Abstract
Background: Antibiotic drug resistance is a serious global health problem that threatens therapeutics against infectious diseases. As antibiotics become less effective every year, our objective was to evaluate the adjuvant activity of methanolic extracts of Moringa oleifera seed combined with antibiotics of clinical use against multidrug-resistant Escherichia coli isolated from street food samples searching for a new alternative to treat infectious diseases commonly treated with antibiotics. Methods: Secondary metabolites of M. oleifera seeds were obtained through maceration (methanol 80%) and detected following qualitative phytochemical assays. MIC, MBC and tolerance level were determined using microdilution tests. Antimicrobial activity was tested by sensitivity analysis, and the adjuvant activity was explored in combination with twelve antibiotics against the E. coli samples. Results: Alkaloids, phenolic compounds, flavonoids, and polyphenols were detected. MIC and MBC values ranged from 31.3 to 62 mg/mL and 62–125 mg/mL, respectively. The extract showed low antimicrobial activity against the multidrug-resistant E. coli, but the inhibitory capacity of ampicillin, cephalexin, and amoxicillin/clavulanic acid was significantly increased when combined with the plant extract. In contrast, the activity of ciprofloxacin, levofloxacin, tetracycline, polymyxin, and nalidixic acid decreased with the extract. Conclusion: Methanolic extracts of M. oleifera seeds represent a potential adjuvant for beta-lactams in the face of the growing problem of global antimicrobial resistance. This study represents the first steps in exploring the adjuvant capacity of plants against resistant environmental pathogens in Mexico.
1. Introduction
Antibiotic resistance (AR) is the ability of pathogens to adapt and resist or inhibit the molecular mechanism of an antimicrobial to which it was susceptible [1]. Through evolutionary events, pathogens have acquired mechanisms that mediate changes in membrane permeability, modification of the antibiotic target, enzymatic degradation of the antibiotic, and changes in a metabolic pathway [2].
AR is considered among the main global threats to public health and economic development [3]. Although there was a decrease after COVID-19, the antimicrobial resistance burden is predicted to increase to 1.91 million attributable deaths and 8.22 million associated deaths by 2050. Importantly, Latin America is among the countries with the highest predicted AR mortality rate (by 2050) [4], and Mexican hospitals reported an increase in AR cases and multidrug-resistant bacteria. Several reports exhibited an inadequate use of these types of drugs, together with inappropriate prescription and excessive use of broad-spectrum antibiotics [5]. AR impacts all treatments that require antibiotics, including urinary tract infections (UTIs) and gastrointestinal diseases.
UTIs are a common infection that causes serious public health issues. It is responsible for 150 million diseases every year worldwide, and 80–90% of all cases are caused by uropathogenic Escherichia coli (E. coli) (UPEC) [6]. E. coli is also responsible for several gastrointestinal diseases, becoming the leading cause of pediatric diarrhea in developing countries, and is responsible for 40 to 70% of a disease called Traveler’s diarrhea [7]. Diarrheagenic E. coli pathotypes (DEPs) include enterotoxigenic E. coli (ETEC), diffusely adherent E. coli (DAEC), and Shiga toxin-producing E. coli (STEC), which have been identified among food prepared and marketed in Mexican streets [8,9,10,11,12].
Notably, E. coli is among the most common pathogens isolated from hospitalized patients and exhibits distinct AR mechanisms [13]. E. coli is the first AR pathogen considered worldwide, responsible for more than 100,000 deaths associated with AR in Latin America [14]. Therefore, as antibiotic-resistant E. coli spreads, finding new alternatives is essential to counter this problem.
Previous experimental evidence suggested that plant secondary metabolites have potential biological activity. Different research groups focused on medicinal plants, and this trend is increasing worldwide, especially in demonstrating their microbicidal activity and characterizing secondary metabolites as potentially therapeutic molecules [15].
Moringa oleifera Lam. (M. oleifera) is a native Indian tree known for its nutritional value and medicinal properties [16]. M. oleifera has been reported to be enriched in essential amino acids, polyphenols, vitamins, minerals, and proteins, conjointly with flavonoids, anthocyanins, alkaloids, and tannic acids [17,18]. In the last decade, researchers revealed the medicinal properties of all tissues of M. oleifera. M. oleifera seeds are of high pharmacological value due to their anti-inflammatory activity [19,20], anti-cancer activity [21], antioxidant activity [22,23,24], and antimicrobial activity [25]. The latter was demonstrated against several pathogens, including E. coli [26,27,28]. Considering the alarming threat that AR represents to the world, the possibility of using M. oleifera extracts as a new natural source to treat infections is highly promising.
Due to the low activity of the extracts independently, the adjuvant activity is now explored [29,30]. The results against bacteria associated with autoimmune inflammatory diseases were promising, especially in combination with chloramphenicol, erythromycin, and streptomycin, antibiotics that target protein synthesis [31]. To our knowledge, there are currently no reports that have evaluated the microbicidal activity of M. oleifera seed from central Mexico against multidrug-resistant pathogens in the same geographic area. Therefore, the study aims to evaluate the inhibition capacity of methanolic M. oleifera seed extracts and their adjuvant activity with conventional antibiotics against environmental multidrug-resistant E. coli from the central area of Mexico.
2. Materials and Methods
2.1. Food Sampling and E. coli Isolation
The bacterial strains were previously isolated from one hundred and eighty-nine food samples prepared and marketed under street conditions in three areas located in the capital of the State of Mexico in a three-year study (2012–2023) [12]. The study areas were located at “Mercado 16 de Septiembre”, “Mercado Juárez”, and “Terminal de Autobuses”, characterized by the presence of food stalls by the roadside and crowded areas. Food stalls were selected randomly, and food items were collected from the stalls exposed to vehicular traffic, without access to safe water and refrigeration, and ready-to-eat food. The one hundred and eighty-nine samples were plated on MacConkey agar (BIOXON, Mexico City, Mexico) using a bacteriological scraper and by scrubbing the food surface. Round and fuchsia colonies were selected as positive E. coli colonies and were reinoculated onto Eosin Methylene Blue agar (EMB, BIOXON, Mexico, Mexico). The colonies with metallic precipitation were selected as positive E. coli samples and were grouped depending on the food type and year of sampling, resulting in eleven samples for further analysis (Table 1). To determine the sample size, the Raosoft sample size calculation method was used with a 5% margin of error and 95% confidence level.

Table 1.
Distribution and description of the samples included in this study.
2.2. Antibiotic Sensibility Testing
To determine the sensitivity profile of the isolated E. coli, the Kirby–Bauer method established by the Clinical Laboratory Standard Institute (CLSI, [32]) was followed. Briefly, the inoculum was prepared at a turbidity of 0.5 MacFarland standard, and plates with Muller Hinton agar (MHA, BIOXON, Mexico City, Mexico) were inoculated using a sterile swab. Absorbent disks (Whatman paper No. 3), prepared with antibiotics of clinical used in Mexico [33], were placed on the inoculated plate and incubated at 37 °C for 24 h. The inhibition areas were measured and recorded as the inhibition zone of the antibiotic (IZA). The concentrations used for the twelve antibiotics was stablished by CLSI [34]: tetracycline (TET, 30 µg), cephalexin (CEP, 30 µg), sulfamethoxazole/trimethoprim (TMP-SMX, 1.25 µg/25 µg), ampicillin (AMP, 10 µg), amikacin (AMK, 30 µg), polymyxin B (POL, 30 µg), levofloxacin (LEV, 5 µg), ciprofloxacin (CIP, 5 µg), nalidixic acid (NA, 30 µg), chloramphenicol (CLO, 10 µg), amoxicillin/clavulanic acid (AMX, 20 µg/10 µg), and gentamicin (GEN, 10 µg).
2.3. Secondary Metabolites Extraction of Moringa oleifera Seeds
M. oleifera seeds were obtained from the central region of Mexico, based on several criterial. In this geographical area, the trees grow under environmental conditions without controlled temperature or precipitation. The trees from where the seeds were collected are from a natural area to which people have free access. The civilian population collects them and sells them in the local markets of the area, where they are consumed due to their various medicinal properties, according to tradition or ethnobotanical documentation. After collecting, the clean and dry seeds were weighed and powdered using a grinder for gains (Surtek, Monterrey, Mexico) and held in an Erlenmeyer flask at room temperature and moisture-free until further analysis.
To prepare the seed extracts, the protocol described in previous studies by Abubakar et al. [35] was followed. Briefly, 25 g of the powdered seeds was mixed with 125 mL of methanol (80%) for 72 h with constant shaking at room temperature. Every 24 h, the solvent was decanted, and a new volume (125 mL) of methanol was added [36].
Afterward, the solvent recovered was filtered with Whatman’s No. 1 filter paper (Sigma-Aldrich, Toluca, Mexico), concentrated at 40 °C using a rotatory evaporator, and dried in the oven for 48 h at 38 °C [37]. Dried extracts were weighed and diluted with methanol (20%) to obtain a final concentration of 2 g/mL.
2.4. Phytochemical Analysis
To screen the major secondary metabolites, present in the M. oleifera seed methanolic extract a 2 g/mL concentrated extract was used for the following protocols:
Alkaloids:
In HCl, 1 mL of the extract was dissolved, and four drops of Mayer reagent were added to the solution. The formation of a white jelly-like precipitate after mixing the reagent with the extract indicates the presence of alkaloids [35].
Polyphenols:
To 1 mL of extract, two drops of FeCl3 were added. A dark green color indicates the presence of polyphenol compounds [35].
Flavonoids:
M. oleifera extract was combined with 5 mL of ammonium solution, and 1 mL of concentrated H2SO4 was added. The transition into a yellow coloration indicates the presence of flavonoids [27].
Tannins:
To 1 mL of the extract, two drops of 1% lead acetate were added. A yellow precipitate indicates the presence of tannins [38].
Anthocyanins:
To 2 mL of the extract, 2 mL of 2N HCl and ammonia were added. The transition of a dark pink into violet-blue shows the presence of anthocyanins [38].
2.5. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), Tolerance Level, and Antimicrobial Activity
The MIC of the extracts against E. coli was determined through a micro-broth dilution assay [39]. The extract concentration spanned from 1 g/mL to 1.95 × 103 µg/mL. In brief, 50 µL of the culture (1.5 × 105 CFU/mL) was added to a sterile 96-well plates, followed by 50 µL of two-fold dilutions of M. oleifera extracts. Wells with the bacterial inoculum without the extracts were used as growth control, and a well without inoculum was used as a sterile control. The plate was incubated at 24 h at 37 °C, and the OD620 was measured. The MIC is defined as the lowest concentration that has an average OD620 within three standard deviations of the negative control wells. MBCs were determined by subculturing the wells with an OD620 < MIC on plates with MHA and incubated overnight at 37 °C. The MBC was established as the concentration that reduces the bacterial number by 99.9%, compared to the starting inoculum [40]. The Tolerance Level of the E. coli strains isolated from food samples against the M. oleifera seeds methanolic extract was determined using the formula [1]:
A bacteriostatic activity is indicated by a tolerance value ≥ 16, whereas a tolerance value ≤ 4 exhibits a bactericidal effect.
The well diffusion method was carried out, preparing the bacterial inoculum to a turbidity equivalent to 0.5 MacFarland standard. MHA were inoculated with the studied bacteria, and the wells were cut using a sterile cork (6 mm diameter) and sealed with diluted MHA. Next, 50 µL of the extract at the MBC was dispensed into each well. The plates were incubated at 37 °C for 24 h. The inhibition zones were measured and recorded as the inhibition zone of the extract (IZE). Methanol at 20% was used as negative [41,42].
2.6. Effect of the Combination of the Moringa oleifera Seed Extract with Antibiotics
To determine the effect of the combination, the synergistic antimicrobial assay protocol described previously by Saquib was followed. Briefly, the plates were prepared as mentioned in Section 2.3, and 5 µL of the extract, at an MBC, was added to each disk. Plates were incubated for 24 h at 37 °C, and the inhibition zones were measured and recorded as the inhibition zone of the combination (IZC). The activity was determined as follows: IZC > IZE + IZA = synergism; IZC = IZE + IZA = additive; IZC < IZE + IZA = antagonism [39].
2.7. Statistical Analysis
The susceptibility response between the groups and the M. oleifera seed methanolic extract was compared using a one-way ANOVA, in order to elucidate which groups exhibited significant differences, post hoc Tukey’s Honestly Significant Differences (HSD) p < 0.005 was applied. Likewise, the IZA, IZE, and IZC measurements of the synergic and additive results were compared against the same sample. The confidence interval was set at 95% and the data was analyzed using Minitab Software (ver. 21.4.0.0), LLC.
3. Results
3.1. E. coli Sampling
One hundred and eighty-nine samples were obtained from the Capital City of the State of Mexico within the three-year study. In general, 62% of the samples contained lactose-fermenting Gram-negative bacteria, resulting in positive for E. coli on EMB agar (Figure S1). As mentioned in Section 2.1, samples were divided into four categories depending on the type of food resulting in eleven samples that contain the positive E. coli strains obtained from the samples collected (Table 1).
A higher percentage of contamination was detected on G3, followed by G1 and G2. The group with the lowest incidence of contamination was G4 (Figure 1). From the total of samples classified into G1, 62% were positive in 2021, 66% in 2022, and 70% in 2023. In the case of G2, 74% were positive in 2021 and 73% in 2023. Additionally, with higher percentages of contamination, G3 exhibited 100%, 80%, and 100%, in 2021, 2022 and 2023, respectively. Finally, G4, with the lower percentages of contamination, revealed 20% in 2021, 41% in 2022, and 44% in 2023. Importantly, after the genotypic identification of the virulence genes, the authors identified the presence of genes related to the pathogenic mechanisms of UPEC, ETEC, and DAEC.
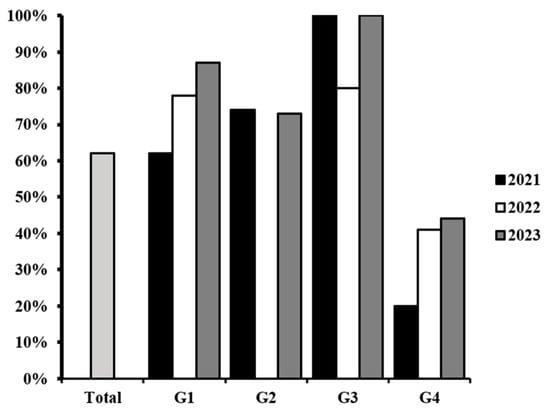
Figure 1.
Percentage of E. coli contamination among the food samples collected (light gray) and by food group (G1, G2, G3, G4) each year.
3.2. Antibiotic Susceptibility
The analysis carried out by Mora et al. highlighted shifting patterns in the antibiotic resistance profile of the isolated E. coli strains (Table S1). In general, the antibiotic sensitivity analysis exhibited higher percentages of resistance against ampicillin (100%), amoxicillin/clavulanic acid (100%), and cephalexin (45%). Moreover, the most effective antibiotics were the fluoroquinolones (levofloxacin, ciprofloxacin), polymyxin, and tetracycline. Importantly, all samples responded differently to the antibiotics and the resistance profiles were less prominent in 2023. Differential antibiotic-resistant profiles were found among the studied E. coli, which varied from one type of food to another. G1 exhibited significant differences between the antibiotic responses compared with the other groups. E. coli strains from G4 revealed lower antibiotic resistance profiles.
3.3. Secondary Metabolites Extraction and Phytochemical Analysis
The phytochemical analyses showed the presence of four of the tested secondary metabolites (Table 2). The alkaloids, phenolic compounds, flavonoids, and tannins test revealed positive results (Figure S2).

Table 2.
Secondary metabolites detected in the methanolic extract of M. oleifera seed by preliminary phytochemical analysis.
3.4. Minimum Inhibitory Concentration, Minimum Bactericidal Concentration and Tolerance Level
The M. oleifera seed methanolic extract inhibited the growth of the antibiotic-resistant E. coli with an MIC value from 31.3 mg/mL to 62 mg/mL, and an MBC value from 62 mg/mL to 125 g/mL (Table 3), apart from G1-23, which exhibited an MIC value of 125 mg/mL and an MBC value of 250 mg/mL. The Tolerance Level was similar for all samples analyzed, with a value ≤4, indicating that the antibacterial efficacy is considered as bactericidal activity (Table 3). Sample groups from different years exhibited the same MIC and MBC values. For example, G3-21, G3-22, and G3-23 revealed an MIC of 31.3 mg/mL and an MBC of 62 mg/mL. The same happens to G2 and G4, apart from G1-23.

Table 3.
Minimal inhibitory concentration (MIC), minimum bactericidal concentration (MB), Tolerance level, and the inhibition zone resulting from the screening of the antimicrobial activity (IZE) of the M. oleifera extracts against the multidrug-resistant E. coli isolated from food samples. Mean and standard deviation of three replicates are shown in IZE.
3.5. Antimicrobial Activity of the M. oleifera Seed Extracts
The antimicrobial activity (inhibition zones of the extract: IZE), shown by the extract was comparatively lower than its combination with antibiotics, as shown in Figure 2b,c.
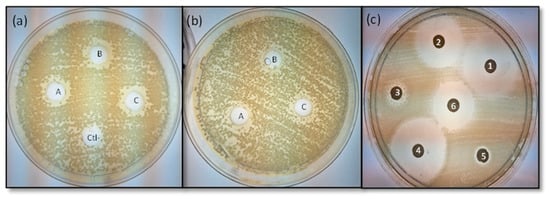
Figure 2.
Evaluation of the antimicrobial activity of M. oleifera seed extracts against the multidrug-resistant E. coli isolated from food samples, (a) G1-23 and (b) G4-22. Combination of M. oleifera with six antibiotics (c). Triplicates are marked with capital letters (A, B, C), and methanol 20% was used as negative control (ctl-). Antibiotics are numbered as follows: (1) levofloxacin, (2) ciprofloxacin, (3) nalidixic acid, (4) chloramphenicol, (5) amoxicillin/clavulanic acid, and (6) gentamicin.
Table 3 presents the means and standard deviations of three replicates of IZE against the multidrug-resistant E. coli strains. When analyzing the antimicrobial effect of the extracts by group, the results exhibited differential behaviors between years (Figure 3). 2021 and 2023 results exhibited higher inhibition values in G2 and G3 samples. In 2023, for G1 and G4, the IZE values were higher compared to the other samples. Interestingly, 2022 results were lower than the other years in all groups.
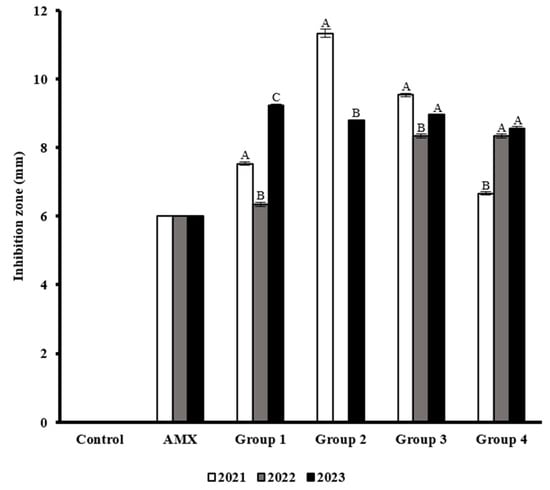
Figure 3.
Measurements of the inhibition diameters produced by M. oleifera extract against multidrug-resistant E. coli strains. The means and standard deviation of three experiments are shown. Statistically significant differences were calculated and the results that do not share the same letter are significantly different (A, B, C).
3.6. Effect of Antibiotic Interaction with M. oleifera Seed Extract
The inhibition diameter measurements of the combination of M. oleifera extracts with antibiotics against the E. coli strains isolated from food over the study period were recorded (Table S2). The analysis exhibited differential results that varied from one sample to another and from one antibiotic to another, with the antagonist being the most frequent (Table S3).
AMP, AMX, and CEP exhibited the most promising results since the IZC value was significantly higher than the IZA value, indicating synergic interactions (Figure 4). The results from 2023 presented important improvements in the inhibition activity of these antibiotics when combined with the seed extract of M. oleifera. In addition, resistant E. coli stains from G3 were significantly inhibited by these antibiotics; G3 includes samples of fruits, juices, and smoothies. Results from 2021 and 2022 strains showed differential behaviors. Results from the CEP analysis exhibited improvements in the growth inhibition of G1-22 (Figure 4a). The AMP results also showed significant differences between the inhibition activity of the antibiotics themselves and the combination, revealing higher inhibition values against G1-21 and G4-22 when AMP was combined with the plant extract (Figure 4b). AMX exhibited potential effects on G1-22 and G4-22 (Figure 4c). Notably, promising results were found against E. coli strains belonging to G1 and G4, samples involving sauces and cooked food such as meat and bread.
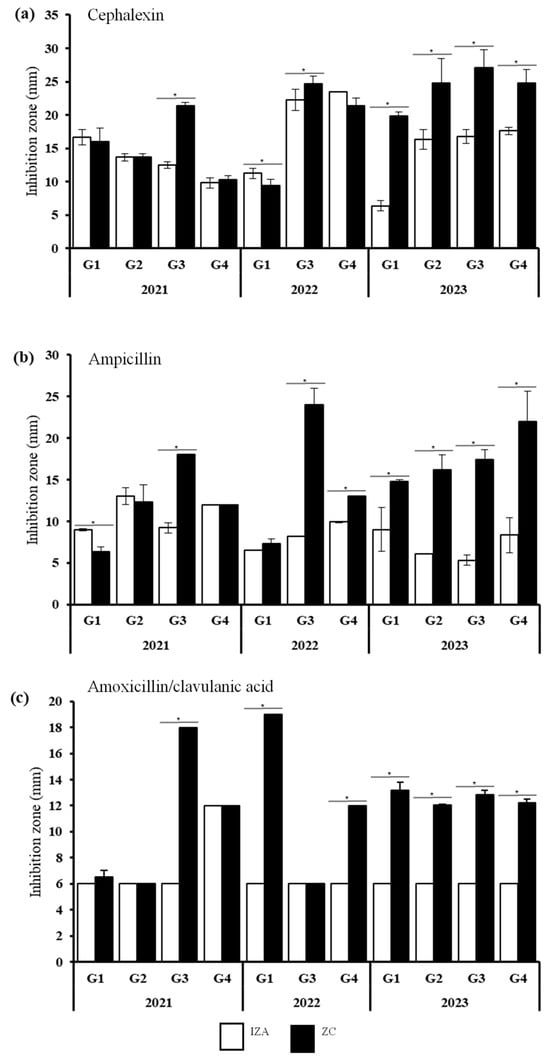
Figure 4.
Synergic effect of the combination of the M. oleifera extract with (a) cephalexin, (b) ampicillin, and (c) amoxicillin/clavulanic acid against the resistant E. coli strains isolated from the food samples. Statistically significant differences between IZA and IZC are indicated as * p < 0.05.
In 2021 and 2022, several results of the extract-drug interaction were classified as antagonist. However, few comparisons exhibited significant differences between the IZA and the IZC value. The analysis of TET, POL, LEV, CIP, and NA exhibited IZA values significantly higher compared to the IZC in at least one sample (Figure 5). The E. coli strains isolated from G1-22 exhibited significantly lower IZC values when POL, LEV, CIP, and NA were combined with the extract (Figure 5a–e). G3-21 also exhibited significant differences in IZA and IZC values against TET, CIP, and NA, revealing a reduction in inhibition activity (Figure 5a,d,e). G1-21, G4-21, and G4-22 showed significantly lower IZC values in at least two of the above-mentioned antibiotics. Notably, CIP and NA exhibited significant antagonist results in four of the five samples mentioned (Figure 5d,e). These results were observed mainly in E. coli strains isolated from food made of sauces, fruits, and smoothies, and cooked food such as meat and bread.
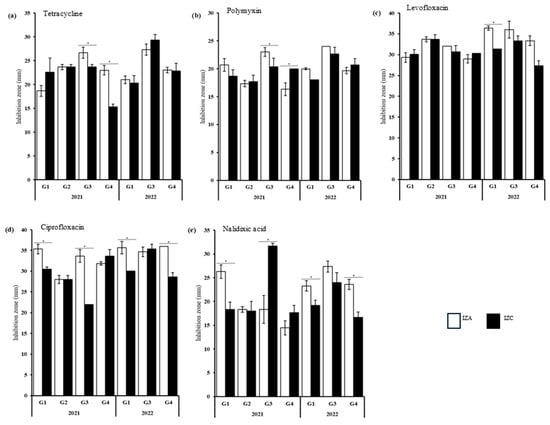
Figure 5.
Antagonist effect of the combination of the M. oleifera extract with (a) tetracycline, (b) polymyxin, (c) levofloxacin, (d) ciprofloxacin, and (e) nalidixic acid against the resistant E. coli strains isolated from the food samples. Statistically significant differences between IZA and IZC are indicated as * p < 0.05.
4. Discussion
Mexico faces a major public health challenge from food contamination by E. coli, and different diarrheal pathotypes of E. coli have been identified among the isolated bacteria. E. coli was found in more than 40% of samples of tomatoes, fresh cheese, pasteurized cheese, and unpasteurized cream with resistant profiles from different local markets in Mexico. Diarrheal pathotypes such as ETEC and UPEC were also detected among them [43,44,45].
The E. coli strains included in this study were previously submitted for several analyses that characterized them as E. coli. Mora et al. found a significant problem of food contamination by E. coli among the samples collected from street vendors in the Capital City of the State of Mexico. During three years of sampling, they observed an increase in contamination from 56% to 70%. Moreover, the authors explored the AR phenotype, finding a differential multidrug-resistant phenotype with higher percentages of resistance to ampicillin, amoxicillin/clavulanic acid, and cephalexin. Additionally, the virulent genes present within the genome of the isolates were explored and genes associated with the pathogenic mechanisms of UPEC, ETEC, and DAEC were detected [12], representing a serious threat to human health.
On the other hand, phytochemicals such as alkaloids, organosulfur, phenolic compounds, and terpenes, have gained interest, and several researchers have validated their antimicrobial activity. They had proven the capacity to block resistant mechanisms including the efflux pumps and enzymes [46,47,48].
Accordingly, a study performed in Egypt explored the antimicrobial activity of the methanolic extract of M. oleifera seed, reporting an inhibition diameter of 14 mm with 2.5 mg/well. Even though our values were slightly lower than those reported by El-Fakharany group, the methanolic extracts of M. oleifera seed collected for this study proved to inhibit the growth of E. coli, even when it exhibited antibiotic-resistant profiles. Additionally, the MIC value from the previously mentioned study was lower than the ones found in this study, indicating a weak antimicrobial activity. However, this can be associated with the nature of the studied strains, since they exhibited multidrug-resistant profiles [49].
Alkaloids, flavonoids, tannins, and phenolic compounds were found in the methanolic extract of M. oleifera seeds from this geographic area. Importantly, no study was found in Mexico on the characterization of the secondary metabolites of M. oleifera seeds, nor the antimicrobial capacity of these compounds against environmental strains. To compare our findings, we used studies from other countries that analyzed the methanolic extracts of M. oleifera seed through phytochemical analyses. The results exhibited similar findings. Three previous studies from Nigeria detected flavonoids and alkaloids among the samples [25,27,50]. Two of them did not detect polyphenols, and the other one did not analyze the presence of this bioactive compound. Anthocyanins were poorly studied; none of the studies evaluated their presence among the samples, which was negative in our analysis (Table 4).

Table 4.
Comparison of the secondary metabolites identified from other studies. Positive results (+), negative results (-), and not determined (NA).
Secondary metabolites such as flavonoids, alkaloids, and phenolic compounds have pharmacokinetic and pharmacodynamic interactions. Depending on the mechanisms of action of the antibiotic, the effect can be synergic, additive or antagonistic. Flavonoids and alkaloids can interfere with drug distribution and metabolism, or modulate receptors on the membrane, activating or inhibiting enzymes, or inhibiting voltage-gate sodium channels [51]. These activities can be associated with our results since the antimicrobial activity of ampicillin, amoxicillin/clavulanic acid, and cephalexin were potentiated when combined with the methanolic extracts.
Beta-lactams are one of the most widely used antibiotics, representing 60% of all prescriptions [52]. Thus, increasing resistance to this family of antibiotics represents a serious threat to human health. This family of antibiotics includes penicillin (ampicillin and amoxicillin) and cephalosporins (cephalexin), antibiotics characterized by the presence of a β-lactam ring on their structure [52].
The most common antibiotic-resistant mechanism associated with beta-lactam resistance is the presence of bacterial enzymes, typically β-lactamases, responsible for destroying the amide bond of the β-lactam ring [46]. However, bacteria have acquired other mechanisms, such as drug-efflux pumps. Efflux pumps became one of the vital mechanisms of resistant bacteria, as it decreases the antibiotic concentration inside the cytoplasm by transporting the drug outside the cell [46].
Considering that none of the beta-lactam genes (blaTEM, blaOXA) were previously amplified during the genotyping analysis carried out by Mora and coworkers [12], other resistant mechanisms have to be involved in the beta-lactam resistance of the isolated E. coli. AcrAB-TolC is one of the major efflux pumps found in E. coli and has been related to beta-lactams expellers [53]. Considering the above findings, in the studied E. coli, resistance to beta-lactams could be mediated by an efflux pump.
Alkaloids are demonstrated to inhibit antibiotic-resistant mechanisms present in E. coli. E. coli ATP-dependent efflux pumps were inhibited by lysergol, a phytochemical that belongs to the alkaloid family. The study evaluates the activity independently and in combination with nalidixic acid against nalidixic acid-resistant E. coli. The results showed a reduction in the MIC value when the antibiotic was combined with the lysergol [54]. Additionally, 6-shogaol, a phenolic compound obtained from ginger, blocks AcrAB-TolC efflux pump function through the interaction with the AcrB homotrimer domain in E. coli [55].
The above leads us to hypothesize that the synergic activity found on AMP, AMX, and CEP with the methanolic extracts of Moringa oleifera seeds might be due to the presence of phytochemicals that might act as Efflux Pumps Inhibitors (EPIs), blocking the antibiotic-resistant mechanisms in the E. coli strains obtained in 2023, and increasing their inhibitory activity (Figure 6).
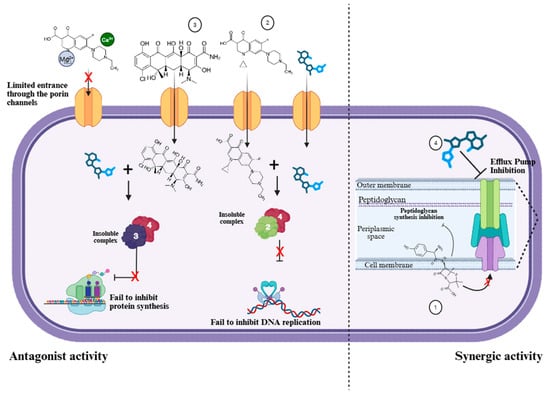
Figure 6.
Model of the possible molecular mechanisms of beta-lactams (amoxicillin) (1), quinolone (ciprofloxacin) (2), and tetracycline (3) antibiotics affected by treatment with the extract of M. oleifera (4). The methanolic extract drags chemical species that might have a bactericidal effect, enhancing the activity of beta-lactams by disrupting the activity of flow pumps such as the RND protein. Moreover, the molecules contained in the extract might form insoluble complexes with quinolones and tetracycline, significantly attenuating their antimicrobial activity. Furthermore, the calcium and magnesium of the seed could form complexes with the antibiotic limiting their entrance through the porin channels.
This activity can be proven by the implementation of a well-studied EPI such as Phenylalanine-Arginine-β-Naphthylamine (PaβN). Previous studies have shown that PaβN enhances the activity of certain antibiotics by reducing antimicrobial resistance, especially in bacteria that express efflux pumps [56].
On the other hand, and interestingly, when the seed extract of M. oleifera was combined with quinolones, the inhibitory activity decreased (Figure 4), even when the antimicrobial activity was considerably effective against the studied E. coli strains [10]. These broad-spectrum antibacterial agents interfere with DNA gyrase and topo IV, specifically in Gram-negative bacteria. Quinolones include nalidixic acid, ciprofloxacin and levofloxacin [57].
Notably, G3-21 and G1-22 were the samples that had the highest incidence of antagonist activity with quinolones. These samples include sauces and fruits, food that lacks heating procedures and requires proper manipulation to guarantee the elimination of contaminants.
Previously, Mora and coworkers found the amplicon of the qnrS gene in the E. coli strains isolated from G1-22 and G4-22 [12], a gene associated with resistance to quinolones. Although the gene was present, the E. coli strains were susceptible to this antibiotic [12]. Based on these findings, it can be hypothesized that the expression of the QnrS protein might be induced by the extract, decreasing the antimicrobial activity. However, further analysis is necessary to identify the expression of this polypeptide or its integration into the bacterial genome.
Moreover, it has been reported that divalent cations can antagonize the antimicrobial activity of quinolones. The combination of magnesium or calcium chloride with quinolones causes a decrease in the MIC values against Gram-negative bacteria due to the formation of magnesium–quinolone complexes that cannot be transported through the porin channels [58]. Therefore, the presence of magnesium or calcium in the seed might be responsible for the antagonist effect of the quinolones [59].
In this study, we analyzed environmental strains that have suffered from a lack of nutrition, selection, and other factors, resulting in bacterial strains that have acquired exogenous genes or have developed mutations to survive.
Ilanko and coworkers also identified antagonist effects when the extract was combined with gentamicin and tetracycline [31]. Both antibiotics’ mechanisms of action are driven by their binding to the 30S ribosomal subunit. It is worth noting that our study also reported antagonist effects when the extract was combined with tetracycline. Therefore, it can be hypothesized that the extract might interfere with the drug action, forming a complex with tetracycline, preventing it from binding to its target site (Figure 6).
5. Conclusions
In this study, we found a natural alternative to treat infections caused by multidrug-resistant bacteria such as E. coli. The extract exhibited the presence of alkaloids, phenolic compounds, flavonoids, and tannins. However, M. oleifera seed methanolic extracts exhibited low antimicrobial activity against the studied E. coli strains but the activity of the antibiotic increased when combined with antibiotics that belong to the β-lactam family. On the other hand, the M. oleifera extracts exhibited antagonistic activity when combined with quinolone. These findings provide the first insight into the possibility of using M. oleifera seed as a natural alternative to treat infections caused by resistant pathogens in the central region of Mexico.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16110238/s1, Figure S1: Growth of colonies of bacteria isolated from food samples during the study; Table S1. Inhibition zone of the antibiotics (IZA) against the eleven samples E. coli included in this study and the interpretation into susceptible (S), intermediate (I), and resistant (R); Figure S2: Phytochemical screening of the secondary metabolites in the M. oleifera seed methanolic extract; Table S2: Inhibition diameter measurements of the combination of the M. oleifera extracts and antibiotics against the multidrug-resistant E. coli strains isolated from food samples during the three years of study; Table S3: Synergistic antimicrobial activity of M. oleifera seed extracts with different antibiotics.
Author Contributions
Conceptualization, D.M.-C. and M.A.O.-T.; data curation, D.M.-C. and S.M.-C.; formal analysis, D.M.-C., S.M.-C. and M.A.O.-T.; investigation, D.M.-C. and P.R.M.-V.; methodology, D.M.-C., P.R.M.-V., J.L.-M., J.J.J.-B., A.P.-G., S.M.-C. and M.A.O.-T.; project administration, D.M.-C. and M.A.O.-T.; software, D.M.-C. and S.M.-C.; supervision, J.L.-M., J.J.J.-B., A.P.-G. and M.A.O.-T.; validation, D.M.-C. and M.A.O.-T.; visualization, D.M.-C., J.L.-M., J.J.J.-B., A.P.-G. and M.A.O.-T.; writing—original draft, D.M.-C. and M.A.O.-T.; writing—review and editing, D.M.-C., S.M.-C. and M.A.O.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to IBT Montserrat Legorreta González, M. en C. Martha Gabriela López Prado, M. en C. Daniel Alejandro Mejía Serrano; IBT María Fernanda Orozco Montes de Oca, M. en C. Juan Jesús Cruz Maldonado, M. en C.F. Cindy Guadalupe Pérez Sotelo, M. en C. Cecilia Franco Mendieta, QFB. Mercedes Rojas Moreno, QFB. Emilia Dolores Ledezma, and Dra. Liliana Santos Zea for expert technical assistance. CEV is indebted to CONAHCyT, México for a doctoral fellowship (1000105) and Tecnológico de Monterrey.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arsene, M.M.J.; Viktorovna, P.I.; Davares, A.K.L.; Parfait, K.; Andreevna, S.L.; Mouafo, H.T.; Rehailia, M.; Vyacheslavovna, Y.N.; Pavlovna, S.I.; Manga, I.A.M.; et al. Antimicrobial and Antibiotic-Resistance Reversal Activity of Some Medicinal Plants from Cameroon against Selected Resistant and Non-Resistant Uropathogenic Bacteria. Front. Biosci. Elite 2022, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Arsene, M.M.J.; Jorelle, A.B.J.; Sarra, S.; Viktorovna, P.I.; Davares, A.K.L.; Ingrid, N.K.C.; Steve, A.A.F.; Andreevna, S.L.; Vyacheslavovna, Y.N.; Carime, B.Z. Short Review on the Potential Alternatives to Antibiotics in the Era of Antibiotic Resistance. J. Appl. Pharm. Sci. 2022, 12, 029–040. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 2 April 2024).
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Svarch, A.E.; Jackeline, M.; Rosales, L.; Contreras, A.V.; Lugo, H.S.; Esmeralda, L.; Vázquez, R.; Sandoval, R.C. Actions to Combat Antimicrobial Resistance in Mexico: A Perspective from COFEPRIS. Rev. Mex. Politica Exter. 2024, 129, 73–86. [Google Scholar]
- Arsene, M.M.J.; Viktorovna, P.I.; Davares, A.K.L.; Esther, N.; Nikolaevich, S.A. Urinary Tract Infections: Virulence Factors, Resistance to Antibiotics, and Management of Uropathogenic Bacteria with Medicinal Plants—A Review. J. Appl. Pharm. Sci. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Estrada-Garcia, T.; Lopez-Saucedo, C.; Thompson-Bonilla, R.; Abonce, M.; Lopez-Hernandez, D.; Santos, J.I.; Rosado, J.L.; DuPont, H.L.; Long, K.Z. Association of Diarrheagenic Escherichia coli Pathotypes with Infection and Diarrhea among Mexican Children and Association of Atypical Enteropathogenic E. coli with Acute Diarrhea. J. Clin. Microbiol. 2009, 47, 93–98. [Google Scholar] [CrossRef]
- Canizalez-Roman, A.; Gonzalez-Nuñez, E.; Vidal, J.E.; Flores-Villaseñor, H.; León-Sicairos, N. Prevalence and Antibiotic Resistance Profiles of Diarrheagenic Escherichia coli Strains Isolated from Food Items in Northwestern Mexico. Int. J. Food Microbiol. 2013, 164, 36–45. [Google Scholar] [CrossRef]
- Dominguez-Gonzalez, K.G.; Aguilar-Chairez, S.; Cerna-Cortes, J.; Soria-Herrera, R.J.; Cerna-Cortes, J.F. Microbiological Quality and Presence of Foodborne Pathogens in Fresh-Squeezed Orange Juice Samples Purchased from Street Vendors and Hygienic Practices in Morelia, Mexico. Food Sci. Technol. 2022, 42, e10222. [Google Scholar] [CrossRef]
- Cerna-Cortes, J.F.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Ramírez-Cruz, E.; Castro-Rosas, J. Presence of Indicator Bacteria, Salmonella and Diarrheagenic Escherichia coli Pathotypes on Mung Bean Sprouts from Public Markets in Pachuca, Mexico. Food Control 2013, 31, 280–283. [Google Scholar] [CrossRef]
- Cerna-Cortes, J.F.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Torres-Vitela, M.d.R.; Villarruel-López, A.; Castro-Rosas, J. Presence of Some Indicator Bacteria and Diarrheagenic E. Coli Pathotypes on Jalapeño and Serrano Peppers from Popular Markets in Pachuca City, Mexico. Food Microbiol. 2012, 32, 444–447. [Google Scholar] [CrossRef]
- Mora-Coto, D.; Moreno-Vélez, P.; Luna-Muñoz, J.; Moreno-Campuzano, S.; Ontiveros-Torres, M.A. Intestinal and Extraintestinal Pathotypes of Escherichia coli Are Prevalent in Food Prepared and Marketed on the Streets from the Central Zone of Mexico and Exhibit a Differential Phenotype of Resistance Against Antibiotics. Antibiotics 2025, 14, 406. [Google Scholar] [CrossRef]
- Rojas-Larios, F.; Martínez-Guerra, B.A.; López-Jácome, L.E.; Bolado-Martínez, E.; Vázquez-Larios, M.d.R.; Velázquez-Acosta, M.d.C.; Romero-Romero, D.; Mireles-Dávalos, C.D.; Quintana-Ponce, S.; Feliciano-Guzmán, J.M.; et al. Active Surveillance of Antimicrobial Resistance and Carbapenemase-Encoding Genes According to Sites of Care and Age Groups in Mexico: Results from the INVIFAR Network. Pathogens 2023, 12, 1144. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Ventura-Martínez, R.; González-Trujano, M.E.; Silveira, D. Editorial: Pharmacological Interaction between Drugs and Medicinal Plants. Front. Pharmacol. 2022, 13, 1081090. [Google Scholar] [CrossRef]
- Prashith Kekuda, T.R.; Mallikarjun, N.; Swathi, D.; Nayana, K.V.; Aiyar, M.B.; Rohini, T.R. Antibacterial and Antifungal Efficacy of Steam Distillate of Moringa oleifera Lam. J. Pharm. Sci. Res. 2010, 2, 34–37. [Google Scholar]
- Anzano, A.; Ammar, M.; Papaianni, M.; Grauso, L.; Sabbah, M.; Capparelli, R.; Lanzotti, V. Moringa oleifera Lam.: A Phytochemical and Pharmacological Overview. Horticulturae 2021, 7, 409. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.C.C.; Aguiar, J.S.; Napoleão, T.H.; Mota, F.V.B.; Barros, A.L.S.; Moura, M.C.; Coriolano, M.C.; Coelho, L.C.B.B.; Silva, T.G.; Paiva, P.M.G. Evaluation of Cytotoxic and Anti-Inflammatory Activities of Extracts and Lectins from Moringa oleifera Seeds. PLoS ONE 2013, 8, e81973. [Google Scholar] [CrossRef]
- Wolff, K.; Jaja-Chimedza, A.; Kim, Y.; Waterman, C.; Poulev, A.; Raskin, I.; Ribnicky, D. Moringa Isothiocyanate-1 Is Bioaccessible and Bioavailable as a Stable Unmodified Compound. Phytochem. Lett. 2020, 38, 33–38. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Asogwa, N.T.; Aja, P.M.; Akunna, G.G.; Awoke, J.N.; Ekeleme-Egedigwe, C.A.; Maduagwuna, E.K.; Folawiyo, A.M.; Besong, E.E.; Ekpono, E.U.; et al. Moringa oleifera Seed Oil Modulates Redox Imbalance and INOS/NF-ĸB/Caspase-3 Signaling Pathway to Exert Antioxidant, Anti-Inflammatory and Antiapoptotic Mechanisms against Anticancer Drug 5-Fluorouracil-Induced Nephrotoxicity in Rats. S. Afr. J. Bot. 2019, 127, 96–103. [Google Scholar] [CrossRef]
- Suphachai, C. Antioxidant and Anticancer Activities of Moringa oleifera Leaves. J. Med. Plants Res. 2014, 8, 318–325. [Google Scholar] [CrossRef]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K.P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Govardhan Singh, R.S.; Negi, P.S.; Radha, C. Phenolic Composition, Antioxidant and Antimicrobial Activities of Free and Bound Phenolic Extracts of Moringa oleifera Seed Flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Akinyeye, A.J.; Solanke, E.O.; Adebiyi, I.O. Phytochemical and Antimicrobial Evaluation of Leaf and Seed of Moringa olifera Extracts. Int. J. Res. Med. Health Sci. 2014, 4, 1–10. [Google Scholar]
- Dinesha, B.L.; Nidoni, U.; Ramachandra, C.T.; Naik, N.; Sankalpa, K.B. Effect of Extraction Methods on Physicochemical, Nutritional, Antinutritional, Antioxidant and Antimicrobial Activity of Moringa (Moringa oleifera Lam.) Seed Kernel Oil. J. Appl. Nat. Sci. 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Emmanuel, S.A.; Abubakar, S. Phytochemical and Antimicrobial Studies of Methanol, Ethyl Acetate, and Aqueous Extracts of Moringa oleifera Seeds. Ame. J. Eth. 2014, 1, 346–354. [Google Scholar]
- Fowoyo, P.; Olalekan Oladoja, E. Phytochemical Screening, Nutritional Composition and Antimicrobial Activity of Moringa oleifera Seed and Leaf Extract against Selected Gastrointestinal Pathogens. Artic. IOSR J. Pharm. Biol. Sci. 2015, 10, 116–124. [Google Scholar]
- Forsman, L.D.; Giske, C.G.; Bruchfeld, J.; Schön, T.; Juréen, P.; Ängeby, K. Meropenem-Clavulanic Acid Has High in Vitro Activity against Multidrug-Resistant Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 3630–3632. [Google Scholar] [CrossRef]
- Alsahli, A.A.; Al Sahli, A.A.; Abdulkhair, W.M. Inhibition of Beta-Lactamase Enzyme of Pseudomonas Aeruginosa by Clavulanic Acid of Rumex vesicarius L. Afr. J. Agric. Res. 2011, 6, 2908–2915. [Google Scholar]
- Ilanko, P.; McDonnell, P.A.; van Vuuren, S.; Cock, I.E. Interactive Antibacterial Profile of Moringa oleifera Lam. Extracts and Conventional Antibiotics against Bacterial Triggers of Some Autoimmune Inflammatory Diseases. S. Afr. J. Bot. 2019, 124, 420–435. [Google Scholar] [CrossRef]
- CLSI M02; Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Vineetha, N.; Sridhar, D.; Vignesh, R.A. Preparation, Standardization of Antibiotic Discs and Study of Resistance Pattern for First-Line Antibiotics in Isolates from Clinical Samples. Int. J. Appl. Res. 2015, 1, 624–631. [Google Scholar]
- CLSI M2-A9; Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—9th ed. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2006; Volume 26.
- Abubakar, I.; Usman, A. Phytochemical and Antibacterial Investigations of Moringa (Moringa oleifera) Leaf Extract on Selected Bacterial Pathogens. J. Microbiol. Antimicrob. 2016, 8, 28–33. [Google Scholar] [CrossRef]
- Jones, W.P.; Kinghorn, A.D. Natural Products Isolation, 2nd ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 323–346. [Google Scholar]
- Doughari, J.H.; Pukuma, M.S.; De, N. Antibacterial Effects of Balanites Aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella Typhi. Afr. J. Biotechnol. 2007, 6, 2212–2215. [Google Scholar] [CrossRef]
- Savithramma, N.; Linga Rao, M.; Ankanna, S. Screening of Medicinal Plants for Secondary Metabolites. Int. Res. Pharm. Sci. 2011, 2, 643–647. [Google Scholar]
- Saquib, S.A.; AlQahtani, N.A.; Ahmad, I.; Kader, M.A.; Al Shahrani, S.S.; Asiri, E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal Pathobionts: An in Vitro Microbiological Study. Antibiotics 2019, 8, 89. [Google Scholar] [CrossRef]
- Daly, S.M.; Sturge, C.R.; Greenberg, D.E. Inhibition of Bacterial Growth by Peptide-Conjugated Morpholino Oligomers. Meth. Mol. Biol. 2017, 1565, 115–122. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Faisal Madhloom, A.; Bashir Hashim Al-Taweel, F.; Sha, A.M.; Raad Abdulbaqi, H. Antimicrobial Effect of Moringa oleifera L. and Red Pomegranate against Clinically Isolated Porphyromonas Gingivalis: In Vitro Study. Arch. Razi Inst. 2022, 77, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Del Refugio Torres-Vitela, M.; Acevedo-Sandoval, O.A.; Rangel-Vargas, E.; Villarruel-López, A.; Castro-Rosas, J. Presence of Shiga Toxin-Producing Escherichia coli, Enteroinvasive E. coli, Enteropathogenic E. coli, and Enterotoxigenic E. coli on Tomatoes from Public Markets in Mexico. J. Food Prot. 2013, 76, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa-Hernandez, M.C.; Cadena-Ramírez, A.; Téllez-Jurado, A.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Chávez-Urbiola, E.A.; Castro-Rosas, J. Presence of Multidrug-Resistant Shiga Toxin–Producing Escherichia coli, Enteropathogenic Escherichia coli, and Enterotoxigenic Escherichia coli on Fresh Cheeses from Local Retail Markets in Mexico. J. Food Prot. 2018, 81, 1748–1754. [Google Scholar] [CrossRef]
- Ríos-Muñiz, D.; Cerna-Cortés, J.F.; Morán-García, N.; Meza-Segura, M.; Estrada-García, T. Escherichia coli Enterotoxigénica y Enteroagregativa: Prevalencia, Patogénesis y Modelos Múridos. Gac. Med. Mex. 2019, 155, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmcol 2021, 12, 720726. [Google Scholar] [CrossRef]
- Vankwani, S.; Ansari, A.; Azhar, A.; Galani, S. Synergistic Evaluation of Moringa oleifera Extract and SS-Lactam Antibiotic to Restore the Susceptibility of Methicillin-Resistant Staphylococcus aureus. Mol. Biol. Rep. 2022, 49, 421–432. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.F.; Taha, M.A.; El-Gazzar, N. Inhibition of Staphylococcus aureus LC 554891 by Moringa oleifera Seed Extract Either Singly or in Combination with Antibiotics. Molecules 2020, 25, 4583. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Elsharkawy, W.B.; El-Maradny, Y.A.; El-Gendi, H. Moringa oleifera Seed Methanol Extract with Consolidated Antimicrobial, Antioxidant, Anti-Inflammatory, and Anticancer Activities. J. Food Sci. 2024, 89, 5130–5149. [Google Scholar] [CrossRef]
- Garga, M.A.; Manga, S.B.; Rabah, A.B.; Tahir, H.; Abdullahi, M.; Ahmad, M.; Abdullahi, H.A.; Bako, I.; Abdurrahman, S.A.; Mukhtar, U.F. Antibacterial Activity and Phytochemical Screening of Moringa oleifera Lam. Leaves and Seeds Extract on Staphylococcus aureus. Int. J. Res. 2019, 7, 276–284. [Google Scholar] [CrossRef]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Narendrakumar, L.; Chakraborty, M.; Kumari, S.; Paul, D.; Das, B. β-Lactam Potentiators to Re-Sensitize Resistant Pathogens: Discovery, Development, Clinical Use and the Way Forward. Front. Microbiol. 2022, 13, 1092556. [Google Scholar] [CrossRef]
- Yamasaki, S.; Zwama, M.; Yoneda, T.; Hayashi-Nishino, M.; Nishino, K. Drug Resistance and Physiological Roles of RND Multidrug Efflux Pumps in Salmonella enterica, Escherichia coli and Pseudomonas aeruginosa. Microbiol. (UK) Microbiol. Soc. 2023, 169, 001322. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and Synergy of Clavine Alkaloid Lysergol and Its Derivatives Against Nalidixic Acid-Resistant Escherichia coli. Chem. Biol. Drug Des. 2013, 81, 484–490. [Google Scholar] [CrossRef]
- Moulick, S.; Bera, R.; Roy, D.N. Bactericidal Action of Ginger (Zingiber officinale Roscoe) Extract against Escherichia coli through Synergistic Modulation of the AcrAB-TolC Efflux Pump and Inhibition of Peptidoglycan Synthesis: In Vitro and in Silico Approaches. Microb. Pathog. 2025, 204, 107624. [Google Scholar] [CrossRef] [PubMed]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial and Antibiotic-Modifying Activities of Three Food Plants (Xanthosoma mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against Multidrug-Resistant (MDR) Gram-Negative Bacteria. BMC Complement. Altern. Med. 2016, 16, 9. [Google Scholar] [CrossRef]
- Spencer, A.C.; Panda, S.S. DNA Gyrase as a Target for Quinolones. Biomedicines 2023, 11, 371. [Google Scholar] [CrossRef]
- Marshall, A.J.H.; Piddock, L.J.V. Interaction of Divalent Cations, Quinolones and Bacteria. J. Antimicrob. Chem. 1994, 34, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, C.; Li, S.; Chu, X.; Sun, K. Nutritional Compositions of Indian Moringa oleifera Seed and Antioxidant Activity of Its Polypeptides. Food Sci. Nutr. 2019, 7, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).