Abstract
Carotenoid pigments are widely distributed in nature and play a crucial role in protecting organisms from photodynamic damage. However, the characterization of carotenoid production in clinically relevant mycobacteria has been limited due to the low sensitivity of conventional detection methods. We present a descriptive analysis of carotenoid production in seven mycobacterial isolates from the scotochromogenic, photochromogenic, and non-chromogenic groups. To achieve this, we used a combination of High-performance liquid chromatography with diode-array detection (HPLC-DAD) and Ultra-high performance liquid chromatography–mass spectrometry (UHPLC-MS) to detect carotenoids pigments. Mycobacterium tuberculosis (MTB) and Mycobacterium bovis (MB) (non-chromogenic mycobacteria) produced β-carotene when cultured in the absence of light, at levels comparable to those of photochromogenic mycobacteria such as M. marinum (MM) and M. kansasii (MK). The highest levels of carotenoids were found in scotochromogenic species M. avium (MAV) and M. gordonae (MGOR). Conversely, M. abscessus (MABS), a non-chromogenic species in which no β-carotene was detected, served as a negative control for matrix effects. As expected, the use of highly sensitive analytical techniques such as HPLC-DAD and UHPLC-MS significantly enhanced the detection of β-carotene compared to visual pigment assessment. These methods allowed the detection of basal β-carotene levels even in mycobacteria classified as non-chromogenic. The proposed analytical approach provides a robust research tool to understand the effects of different stimulus that may alter the cell physiology in terms of pigment production.
1. Introduction
Carotenoids are one of the most important groups of natural pigments due to their wide distribution, structural diversity, and numerous functions [1]. β-carotene, a pigment within this group, is a tetraterpene that is biochemically synthesized from eight isoprene units. These units are cyclized to form beta rings at both ends of the molecule and contain a linear structure of C40 hydrocarbons with three to fifteen conjugated double bonds [2], (Figure 1). The number of double bonds determines the spectral properties of the carotenoids, allowing them to absorb light at wavelengths between 400 and 500 nm, which confers a characteristic color to the colony or microorganisms that synthesize them (Figure 2) [3]. Regarding their functions, carotenes are involved in numerous biochemical processes, such as subcellular orientation and regulation, free radical scavenging and cell protection against photodynamic lesions and pH changes [4,5]. The production of these pigments is therefore an important characteristic of several microorganisms facing these challenges such as yeasts, filamentous fungi, micro-algae and bacteria [6,7].
Figure 1.
Chemical structure of the beta-carotene showing the carbonated backbone chains of the molecule [3].
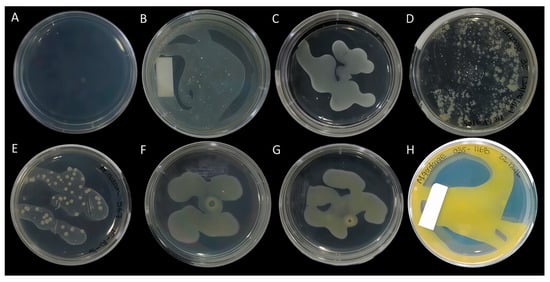
Figure 2.
Visual observation of mycobacterial pigments. Clinical isolates (A) control media; (B) M. tuberculosis; (C) M. abscessus; (D) M. bovis; (E) M. avium; (F) M. marinum; (G) M. kansasii; (H) M. gordonae.
In the genus Mycobacterium, the presence of carotenes has been widely described [2,3,8,9]. Beyond their phenotypic relevance, carotenoid biosynthesis in mycobacteria is genetically encoded by a conserved crt gene cluster, comprising the enzymes crtE (geranylgeranyl diphosphate synthase), crtB (phytoene synthase), crtI (phytoene desaturase), and crtY (lycopene cyclase), which collectively mediate the sequential conversion of isoprenoid intermediates derived from the DOXP pathway into β-carotene [3]. Comparative analyses of Mycobacterium marinum (MM), M. kansasii (MK), and M. vaccae (MV) have demonstrated that these genes are organized in a similar genomic arrangement, supporting the notion that carotenoid production is a stable and conserved trait among pigment-producing mycobacteria. However, as noted by Robledo et al. [3], some species within the genus lack visible pigmentation, suggesting that while the genetic machinery for carotenoid synthesis may persist, its transcriptional activation or enzymatic efficiency differs across species. This pattern indicates that evolutionary conservation of the crt cluster is coupled with regulatory diversification, allowing photochromogenic species such as MM and MK to produce β-carotene under light stimulation, whereas nonchromogenic species like M. tuberculosis (MTB) and M. bovis (MB) likely retain these genes for non-photogenic functions related to oxidative stress protection. This genetic heterogeneity provides a molecular explanation for the phenotypic variation described by Runyon, thereby linking genotypic regulation with pigment expression in the genus Mycobacterium.
Over the last five decades, the Runyon system has therefore been used to classify mycobacteria based on visually detected pigment production (Figure 2) into the following categories: (i) scotochromogenic, those whose production is independent of light stimulation; (ii) photochromogenic, those that produce pigmentation in response to exposure to light; (iii) those without visually detectable pigment (non-chromogenic) regardless of light stimulation. This classification system was complemented by the analysis of the proliferation rate: slow-growing (taking more than a week to develop colonies) or fast-growing (taking less than a week to develop colonies) [10]. These characteristics, together with a series of biochemical tests, acquired taxonomic value and contributed to species identification. Despite still being useful today, this method is unsatisfactory due to its basis on visual detection, which lacks sensitivity and reproducibility in determining the presence or absence of pigment. Thus, thin layer chromatography (TLC) was incorporated to detect carotenoids in different mycobacterial species [11,12]. Although this resulted in a significant improvement, TLC only allowed qualitative determinations, or, in the best situation, semi-quantitative determinations of carotenes, with poor sensitivity.
High-performance liquid chromatography HPLC-based analyses of different types of crude samples for the detection of carotenoids are important in several applications, such as food chemistry and medicine [13,14,15]. This method offers significant advantages in terms of simplicity, speed, cost, sensitivity, specificity, precision, and sample preservation. The use of a diode array detector (DAD) coupled to HPLC enables continuous data collection, which helps determine peak purity. Liquid chromatography–mass spectrometry (LC-MS) is even more specific and sensitive, providing information on carotenoid molecular mass and fragmentation patterns, with a detection threshold in the range of 500 fmol for individual carotenoids [15].
Based on the need of more sensitive and reproducible methods to detect β-carotene for identifying mycobacterial species pathogenic to humans, we developed an optimized pipeline for its detection. The method was validated for detecting basal levels of β-carotene production in seven different species: MTB, MB, MM, MK, M. avium (MAV), M. gordonae (MGOR) and M. abscessus (MABS).
2. Materials and Methods
2.1. Mycobacterial Material
Seven representative species of mycobacteria were used in this study. Three different isolates were obtained from clinical samples, which were stored at −70 °C in the strain bank of the Bacteriology and Mycobacteria Unit of the Corporation for Biological Research (CIB) in Medellín, Colombia. The species of clinical interest selected were MTB, MB, MM, MK, MAV, MGOR and MABS (Table A1).
2.2. Sample Preparation
Each isolate from the strain bank was reconstituted in Middlebrook 7H9 medium for its functional and metabolic reactivation over a period ranging from one to two weeks, depending on the mycobacterial species. Then, a scale culture was started, for which an initial inoculum of 1 mL of bacterial cells was placed in 50 mL of medium until it reached a McFarland turbidity standard of 1, equivalent to 3 × 108 CFU/mL, and subsequently transferred to 500 mL of medium. All the cultures in each of their stages were kept in conditions of total darkness, constant shaking (85 RPM) and incubation at 37 °C, until reaching a stationary growth phase, considering two weeks for the fast-growing species and four weeks for the slow-growing species. For β-carotene extraction, 3 × 108 CFU/mL in the stationary phase were harvested by centrifugation (Sorvall legend RT refrigerated, Boston, MA, USA) at 4150 RPM × 15 min at 4 °C, and the cell pellet was separated from the supernatant. The cell pellet was homogenized with 1 mL of sterile isotonic saline solution (NaCl 0.85%) and 200 µL of lysozyme was added at 20 mg/mL (Sigma, Darmstadt, Germany; L-6876), followed by incubation at 37 °C overnight in the dark. β-carotene extraction was based on a standard method (13), which involves saponification of the cell pellet with 100 mL of absolute ethanol (Cat. 107017, Merck, Darmstadt, Germany;) and 20 mL of potassium hydroxide (KOH) (Cat. 105033, Merck, Germany) at 60%, mixed with 1 g of ascorbic acid (Cat. 47863, Sigma, Germany) to prevent oxidation during the extraction process. The mixture was incubated at 30 °C for 1 h, then passed 3 times through a separatory funnel with 50 mL volumes of hexane until the solution was decolorized. The pigmented fraction corresponding to the hexane phase, was recovered each time. The combined hexane phases were collected in an amber flask and evaporated under reduced pressure at 40 °C and 40 RPM in a rotary evaporator (BUCHI, Flawil, Switzerland; R-210) until complete.
2.3. HPLC-DAD Analysis
The analysis was performed on an Agilent 1200 series chromatograph (Germany) with a C30 column (5 μm; 150 × 4.6 mm Thermo Scientific, Waltham, MA, USA) and equipped with a diode-array detector. An isocratic method 75:24.9:0.1 ethanol, hexane and 2-propanol, respectively, over 8 min at a flow rate of 1 mL min−1 at 20 °C was used as the mobile phase. Detection was performed at 450 nm. For method validation, the following aspects were considered: linearity, limits of detection and quantification, precision and accuracy, percentage of recovery and stability. In this study, particular emphasis was placed on the linearity parameter because it ensures the reliability and reproducibility of quantification across the tested concentration range. Establishing linearity was critical to confirm that the detector response was directly proportional to β-carotene concentration, which in turn allowed accurate comparisons of pigment levels among different Mycobacterium species. This parameter also minimized potential bias from matrix effects and provided the necessary robustness for subsequent biological interpretations. As a reference, the commercial β-carotene (type II, synthetic, ≥95%, C4582, Sigma-Aldrich, Steinheim, Germany) was used to prepare the standard curve for quantification. For method calibration, a standard curve with a scale that moved from 0.0098 µg to 10 µg of β-carotene was prepared by mobile phase dilution from the standard solution. HPLC methods use either isocratic or gradient mobile phases in reversed-phase or normal-phase mode [13,16]. Since carotenoids are easily oxidized, it is useful to add antioxidants to the extraction solvent and mobile phase and to keep a low, constant column temperature [17].
2.4. UHPLC-MS Analysis
To unambiguously confirm the identity of the pigment, mass spectra of carotenoids were recorded using a Shimadzu UHPLC with an LCMS 2020 mass detector. The DL temperature was 275 °C, and the heat block was set to 425 °C, with a nebulizing gas flow of 1.5 L/min. Mass spectra were recorded over a range of 200 to 600. Chromatographic separation was achieved using an XDB-C18 column (1.8 μm; 2.1 × 50 mm ZORBAX Eclipse) at 40 °C with an isocratic method 55:45 acetonitrile and methanol, respectively, over 10 min at a flow rate of 0.5 mL min−1.
A β-carotene standard was used to determine the peak retention times. HPLC column properties were checked at regular intervals and re-evaluated using the standard. Pigment peak identification was performed using HPLC-DAD with the calibration curve of the standard, allowing for sample quantification and confirming the presence of β-carotene via UHPLC-MS.
3. Results
3.1. Analytical Characteristics of the Beta-Carotene Detection Method by HPLC-DAD
For the linearity parameter, calibration curves were prepared in triplicate within the defined range. All curves demonstrated excellent linearity (R2 > 0.999) across the analyzed range, from 0.0098 µg/mL to 10 µg/mL (Table A4, Figure A1). The limit of detection (LOD) was determined as 0.003 µg/mL (signal area of 1584 AU), and the limit of quantification (LOQ) as 0.01 µg/mL (signal area of 3168 AU). Both values fall below the lowest point of the calibration range, confirming the sensitivity of the method. In Mycobacterium, there are no previous references for the minimum detectable levels of β-carotene; therefore, the LOQ obtained here demonstrates that the proposed method is suitable for detecting β-carotene without matrix interference. It is important to note that previously reported LOD/LOQ values for β-carotene from other biological sources are expressed in terms of concentration (0.157 µg/mL for LOD and 0.52 µg/mL for LOQ) [18].
Precision and accuracy were assessed in triplicate, considering both repeatability and reproducibility [%Re]) and evaluated using the coefficient of variation and relative error percentages, which were less than 20% (according to the Bioanalytical Method Validation, FDA. 2018), corresponding to the maximum accepted variation (Table A5, Figure A2). Three concentrations were used (0.039 µg/mL, 0.625 µg/mL and 10 µg/mL of the standard) to determine the %Re, and we worked with standards in a diluent solution as a reference value, which was equivalent to 100%. The average %Re of the applied method was 98.6% with a coefficient of variation of 11.4%. The matrix for this case did not show appreciable interferences, as evidenced by this result (≈100%). The value of 100% indicates that no measurable matrix interference was observed, and that the recovery rate was equivalent to that obtained with the standard alone. Thus, the term reflects both the recovery efficiency and the absence of matrix effects in our quantification.
3.2. Quantification of B-Carotene by HPLC-DAD
Among the 21 isolates evaluated from seven species of mycobacteria, two isolates from two species evaluated showed high β-carotene content, around 4 µg/mL. Figure 3 shows the β-carotene content per species. MGOR exhibited the highest detected amounts (Figure A3), while MABS was used as the control since no pigment was detected in the evaluated isolates. Importantly, basal levels of β-carotene were also observed in the photochromogenic species MM and MK, even under dark growth conditions.
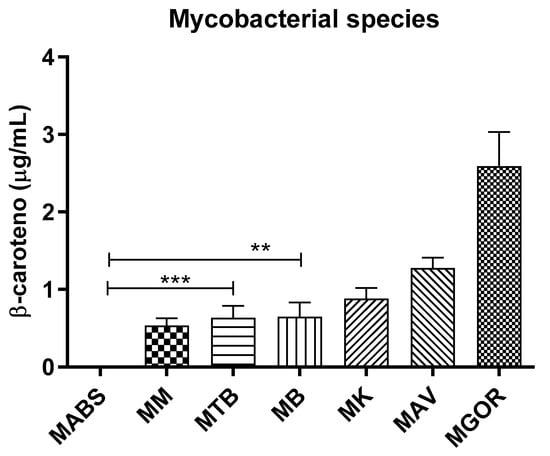
Figure 3.
Inter-species production levels of β-carotene in mycobacteria of clinical interest obtained by HPLC-DAD. MABS (M. abscessus); MM (M. marinum); MTB (M. tuberculosis); MB (M. bovis); MK (M. kansasii); MAV (M. avium); MGOR (M. gordonae). Statistical significance was determined by one-way ANOVA and performed in GraphPad Prism 8.0, *** p < 0.001; ** p < 0.01.
Despite detecting pigments in six of the seven evaluated species, intraspecies analysis revealed significant differences between isolates. Figure 4 shows the detection of β-carotene for each isolate within each species. For MTB and MB, one isolate from each species exhibited either no or low β-carotene detection (Figure A4). However, the other two examined isolates showed pigment levels comparable to the average range found among species classified as chromogenic or photochromogenic (Figure A5), corresponding to 0.7–1.3 µg/mL. Calculations were based on a standard curve generated using commercial β-carotene (Table A4, Figure A1).
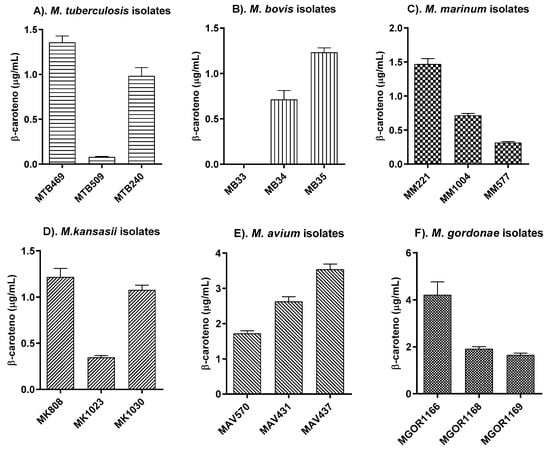
Figure 4.
Production levels of intraspecies β-carotene among isolates of mycobacteria of clinical interest obtained by HPLC-DAD. (A) M. tuberculosis; (B) M. bovis; (C) M. marinum; (D) M. kansasii; (E) M. avium; (F) M. gordonae.
3.3. Detection of β-Carotene Confirmed by UHPLC-MS
Samples quantified by HPLC-DAD were analyzed using the UHPLC-MS method to confirm whether the detected pigment corresponded to β-carotene. Table 1 presents both the quantified numerical values and qualitative data regarding the presence or absence of pigment in each isolate of each species. The analytical characteristics of the UHPLC-MS β-carotene detection method include general parameters (Table A2), system suitability (Table A3), selectivity (Appendix A.4), and detection limits (Appendix A.5).

Table 1.
Detection levels by HPLC-DAD of β-carotene and confirmation by UHPLC-MS in the 3 isolates of the mycobacteria of clinical interest.
4. Discussion
To assess the ability of the proposed HPLC-DAD method to detect β-carotene in clinically relevant samples, analytes were initially extracted from cultures of three different clinical isolates per species, each tested in triplicate. MGOR, a primarily environmental and opportunistic scotochromogenic species, exhibited the highest β-carotene levels (3 µg/mL) among the tested species, corresponding to the amount produced through constitutive biosynthesis. For the photochromic species MM and MK, the obtained values were 0.7 µg/mL and 1.22 µg/mL, respectively. These lower values, compared to those of MGOR, are comparable to those of non-chromogenic species. In addition, this is consistent with the fact that the extracts were not exposed to light during their preparation. Remarkably, since the 1970s, the presence of β-carotene has been documented in both species, but there is no reference to quantified detectable levels [12,19].
Gao et al. [20] showed that the absence of pigment increases cellular susceptibility to singlet oxygen and identified two homologous (putative) genes within an operon in MTB, which may be associated with pigment synthesis. These results are consistent with the baseline β-carotene levels observed in both MTB and MB (members of the MTB complex), which are comparable to those found in MM (Figure 3). However, some variability was observed among MTB and MB isolates, despite their being cultured under identical amplification and growth conditions. This variability could reflect differences among the original clinical sources, as mycobacterial isolates often display phenotypic diversity influenced by their genetic backgrounds, infection histories, and adaptive responses to host environments. Nonetheless, these interpretations remain speculative in the absence of genotypic data. To determine whether such heterogeneity arises from true genetic or regulatory differences, future studies should incorporate whole-genome sequencing or transcriptomic profiling to identify potential determinants underlying pigment variability.
Regarding MAV, it is one of the most important NTMs causing mycobacteriosis and is classified as a non-chromogenic species. However, both pigmented and non-pigmented colonies have been observed in isolates of this species and have been associated with antibiotic resistance [21]. At the basal level, MAV produces significant amounts of β-carotene compared to the species included in this analysis, linking it to scotochromogenic species with constitutive pigment production (Table 1). For MABS, β-carotene was not detected at the basal level within the range and conditions established for this method, which is consistent with its classification as a non-chromogenic species. However, a study by Saviola & Felton [22] reported that MABS presents pigmentation under acidic pH conditions.
These inter-species comparisons provide a baseline for understanding β-carotene production across clinically and environmentally relevant mycobacteria. Building upon this, a closer examination of intra-species variability revealed additional layers of complexity. Notably, significant differences were observed among clinical isolates belonging to the same species (Figure 4). For instance, within the MB group, the coefficient of variation in β-carotene concentrations reached 23.53%, reflecting substantial inconsistency among replicates of MB34, while no analyte was detected in MB33 using the applied method.
Although most species presented acceptable coefficients of variation (<20%), MAV 570 approached this upper threshold (18.11%), further reinforcing the presence of intra-species heterogeneity (Table 1). This challenges the assumption that pigment production is homogeneously expressed within a species, particularly for members of the MTB complex. Such findings suggest a strain-specific metabolic plasticity that may reflect differences in gene regulation, mutations in biosynthetic pathways, or environmental adaptation.
Importantly, the presence or absence of β-carotene as detected by HPLC-DAD was confirmed using UHPLC-MS, which provided rapid, high-specificity spectra for the target compound (m/z 536). This cross-validation confirmed that the detected signals corresponded to β-carotene and not to other structurally similar molecules, reinforcing the biological relevance of the findings.
The clinical isolates used in this research were obtained from the CIB collection of microorganisms, in which they are stored at −70 °C since the time of isolation and identification, without being exposed to successive passages. This preservation method minimizes alterations in phenotypic expression, allowing the retention of pigments anchored in the cell membrane as a result of mechanisms activated prior to cryopreservation. The mechanisms inducing pigment synthesis in these isolates remain unknown, including potential factors such as exposure to UV light [18], pH changes [9,22] and intracellular response in macrophages [20].
While pigmentation remains a fundamental taxonomic criterion in the classification of mycobacteria, our findings reinforce that visual inspection alone is insufficient to confirm pigment production. The application of HPLC-DAD in this study enabled the detection of basal β-carotene levels in species considered non-pigmented, such as MTB and MB. In carotenoid analysis, saponification is commonly used to release esterified forms and reduce the risk of artifact formation. However, several studies have shown that saponification can adversely affect analytical precision [23]. Moreover, because carotenoids are often bound to fatty acids within bacterial membranes, saponification may interfere with pigment identification and yield inconsistent results [1]. Scott et al. [24] demonstrated that sample preparation contributes significantly to overall analytical variability, with saponification conditions—including time, temperature, potassium hydroxide concentration, number of extraction cycles, and initial sample volume, playing critical roles in result accuracy. In our study, reliance on a single saponification protocol may therefore have predisposed the results to methodological bias and partially accounted for the variability observed among isolates.
In the present study, different extraction protocols were not compared, and thus the influence of the selected method on β-carotene quantification remains undetermined. However, the adopted extraction procedure, based on the method described by Radu et al. [25], enabled consistent detection in most analyzed samples. Notably, the absence of detectable β-carotene in MABS confirmed its suitability as a negative reference in method validation, both in visual and chromatographic assessments.
Validation parameters applied to the HPLC-DAD method demonstrated that it is a linear, accurate, and precise approach, with a metabolite recovery rate close to 100%. Moreover, the UHPLC-MS analysis confirmed the specificity of the β-carotene detected, validating its presence even in non-chromogenic species such as MB and MTB. These findings expand our understanding of pigment expression in the Mycobacterium genus and demonstrate the value of chromatographic detection in surpassing the limitations of phenotypic assessments.
Further studies are needed to compare isolates from diverse clinical conditions and determine whether β-carotene production correlates with host–pathogen interactions or disease progression. Additionally, the potential regulatory and protective functions of carotenoids in response to oxidative damage should be investigated, as these compounds may contribute to bacterial persistence in hostile environments. In this study, photochromogenic species were intentionally not exposed to light, as our objective was to quantify basal pigment production under standardized conditions, allowing direct comparison across all species. Nevertheless, we acknowledge that light exposure typically induces higher carotenoid production in these species. Future studies will therefore compare β-carotene levels in photochromogenic isolates under both light-exposed and non-exposed conditions. This comparative approach will not only enhance the robustness of our findings but also position pigment quantification as a potential research tool for exploring light-dependent regulatory mechanisms in mycobacteria, rather than as an immediate diagnostic strategy.
Building upon this premise, the photoinducible behavior observed in certain species has been linked to the transcriptional activation of the crt operon upon light exposure, particularly in MM and MK. In their comprehensive review, Robledo et al. [3] compiled molecular and genetic evidence suggesting that these photochromogenic species possess a conserved crtE–crtB–crtI–crtY gene cluster, whose expression is modulated by light through a phytochrome-like regulatory system. Although their analysis did not include experimental validation, it established a conceptual framework in which β-carotene synthesis in photochromogenic mycobacteria is understood as a genetically mediated, light-responsive process rather than as passive pigment accumulation.
In line with this model, complementary biochemical studies on MK revealed that this species predominantly synthesizes α-carotene (~85%) and lycopene (~15%) as its main carotenoids, while other derivatives appear only in trace amounts [11,26]. This compositional pattern indicates a highly efficient conversion of precursor molecules into terminal products, namely α- and β-carotene, and possibly leprotene. Among these, β-carotene emerges as the principal end product and the pigment most directly involved in photooxidative protection. These findings collectively support the notion that light-induced carotenoid biosynthesis in MK constitutes a genetically regulated adaptive response, integrating environmental cues with metabolic efficiency to strengthen bacterial resilience under oxidative stress conditions.
Expanding on these biochemical insights, recent genomic and transcriptomic analyses have refined the understanding of carotenoid biosynthesis regulation in photochromogenic mycobacteria. According to Janisch et al. [27], two distinct crt gene clusters (crtE–crtB–crtI–crtY and crtW–crtZ) are differentially expressed under light exposure, indicating the existence of specialized biosynthetic routes for various carotenoid derivatives. The study also identified a putative photoreceptor-regulated network that controls crt transcription through a light-inducible, MarR-type transcriptional regulator homologous to CrtR found in other Actinobacteria, suggesting that the light-dependent activation of carotenogenesis in MK involves a dual regulatory system integrating both photoreception and metabolic control.
These findings are consistent with evidence from Henke et al. [28], which demonstrated that CrtR functions as a negative regulator of carotenoid biosynthesis under dark conditions and is inactivated by light-induced conformational changes. The conservation of CrtR-like proteins across Actinobacteria, including mycobacteria, supports the hypothesis that photochromogenic species such as MK utilize analogous regulatory pathways for environmental adaptation, linking gene expression to photic stimuli.
Furthermore, comparative genomic insights from Giraud et al. [29] provide additional support for this model. The study showed that the presence of multiple crt operons enables functional diversification of carotenoid biosynthesis, allowing bacteria to synthesize pigments with distinct photoprotective and structural roles. This evolutionary redundancy may also occur in mycobacteria, where maintaining parallel crt clusters could provide metabolic flexibility and enhanced adaptability to fluctuating light or oxidative conditions.
Beyond its function as a visual marker, β-carotene may play a protective role in bacterial physiology, particularly under oxidative stress conditions. In other pathogenic bacteria, such as Staphylococcus aureus, carotenoids have been shown to neutralize reactive oxygen species (ROS) generated by host immune cells, enhancing bacterial survival within macrophages [30]. Although this function has not been fully characterized in mycobacteria, the detection of β-carotene in species such as MTB and MB, both capable of intracellular persistence, raises the possibility that carotenoid biosynthesis contributes to redox homeostasis during infection. This is consistent with previous findings indicating that pigment-deficient mutants are more susceptible to oxidative damage [31], suggesting a potential adaptive role for carotenoids in the mycobacterial response to host defenses.
The detection of β-carotene in both chromogenic and non-chromogenic mycobacteria using HPLC-DAD and UHPLC-MS suggests that carotenoid profiling represents a valuable research and taxonomic tool, particularly for differentiating closely related NTM species. In species such as MGOR, MK, and MM, pigmentation is traditionally used for classification under the Runyon system. However, as demonstrated in this study, pigment production can vary between isolates and may not always be visually detectable under standard laboratory conditions. This intra-species variability, together with basal carotenoid expression in non-chromogenic species like MTB and MB, underscores the limitations of visual classification systems and highlights the use of chromatographic carotenoid quantification as a complementary approach for species characterization and metabolic assessment.
In conclusion, this study introduces a robust, sensitive, and reproducible analytical approach that reveals low-level β-carotene production in mycobacteria, extending beyond traditional chromogenic classifications. By demonstrating both inter- and intra-species variability and confirming the presence of carotenoids in species historically considered non-pigmented, these findings refine the current phenotypic understanding of Mycobacterium spp. Rather than proposing an immediate diagnostic application, this method should be viewed as a research tool to investigate the biochemical diversity, metabolic adaptation, and potential photoprotective functions of carotenoids across mycobacterial species. The observed heterogeneity in pigment expression further suggests that carotenoid biosynthesis may reflect distinct regulatory mechanisms and adaptive responses to environmental cues, offering new avenues for exploring metabolic regulation and evolutionary differentiation within the genus Mycobacterium.
This study has several limitations that should be considered when interpreting the findings. First, the isolates analyzed were obtained exclusively from the strain collection of the Bacteriology and Mycobacteria Unit at the Corporación para Investigaciones Biológicas (CIB) in Medellín, Colombia. Consequently, the results may not capture the full intra-species variability that exists among geographically distinct populations. Future investigations should include strains from different regions and ecological contexts to validate these observations.
Second, all cultures were grown under dark conditions to ensure standardized comparison across species. Although photochromogenic organisms such as MM and MK exhibit basal carotenoid production even in the absence of light, this design precluded evaluation of light-induced pigment biosynthesis. Further studies incorporating light–dark experimental contrasts are warranted to elucidate the dynamics of photoinducible regulatory systems under physiologically relevant conditions.
Third, carotenoid extraction was performed using a single saponification protocol. While saponification is a widely accepted approach in carotenoid analysis, it may alter pigment stability or recovery efficiency, potentially introducing methodological bias. Employing complementary extraction methods or non-saponifying techniques in future studies could help confirm the reproducibility and accuracy of pigment quantification.
Finally, although the analytical method proved sensitive for detecting β-carotene at low concentrations and revealed carotenoid presence in species traditionally regarded as non-pigmented, its practical implementation currently requires relatively high bacterial biomass. Culturing an isolate remains necessary for reliable pigment detection in clinical specimens.
Author Contributions
A.M.M.: Writing—original draft. Writing—review and editing. Visualization. Methodology. Investigating. Formal analysis. Validation. Funding acquisition. J.D.Z.S.: Methodology. Investigating. Formal analysis. Writing—review and editing. V.G.T.: Methodology. Investigating. Writing—review and editing. J.A.R.R.: Writing—review and editing. L.E.B.: Funding acquisition. Project administration. Validation. Writing—original draft. Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia, Tecnología e Innovación de Colombia, grant number 221365843006.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank the Centro de la Ciencia y la Investigación Farmacéutica (CECIF) at Universidad CES for its valuable support throughout the development of this research. We would also like to acknowledge the support provided by Minciencias, which greatly contributed to the development of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CFU | Colony-Forming Units |
| DAD | Diode-Array Detector |
| HPLC | High-Performance Liquid Chromatography |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode-Array Detection |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MB | Mycobacterium bovis |
| MABS | Mycobacterium abscessus |
| MAV | Mycobacterium avium |
| MGOR | Mycobacterium gordonae |
| MK | Mycobacterium kansasii |
| MM | Mycobacterium marinum |
| MTB | Mycobacterium tuberculosis |
| NTM | Nontuberculous Mycobacteria |
| TLC | Thin-Layer Chromatography |
| UHPLC-MS | Ultra-High-Performance Liquid Chromatography–Mass Spectrometry |
| Re% | Recovery percentage |
| R2 | Coefficient of determination |
| ROS | Reactive Oxygen Species |
Appendix A
Appendix A.1. Mycobacterial Species and Isolates Codes

Table A1.
Clinically relevant mycobacterial species and isolates *.
Table A1.
Clinically relevant mycobacterial species and isolates *.
| Species | Isolates |
|---|---|
| Mycobacterium tuberculosis (MTB) | MTB469 |
| MTB509 | |
| MTB204 | |
| Mycobacterium bovis (MB) | MB33 |
| MB34 | |
| MB35 | |
| Mycobacterium marinum (MM) | MM221 |
| MM577 | |
| MM1004 | |
| Mycobacterium kansasii (MK) | MK808 |
| MK1023 | |
| MK1030 | |
| Mycobacterium avium (MAV) | MAV431 |
| MAV437 | |
| MAV570 | |
| Mycobacterium gordonae (MGOR) | MGOR1166 |
| MGOR1168 | |
| MGOR1169 | |
| Mycobacterium abscessus (MABS) | MABS1158 |
| MABS1124 | |
| MABS1064 |
* The isolate codes correspond to their identification in the CIB strain collection.
Appendix A.2. Conditions and Parameters of the UHPLC-MS

Table A2.
Chromatographic conditions and the parameters of the UHPLC-MS detector.
Table A2.
Chromatographic conditions and the parameters of the UHPLC-MS detector.
| Parameters | Characteristics |
|---|---|
| Mobile phase | Acetonitrile: Methanol 55:45 |
| Flux | 0.5 mL/min |
| Injection Volume | 5 uL |
| Column | ZORBAX Eclipse XDB-C18 2.1 × 50 mm 1.8 Micron |
| Column Temperature | 40 °C |
| Retention time | 8 min |
| Run Time | 10 min |
| Mass detector parameters: | |
| Interface | |
| ESI | |
| DL temperature | 275 °C |
| Nebulizing gas flow | 1.5 L/min |
| Heat block | 425 °C |
| Drying gas flow | 10 L/min |
| Event 1 SIM (m/z) | 536.35 |
| Event 2 Scan (m/z) | 200–600 |
Appendix A.3. System Suitability
One µg/mL beta-carotene standard solution (Type II, synthetic, ≥95%, C4582, Sigma-Aldrich, Steinheim, Germany) and a sensitivity standard solution containing 0.0012 µg/mL were prepared. Each solution was then injected in duplicate at the beginning and end of each sequence. The mass-to-charge ratio (m/z) was determined for the beta-carotene standard, and the signal-to-noise ratio was determined for the sensitivity standard. Subsequently, the retention time of the beta-carotene peak in the standard was recorded, and the error was calculated relative to the retention time of the initial standard.

Table A3.
Parameters and acceptance criteria for system suitability.
Table A3.
Parameters and acceptance criteria for system suitability.
| Parameter | Acceptance Requirements |
|---|---|
| Mass accuracy for the standard | 535.85–536.85 m/z |
| Signal/noise ratio (S/N) | ≥2.0 |
| Percentage of error in final retention time | ≤10% |
Appendix A.4. Selectivity
Two matrix solutions (A and B) were independently prepared at different times following the same sample preparation protocol. From each matrix solution, three test samples were obtained by spiking with the β-carotene standard to achieve a final concentration of 10 µg/mL. These samples were injected into the instrument, and for each, two determinations were performed to assess the mass-to-charge ratio (m/z) and retention time for the detection of the β-carotene peak, alongside the 1 µg/mL standard.
Selectivity acceptance criteria were applied to both spiked samples (matrix with added analyte) and blank samples (matrix without β-carotene), as follows:
Spiked samples: A characteristic signal must be present, with a retention time percentage error (%RE) ≤ 10% relative to the retention time of the initial standard, and a measured m/z within the range of 535.85–536.85.
Blank samples: No characteristic signal should be present; any potential interference at the analyte’s retention time must exhibit a signal-to-noise ratio (S/N) < 2.0.
Appendix A.5. Detection Limit
Two matrix solutions (A and B) were independently prepared at different times, following the same sample preparation protocol. From each matrix, three test samples were spiked with the β-carotene standard to obtain a final concentration of 0.015 µg/mL. Each sample was injected into the instrument, and two determinations of the signal-to-noise (S/N) ratio were performed for the analyte. The acceptance criterion for detection was an S/N ratio ≥ 2.0.
Appendix B
Appendix B.1. Standar Curve with Commercial β-Carotene by HPLC-DAD

Table A4.
Standard curve calculations using commercial β-carotene.
Table A4.
Standard curve calculations using commercial β-carotene.
| Concentration (μg/mL) | Average Areas | Experimental Mean Concentration |
|---|---|---|
| 0.0098 | 3.547 | 0.0096 |
| 0.0195 | 6.850 | 0.0199 |
| 0.039 | 12.447 | 0.0371 |
| 0.078 | 25.840 | 0.0789 |
| 0.156 | 51.883 | 0.1606 |
| 0.312 | 108.937 | 0.3368 |
| 0.624 | 203.580 | 0.6300 |
| 1.248 | 402.617 | 1.2504 |
| 2.496 | 811.213 | 2.5284 |
| 4.992 | 1570.057 | 4.8936 |
| 9.984 | 3226.123 | 10.0449 |
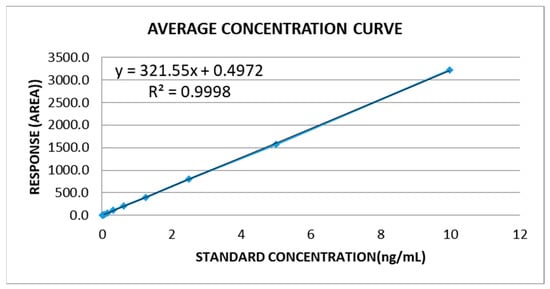
Figure A1.
Standard curve generation using commercial β-carotene as the reference standard for HPLC-DAD.
Appendix B.2. Precision and Accuracy Parameters for HPLC-DAD

Table A5.
Inter-assay precision and accuracy (n = 9, 3 trials).
Table A5.
Inter-assay precision and accuracy (n = 9, 3 trials).
| Theoretical Concentration (µg/mL) | Determined Concentration (µg/mL) | |
|---|---|---|
| 0.1563 | Mean= | 0.17 |
| %CV= | 9.0 | |
| %ER= | 11.9 | |
| 2.5 | Mean= | 2.35 |
| %CV= | 9.6 | |
| %ER= | 5.8 | |
| 10.0 | Mean= | 10.90 |
| %CV= | 3.3 | |
| %ER= | 9.0 | |
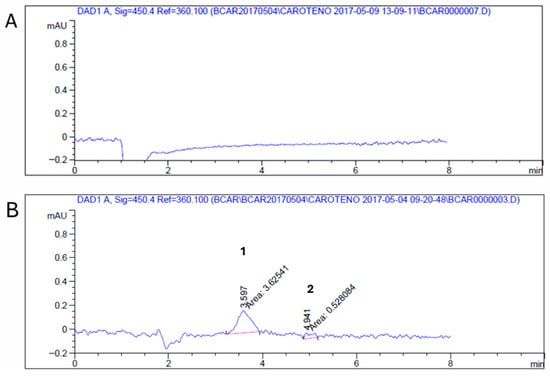
Figure A2.
Representative chromatograms for the determination of the limit of detection (LOD) and limit of quantification (LOQ) of β-carotene. Both chromatograms are shown with the same retention time scale (0–10 min) for direct comparison. (A) Reference matrix without detectable β-carotene signal. (B) Standard sample at the LOQ level, where (1) indicates the β-carotene detection peak (retention time ~3.6 min) and (2) indicates the noise area used for signal-to-noise evaluation.
Appendix B.3. HPLC-DAD and UHPLC-MS Specters for Chromogenic, Non-Chromogenic and Photochromogenic Mycobacterial Species
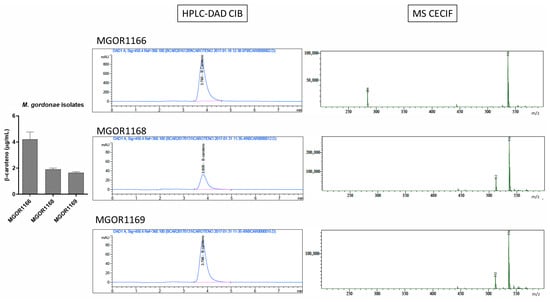
Figure A3.
Chromatographic and mass spectrometry spectra obtained for the scotochromogenic species Mycobacterium gordonae (MGOR).
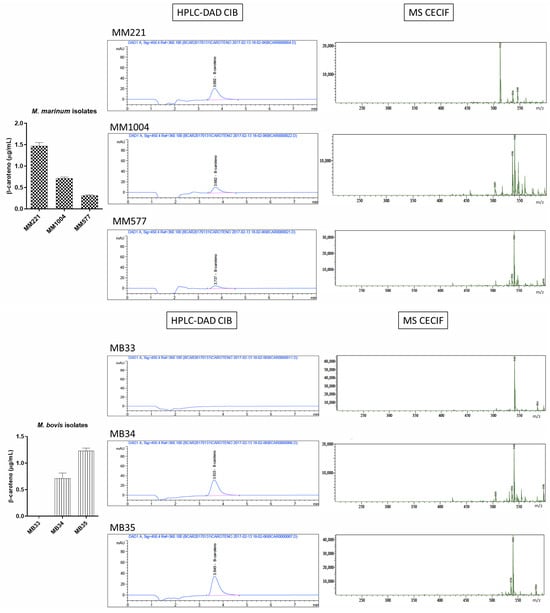
Figure A4.
Chromatographic and mass spectrometry spectra obtained for non-chromogenic species: Mycobacterium tuberculosis (MTB) and Mycobacterium bovis (MB).
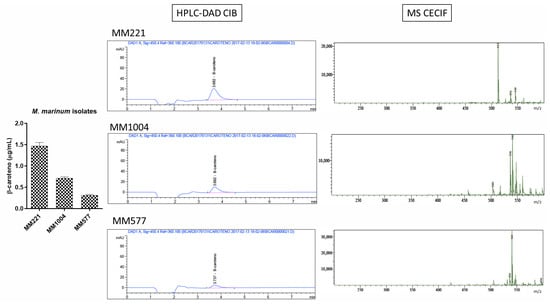
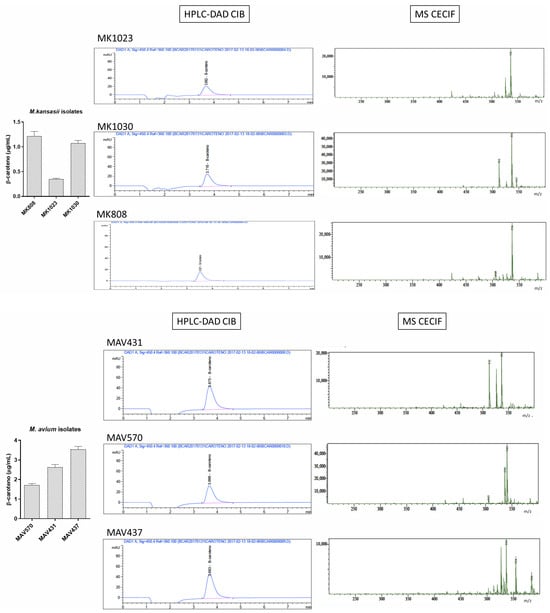
Figure A5.
Chromatographic and mass spectrometry spectra obtained for photochromogenic species: Mycobacterium marinum (MM), Mycobacterium kansasii (MK), and Mycobacterium avium (MAV).
References
- López, G.D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef]
- Brennan, P.J.; Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef]
- Robledo, J.A.; Murillo, A.M.; Rouzaud, F. Physiological role and potential clinical interest of mycobacterial pigments. IUBMB Life 2011, 63, 71–78. [Google Scholar] [CrossRef]
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Phadwal, K. Carotenoid biosynthetic pathway: Molecular phylogenies and evolutionary behavior of crt genes in eubacteria. Gene 2005, 345, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Alves da Costa Cardoso, L.; Feitosa Kanno, K.Y.; Grace Karp, S. Microbial production of carotenoids A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Ichiyama, S.; Shimokata, K.; Tsukamura, M. Relationship between Mycobacterial Species and Their Carotenoid Pigments. Microbiol. Immunol. 1988, 32, 473–479. [Google Scholar] [CrossRef]
- Saviola, B. Pigments and Pathogenesis. J. Mycobact. Dis. 2014, 4, 168. [Google Scholar] [CrossRef]
- Runyon, E.H. Pigment variations in photochromogenic mycobacteria with special reference to M. vaccae. Pneumonologie 1970, 142, 90–93. [Google Scholar] [CrossRef]
- David, H.L. Carotenoid Pigments of Mycobacterium kansasii. Appl. Microbiol. 1974, 28, 696–699. [Google Scholar] [CrossRef]
- Tárnok, I.; Tárnok, Z.S. Carotene and xanthophylls in mycobacteria. I. Technical procedures; thin-layer chromatographic patterns of mycobacterial pigments. Tubercle 1970, 51, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lao, Y.M.; Zhou, J.; Zhang, H.J.; Cai, Z.H. Simultaneous determination of 13 carotenoids by a simple C18 column-based ultra-high-pressure liquid chromatography method for carotenoid profiling in the astaxanthin-accumulating Haematococcus pluvialis. J. Chromatogr. A 2017, 1488, 93–103. [Google Scholar] [CrossRef]
- López-García, D.; de Molina, M.G. Co-Producing Agro-Food Policies for Urban Environments: Toward Agroecology-Based Local Agri-food Systems. In Urban Agroecology; CRC Press: Boca Raton, FL, USA, 2020; pp. 189–208. [Google Scholar] [CrossRef]
- Su, Q.; Rowley, K.G.; Balazs, N.D.H. Carotenoids: Separation methods applicable to biological samples. J. Chromatogr. B 2002, 781, 393–418. [Google Scholar] [CrossRef]
- Miller, K.W.; Yang, C.S. An isocratic high-performance liquid chromatography method for the simultaneous analysis of plasma retinol, α-tocopherol, and various carotenoids. Anal. Biochem. 1985, 145, 21–26. [Google Scholar] [CrossRef]
- Hart, D.J.; Scott, K.J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem. 1995, 54, 101–111. [Google Scholar] [CrossRef]
- Tsukamura, M. Carotenoids of Photochromogens of “Unclassified Mycobacteria”. J. Biochem. 1962, 51, 169–171. [Google Scholar] [CrossRef]
- Tárnok, I.; Röhrscheidt, E.; Tarnok, Z.S. Carotenes and xanthophylls in mycobacteria. III. Influence of light on the pigment synthesis of chromogenic bacteria. Tubercle 1973, 54, 225–233. [Google Scholar] [CrossRef]
- Gao, L.Y.; Groger, R.; Cox, J.S.; Beverley, S.M.; Lawson, E.H.; Brown, E.J. Transposon Mutagenesis of Mycobacterium marinum Identifies a Locus Linking Pigmentation and Intracellular Survival. Infect. Immun. 2003, 71, 922–929. [Google Scholar] [CrossRef]
- Stormer, R.S.; Falkinham, J.O. Differences in antimicrobial susceptibility of pigmented and unpigmented colonial variants of Mycobacterium avium. J. Clin. Microbiol. 1989, 27, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Saviola, B.; Felton, J. Acidochromogenicity is a common characteristic in nontuberculous mycobacteria. BMC Res. Notes 2011, 4, 466. [Google Scholar] [CrossRef]
- Konings, E.J.M.; Roomans, H.H.S. Evaluation and validation of an LC method for the analysis of carotenoids in vegetables and fruit. Food Chem. 1997, 59, 599–603. [Google Scholar] [CrossRef]
- Scott, K.J.; Finglas, P.M.; Scale, R.; Hart, D.J.; de Froidmont-Görtz, I. Interlaboratory studies of HPLC procedures for the analysis of carotenoids in foods. Food Chem. 1996, 57, 85–90. [Google Scholar] [CrossRef]
- Radu, G.; Litescu, S.C.; Camelia, A.; Teodor, E.D.; Badea, G. Beta-carotene and lycopene determination in new enriched bakery products by HPLC-DAD method. Rom. Biotechnol. Lett. 2012, 17, 7005–7012. [Google Scholar]
- David, H.L. Biogenesis of β-Carotene in Mycobacterium kansasii. J. Bacteriol. 1974, 119, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Janisch, N.; Levendosky, K.; Budell, W.C.; Quadri, L.E.N. Genetic Underpinnings of Carotenogenesis and Light-Induced Transcriptome Remodeling in the Opportunistic Pathogen Mycobacterium kansasii. Pathogens 2023, 12, 86. [Google Scholar] [CrossRef]
- Henke, N.A.; Austermeier, S.; Grothaus, I.L.; Götker, S.; Persicke, M.; Peters-Wendisch, P.; Wendisch, V.F. Corynebacterium glutamicum CrtR and Its Orthologs in Actinobacteria: Conserved Function and Application as Genetically Encoded Biosensor for Detection of Geranylgeranyl Pyrophosphate. Int. J. Mol. Sci. 2020, 21, 5482. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Hannibal, L.; Fardoux, J.; Jaubert, M.; Jourand, P.; Dreyfus, B.; Sturgis, J.N.; Verme, A. Two Distinct crt Gene Clusters for Two Different Functional Classes of Carotenoid in Bradyrhizobium. J. Biol. Chem. 2004, 279, 15076–15083. [Google Scholar] [CrossRef]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-Y.; Laval, F.; Lawson, E.H.; Groger, R.K.; Woodruff, A.; Morisaki, J.H.; Cox, J.S.; Daffe, M.; Brown, E.J. Requirement for kasB in Mycobacterium mycolic acid biosynthesis, cell wall impermeability and intracellular survival: Implications for therapy. Mol. Microbiol. 2003, 49, 1547–1563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).