In Vitro Evaluation of the Antiviral Properties of Exogenous mRNA Encoding the Human MxA Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Exogenous mRNA by In Vitro Transcription
2.2. Cell Lines
2.3. ELISA and Immunofluorescence Staining
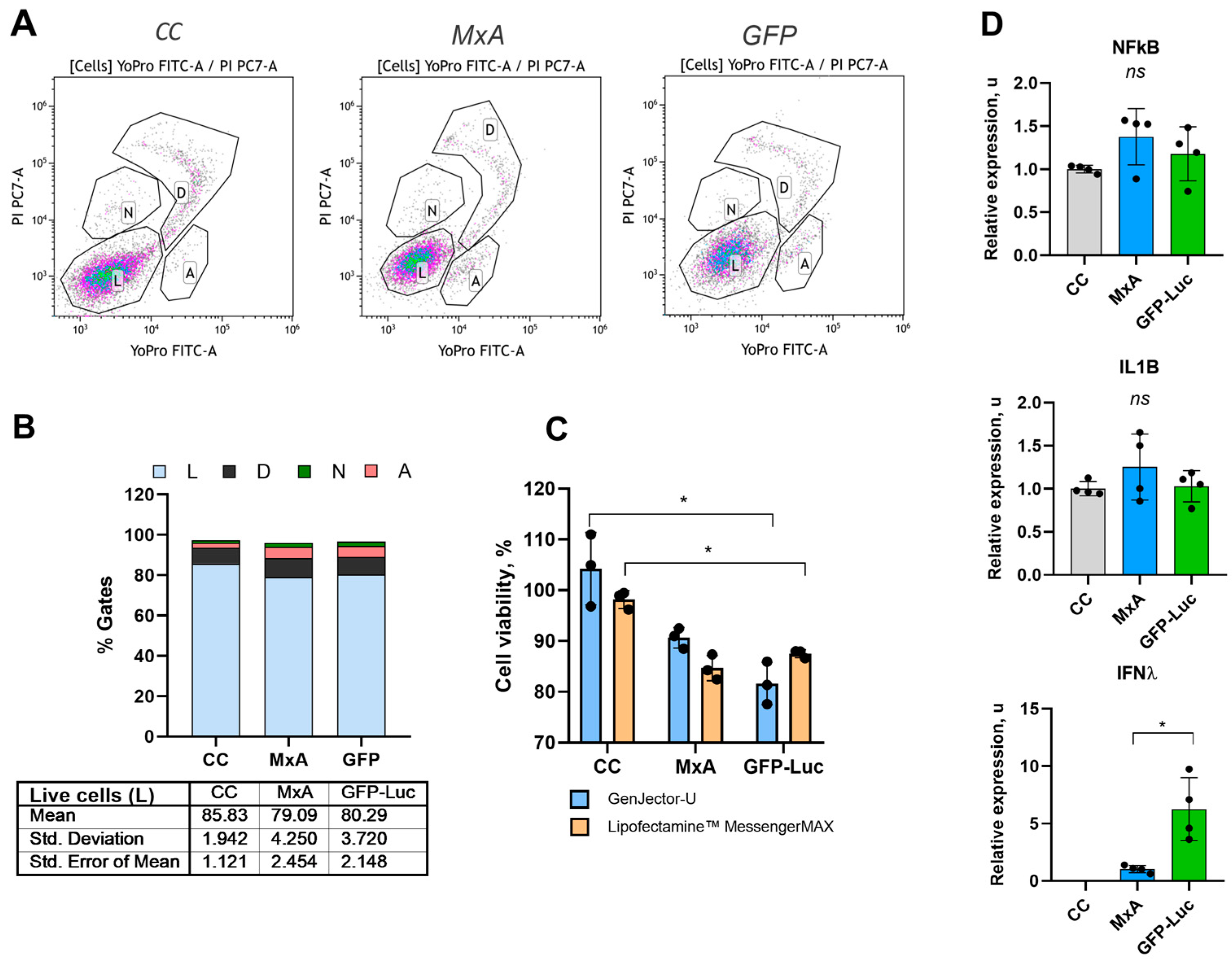
2.4. Cell Viability Assessment
2.5. RT-PCR Analysis
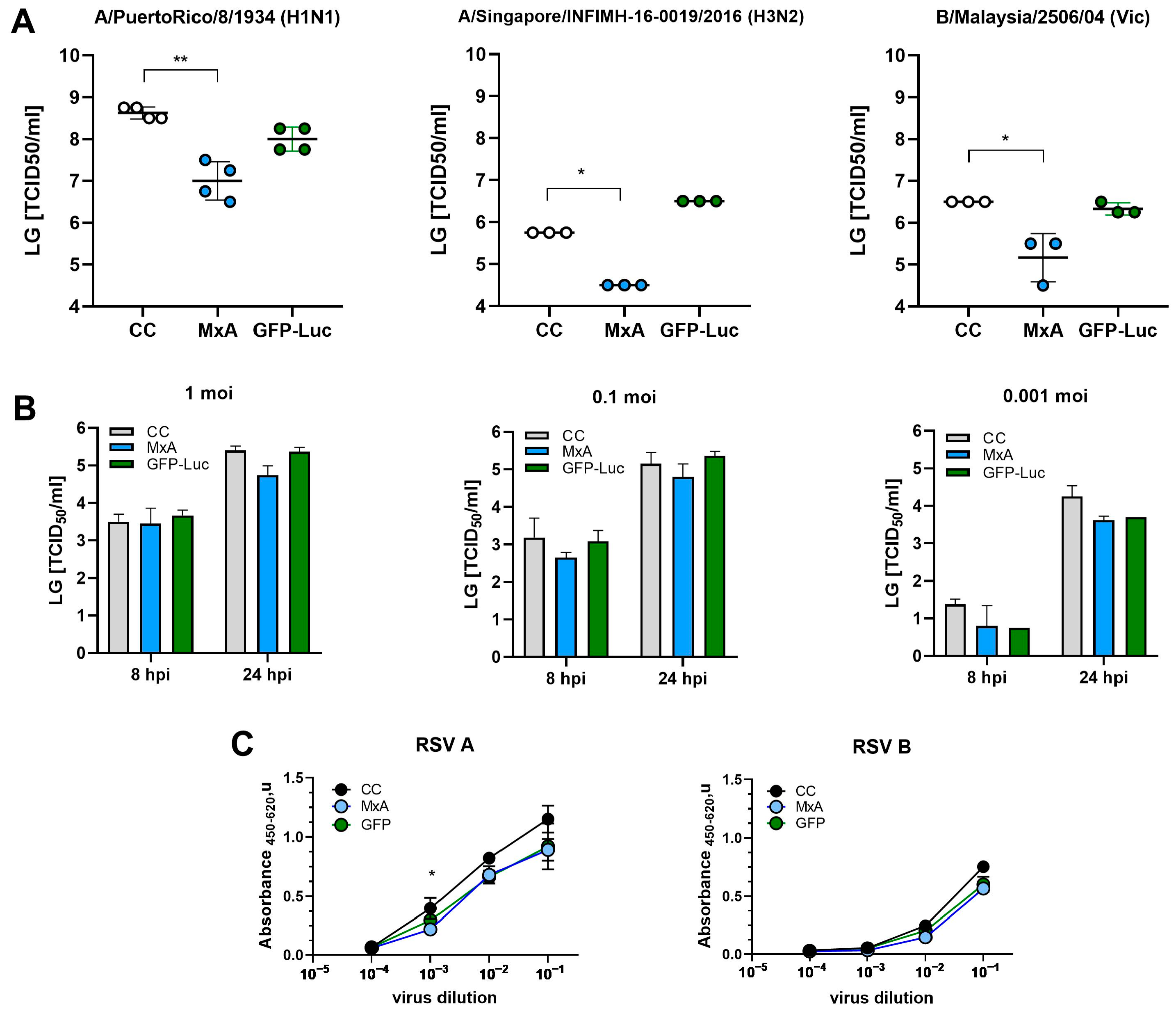
2.6. Antiviral Activity
2.7. Statistical Data Processing
3. Results
3.1. Generating Functional mRNA
3.2. Immunogenicity
3.3. Antiviral Activity of MxA-mRNA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stertz, S.; Reichelt, M.; Krijnse-Locker, J.; Mackenzie, J.; Simpson, J.C.; Haller, O.; Kochs, G. Interferon-induced, antiviral human MxA protein localizes to a distinct subcompartment of the smooth endoplasmic reticulum. J. Interferon Cytokine Res. 2006, 26, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Gao, S. MxA: A broadly acting effector of interferon-induced human innate immunity. Vis. Cancer Med. 2022, 3, 2. [Google Scholar] [CrossRef]
- Verhelst, J.; Hulpiau, P.; Saelens, X. Mx proteins: Antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef]
- Haller, O.; Kochs, G. Mx genes: Host determinants controlling influenza virus infection and trans-species transmission. Hum. Genet. 2020, 139, 695–705. [Google Scholar] [CrossRef]
- Mänz, B.; Dornfeld, D.; Götz, V.; Zell, R.; Zimmermann, P.; Haller, O.; Kochs, G.; Schwemmle, M. Pandemic influenza A viruses escape from restriction by human MxA through adaptive mutations in the nucleoprotein. PLoS Pathog. 2013, 9, e1003279. [Google Scholar] [CrossRef]
- Gao, S.; von der Malsburg, A.; Dick, A.; Faelber, K.; Schröder, G.F.; Haller, O.; Kochs, G.; Daumke, O. Structure of myxovirus resistance protein a reveals intra-and intermolecular domain interactions required for the antiviral function. Immunity 2011, 35, 514–525. [Google Scholar] [CrossRef]
- Dick, A.; Graf, L.; Olal, D.; von der Malsburg, A.; Gao, S.; Kochs, G.; Daumke, O. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J. Biol. Chem. 2015, 290, 12779–12792. [Google Scholar] [CrossRef]
- McKellar, J.; Arnaud-Arnould, M.; Chaloin, L.; Tauziet, M.; Arpin-André, C.; Pourcelot, O.; Blaise, M.; Moncorgé, O.; Goujon, C. An evolutionarily conserved N-terminal leucine is essential for MX1 GTPase antiviral activity against different families of RNA viruses. J. Biol. Chem. 2023, 299, 102747. [Google Scholar] [CrossRef]
- Patzina, C.; Haller, O.; Kochs, G. Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza A virus. J. Biol. Chem. 2014, 289, 6020–6027. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Patzina, C.; Emerman, M.; Haller, O.; Malik, H.S.; Kochs, G. Evolution-guided identification of antiviral specificity determinants in the broadly acting interferon-induced innate immunity factor MxA. Cell Host Microbe 2012, 12, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ryu, J.H. Influenza viruses: Innate immunity and mRNA vaccines. Front. Immunol. 2021, 12, 710647. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Haller, O.T.T.O.; Staeheli, P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 1992, 66, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Zimmermann, O.; Homann, J.M.; Bangert, A.; Müller, A.M.; Hristov, G.; Goeser, S.; Wiehe, J.M.; Zittrich, S.; Rottbauer, W.; Torzewski, J.; et al. Successful Use of mRNA-Nucleofection for Overexpression of Interleukin-10 in Murine Monocytes/Macrophages for Anti-inflammatory Therapy in a Murine Model of Autoimmune Myocarditis. J. Am. Heart Assoc. 2012, 1, e003293. [Google Scholar] [CrossRef]
- Jirikowski, G.F.; Sanna, P.P.; Maciejewski-Lenoir, D.; Bloom, F.E. Reversal of diabetes insipidus in Brattleboro rats: Intrahypothalamic injection of vasopressin mRNA. Science 1992, 255, 996–998. [Google Scholar] [CrossRef]
- Macey, M.G. Principles of flow cytometry. In Flow Cytometry: Principles and Applications; Humana Press: Totowa, NJ, USA, 2007; pp. 1–15. [Google Scholar] [CrossRef]
- Plotnikova, M.A.; Klotchenko, S.A.; Vasin, A.V. Development of a multiplex quantitative PCR assay for the analysis of human cytokine gene expression in influenza A virus-infected cells. J. Immunol. Methods 2016, 430, 51–55. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Krivitskaya, V.; Petrova, E.; Sorokin, E.; Tsareva, T.; Sverlova, M.; Komissarova, K.; Sominina, A.; Danilenko, D. Characterization of a panel of monoclonal antibodies targeting the F-protein of the respiratory syncytial virus (RSV) for the typing of contemporary circulating strains. Trop. Med. Infect. Dis. 2023, 9, 1. [Google Scholar] [CrossRef]
- Mamaghani, S.; Penna, R.R.; Frei, J.; Wyss, C.; Mellett, M.; Look, T.; Weiss, T.; Guenova, E.; Kündig, T.M.; Lauchli, S.; et al. Synthetic mRNAs containing minimalistic untranslated regions are highly functional in vitro and in vivo. Cells 2024, 13, 1242. [Google Scholar] [CrossRef]
- Leppek, K.; Das, R.; Barna, M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Weissman, D. Naturally occurring nucleoside modifications suppress the immunostimulatory activity of RNA: Implication for therapeutic RNA development. Curr. Opin. Drug Discov. Dev. 2007, 10, 523. [Google Scholar]
- Wang, Y.S.; Kumari, M.; Chen, G.H.; Hong, M.H.; Yuan, J.P.Y.; Tsai, J.L.; Wu, H.C. mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar] [CrossRef]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl (3′-O-methyl) GpppG and 7-methyl (3′-deoxy) GpppG. RNA 2001, 7, 1486–1495. [Google Scholar]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck Jr, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef]
- Lukacikova, L.; Oveckova, I.; Betakova, T.; Laposova, K.; Polcicova, K.; Pastorekova, S.; Pastorek, J.; Tomaskova, J. Antiviral effect of interferon lambda against lymphocytic choriomeningitis virus. J. Interferon Cytokine Res. 2015, 35, 540–553. [Google Scholar] [CrossRef]
- Wang, J.; Oberley-Deegan, R.; Wang, S.; Nikrad, M.; Funk, C.J.; Hartshorn, K.L.; Mason, R.J. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-λ1) in response to influenza A infection. J. Immunol. 2009, 182, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Bizzotto, J.; Sanchis, P.; Abbate, M.; Lage-Vickers, S.; Lavignolle, R.; Toro, A.; Olszevicki, S.; Sabater, A.; Cascardo, F.; Vazquez, E.; et al. SARS-CoV-2 infection boosts MX1 antiviral effector in COVID-19 patients. Iscience 2020, 23. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Feng, D.; de Vlas, S.J.; Wang, H.; Fontanet, A.; Zhang, P.; Plancoulaine, S.; Tang, F.; Zhan, L.; Yang, H.; et al. Association of SARS susceptibility with single nucleic acid polymorphisms of OAS1 and MxA genes: A case-control study. BMC Infect. Dis. 2006, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Sa Ribero, M.; Jouvenet, N.; Dreux, M.; Nisole, S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef]
- Atreya, P.L.; Kulkarni, S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology 1999, 261, 227–241. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikova, M.A.; Romanovskaya-Romanko, E.A.; Pulkina, A.A.; Shuklina, M.A.; Shurygina, A.-P.S.; Klotchenko, S.A. In Vitro Evaluation of the Antiviral Properties of Exogenous mRNA Encoding the Human MxA Protein. Microbiol. Res. 2025, 16, 32. https://doi.org/10.3390/microbiolres16020032
Plotnikova MA, Romanovskaya-Romanko EA, Pulkina AA, Shuklina MA, Shurygina A-PS, Klotchenko SA. In Vitro Evaluation of the Antiviral Properties of Exogenous mRNA Encoding the Human MxA Protein. Microbiology Research. 2025; 16(2):32. https://doi.org/10.3390/microbiolres16020032
Chicago/Turabian StylePlotnikova, Marina A., Ekaterina A. Romanovskaya-Romanko, Anastasia A. Pulkina, Marina A. Shuklina, Anna-Polina S. Shurygina, and Sergey A. Klotchenko. 2025. "In Vitro Evaluation of the Antiviral Properties of Exogenous mRNA Encoding the Human MxA Protein" Microbiology Research 16, no. 2: 32. https://doi.org/10.3390/microbiolres16020032
APA StylePlotnikova, M. A., Romanovskaya-Romanko, E. A., Pulkina, A. A., Shuklina, M. A., Shurygina, A.-P. S., & Klotchenko, S. A. (2025). In Vitro Evaluation of the Antiviral Properties of Exogenous mRNA Encoding the Human MxA Protein. Microbiology Research, 16(2), 32. https://doi.org/10.3390/microbiolres16020032





