J.S. reports leading the data collection, management, analysis, interpretation, and coordination with co-authors. S.R. designed and performed the laboratory testing and organized the manuscript writing. H.T.B. performed the data collection and organized the manuscript writing. The manuscript was reviewed by A.H., S.K.P., S.A.N., S.A., N.H., Z.H.H., A.R., S.M.S.R., M.A.H., S.Y.K., J.S., S.G., A.S., F.M. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
The capacity development and data digitization on the WHONET software was performed by the Capturing Data on Antimicrobial Resistance Patterns and Trends in Use in Regions of Asia (CAPTURA) project, funded by the Fleming Fund regional grant, and implemented by the International Vaccine Institute, Seoul, South Korea.
Institutional Review Board Statement
The CAPTURA project was exempt from ethical review by the Institutional Review Board (IRB) of the IVI because the project did not involve intervention or interaction with individuals and the information collected was not individually identifiable. This exemption is per the IVI IRB SOP D-RB-4-003. The CAPTURA project undertook the retrospective data collection and curation, and the authors used the digitized data to prepare this manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available in this link: http://qaapt.com/mmch/manuscript2025_dataset.csv.
Acknowledgments
We would like to express our gratitude to the microbiologists, data entry operators, and laboratory technologists at the microbiology laboratory. We also acknowledge Md. Abul Hasnat, Consultant, CAPTURA, for his invaluable assistance in digitizing the data.
Conflicts of Interest
Author Alina Shaw was employed by the company Public Health Surveillance Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmed, I.; Rabbi, M.B.; Sultana, S. Antibiotic resistance in Bangladesh: A systematic review. Int. J. Infect. Dis. 2019, 80, 54–61. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antimicrobial Resistance 2018 July 17, 2021. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 26 August 2024).
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Mogasale, V.V.; Saldanha, P.; Pai, V.; Rekha, P.D.; Mogasale, V. A descriptive analysis of antimicrobial resistance patterns of WHO priority pathogens isolated in children from a tertiary care hospital in India. Sci. Rep. 2021, 11, 5116. [Google Scholar] [CrossRef]
- Luepke, K.H.; Mohr, J.F. The antibiotic pipeline: Reviving research and development and speeding drugs to market. Expert. Rev. Anti-Infect. Ther. 2017, 15, 425–433. [Google Scholar] [CrossRef]
- Hassan, R.; Gilany, A.H.; Elaal, A.M.; El-Mashad, N.; Abdelazim, D. Antibiotic Resistance Pattern of Bacteria Causing Hospital Acquired Infections in the New Mansoura General Hospital, Egypt. Arch. Community Med. 2021, 3, 16–21. [Google Scholar]
- Azimi, T.; Maham, S.; Fallah, F.; Azimi, L.; Gholinejad, Z. Evaluating the antimicrobial resistance patterns among major bacterial pathogens isolated from clinical specimens taken from patients in Mofid Children’s Hospital, Tehran, Iran: 2013–2018. Infect. Drug Resist. 2019, 12, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Abebe, M.; Tadesse, S.; Meseret, G.; Derbie, A. Type of bacterial isolates and antimicrobial resistance profile from different clinical samples at a Referral Hospital, Northwest Ethiopia: Five years data analysis. BMC Res. Notes 2019, 12, 568. [Google Scholar] [CrossRef]
- Lin, M.; Weinstein, R.; Hayden, M.K. Multidrug-resistant organisms: Epidemiology and control. In Bennett & Brachman’s Hospital Infections, 6th ed.; Wolters Kluwer Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 198–199. [Google Scholar]
- Bernatchez, S.F. Reducing antimicrobial resistance by practising better infection prevention and control. Am. J. Infect. Control 2023, 51, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Storr, J.; Twyman, A.; Zingg, W.; Damani, N.; Kilpatrick, C.; Reilly, J.; Price, L.; Egger, M.; Grayson, M.L.; WHO Guidelines Development Group; et al. Core components for effective infection prevention and control programmes: New WHO evidence-based recommendations. Antimicrob. Resist. Infect. Control 2017, 6, 6. [Google Scholar] [CrossRef]
- Harun, M.G.D.; Anwar, M.M.U.; Sumon, S.A.; Hassan, M.Z.; Haque, T.; Mah-E-Muneer, S.; Rahman, A.; Abdullah, S.A.H.M.; Islam, M.S.; Styczynski, A.R.; et al. Infection prevention and control in tertiary care hospitals of Bangladesh: Results from WHO infection prevention and control assessment framework (IPCAF). Antimicrob. Resist. Infect. Control 2022, 11, 125. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- OECD. Stemming the Superbug Tide; OECD: Paris, France, 2018. [Google Scholar]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. Available online: https://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html (accessed on 26 August 2024).
- Ministry of Health and Family Welfare (MoHFW). G of B. National Action Plan: Antimicrobial Resistance Containment in Bangladesh 2017–22. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/antimicrobial-resistance-containment-in-bangladesh-2017-2022.pdf?sfvrsn=bfa46b_3&download=true (accessed on 22 August 2024).
- Habib, Z.H.; Rasul, S.B.G.; Alam, M.A.; Bably, N.N.; Khan, I.A.; Rizvi, S.M.S.; Shirin, T.; Alam, A.N.; Uzzaman, M.S.; Alamgir, A.; et al. The findings of Antimicrobial Resistance Surveillance in Bangladesh (2016–2020). medRxiv 2021. [Google Scholar] [CrossRef]
- Afroz, S.; Habib, Z.H.; Billah, S.M.B.; Akhter, H.; Jahan, H.; Parveen, R. Spectrum and Antibiotic Resistance Pattern of Bacteria Causing Urinary Tract Infections (UTI) in a Tertiary Care Hospital. J. Surg. Sci. 2020, 23, 13–18. [Google Scholar] [CrossRef]
- Nobel, F.; Akter, S.; Jebin, R.; Sarker, T.; Rahman; Zamane, S.; Islam, K.; Sabrina, S.; Akther, N.; Islam, A.; et al. Prevalence of multidrug resistance patterns of Escherichia coli from suspected urinary tract infection in Mymensingh city, Bangladesh. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 256. [Google Scholar] [CrossRef]
- Bangladesh Post. Bangladesh Post 2020, PM Joins as Co-Chair of Antimicrobial Resistance Group, Bangladesh Post. Available online: https://bangladeshpost.net/posts/pm-joins-as-co-chair-of-antimicrobial-resistance-group-47600 (accessed on 22 August 2024).
- WHONET. Internet. Available online: https://whonet.org (accessed on 10 August 2024).
- SQLite Database Browser. Internet. Available online: https://www.sqlite.org/about.html (accessed on 4 April 2024).
- Quick Analysis of Antimicrobial Patterns and Trends (QAAPT). Internet. Available online: https://qaapt.com (accessed on 5 March 2024).
- Stata Corporation. Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Haque, R.; Akter, M.L.; Salam, M.A. Prevalence and susceptibility of uropathogens: A recent report from a teaching hospital in Bangladesh. BMC Res. Notes 2015, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, S.W.; Kim, K.W.; Song, D.Y.; Kwon, K.T. Third-generation cephalosporin resistance of community-onset Escherichia coli and Klebsiella pneumoniae bacteremia in a secondary hospital. Korean J. Int. Med. 2014, 29, 49. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, R.; Alroy, K.A.; Van Beneden, C.A.; Friedman, M.S.; Kennedy, E.D.; Rahman, M.; Balajee, A.; Muraduzzaman, A.; Shirin, T.; Flora, M.S.; et al. Etiology of Severe Acute Respiratory Infections, Bangladesh, 2017. Emerg. Infect. Dis. 2021, 27, 324–326. [Google Scholar] [CrossRef]
- Tanni, A.A.; Hasan, M.d.M.; Sultana, N.; Ahmed, W.; Mannan, A. Prevalence and molecular characterization of antibiotic resistance and associated genes in Klebsiella pneumoniae isolates: A clinical observational study in different hospitals in Chattogram, Bangladesh. PLoS ONE 2021, 16, e0257419. [Google Scholar] [CrossRef]
- Hu, F.-P.; Guo, Y.; Zhu, D.-M.; Wang, F.; Jiang, X.-F.; Xu, Y.-C.; Zhang, X.-J.; Zhang, C.-X.; Ji, P.; Xie, Y.; et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin. Microbiol. Infect. 2016, 22, S9–S14. [Google Scholar] [CrossRef]
- Ifa, I.A.; Paul, S.K.; Hossain, M.A.; Haque, N.; Ahmed, S.; Nasreen, S.A.; Ahamed, F.; Roy, S.; Sakib, N.; Abedin, S.; et al. Isolation of Acinetobacter species from Clinical Specimens with Detection of Their Antimicrobial Susceptibility Pattern from a Tertiary Care Hospital, Bangladesh. Mymensingh Med. J. 2020, 29, 622–627. [Google Scholar] [PubMed]
- Rahman, M.H.; Ahmed, M.; Sarkar, D.; Rahman, M.A. Prevalence and antibiotic susceptibility pattern of pathogens from urinary tract infections (UTI) in a private diagnostic laboratory in Bangladesh. Asian J. Med. Biol. Res. 2020, 6, 564–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).