Microbial Consortia in the Remediation of Single-Use Waste: The Case of Face Masks
Abstract
1. Introduction
2. Materials and Methods
2.1. Sediment Sample Collection
2.2. Microcosm Generation Prior to Isolation and Scaling
2.3. Maintenance and Scaling of Parent Bioreactors
2.4. Selection of Carbon Source
2.5. Inoculation
2.6. Evaluation of Substrate Biodegradation
2.7. Physical Evaluation of Substrate Biodegradation
2.8. Growth Kinetics of the Microbial Consortium Using McFarland Scale and Spectrophotometry
2.9. Identification of Microorganisms
3. Results and Discussion
3.1. Biodegradation of Disposable Face Masks
3.2. Morphological Changes in Face Mask Microfibers
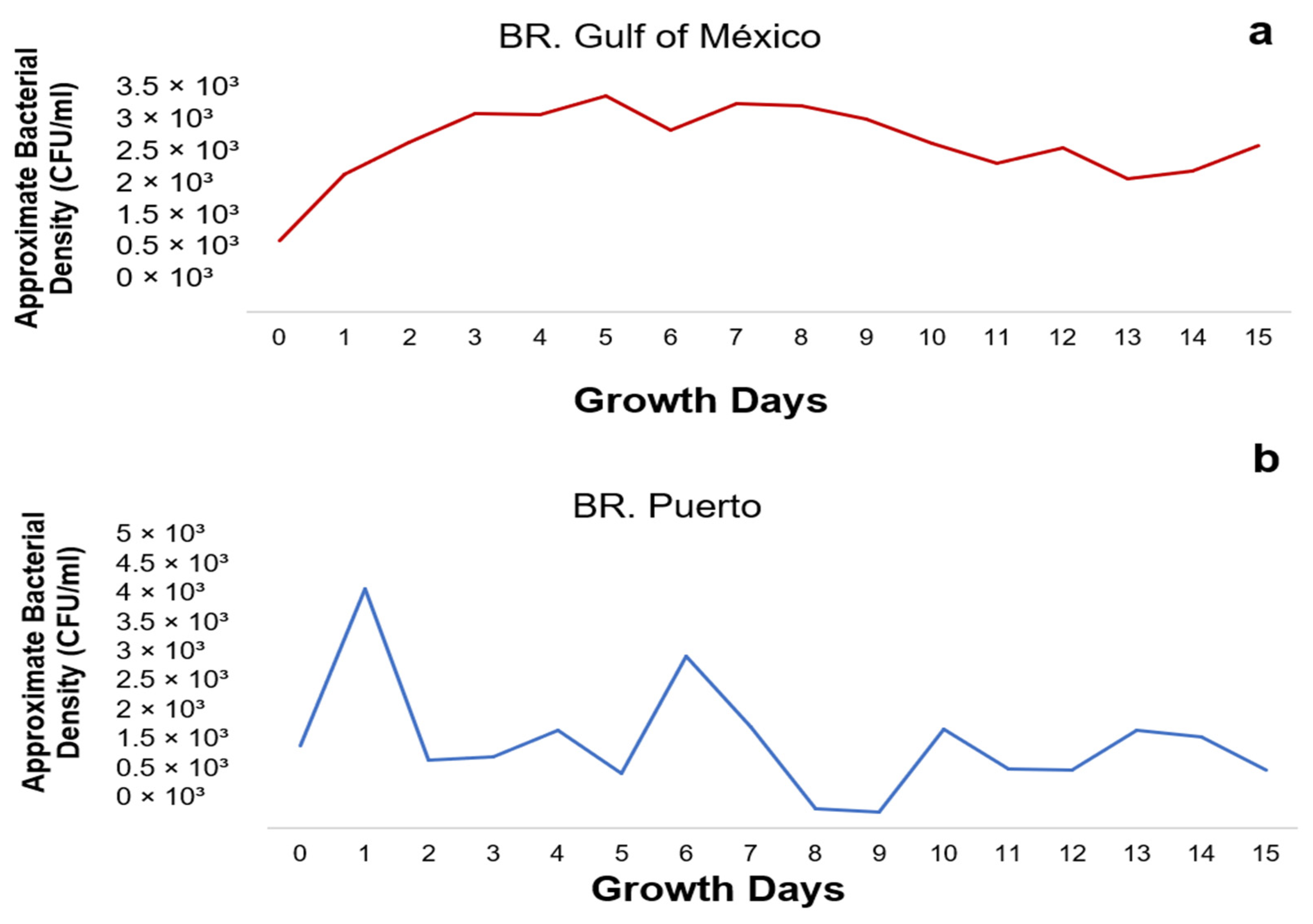
3.3. Growth Kinetics of the Gulf of Mexico Consortium (BR1)
3.4. Growth Kinetics of the Puerto Consortium (BR2)
3.5. Identification of Bacterial Consortia from Puerto and Gulf of Mexico
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segura, D.; Noguez, R.; Espín, G. Contaminación ambiental y bacterias productoras de plásticos biodegradables. Biotecnología 2007, 14, 361–372. [Google Scholar]
- Pérez, J.P. La industria del plástico en México y el mundo. Comer. Exter. 2014, 64, 6–9. [Google Scholar]
- Picó, Y.; Barceló, D. Analysis and prevention of microplastics pollution in water: Current perspectives and future directions. ACS Omega 2019, 4, 6709–6719. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Servín, T.E.; Nava, L.H.; Romero, G.A.T.; Sánchez, G.F.J.; Huerta, G.G. Equipo de protección personal y COVID-19. Cir. Gen. 2020, 42, 116–123. [Google Scholar]
- Anzures, A.; Govela, A.; Hernández, A.F.I.; Martínez, A.M.M.; Rangel, N. Efecto protector de los cubrebocas en épocas de COVID-19: Estudio experimental. Arch. Med. Salud Educ. Médica 2022, 1, 2–9. [Google Scholar]
- Allsopp, M.; Walters, A.; Santillo, D.; Johnston, P. Contaminación por Plásticos en los Océanos del Mundo. 2007. Available online: http://www.bionica.info/biblioteca/allsopp2007contaminacion.pdf (accessed on 12 September 2022).
- Flores, P. La problemática del consumo de plásticos durante la pandemia de la COVID-19. South Sustain. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Rodríguez, N.L.S.; Vera, M.D.F. Repercusión en las Costas Marinas Asociada al Uso de Equipo de Protección Personal y Micro plásticos Durante la Pandemia COVID-19: Revisión Sistemática. Ph.D. Thesis, Universidad César Vallejo, Vallejo, Peru, 2022. [Google Scholar]
- Acuña, A.; Pucci, G.; Morales, M.J.; Pucci, O. Biodegradación de petróleo y sus derivados por la comunidad bacteriana en un suelo de la Patagonia Argentina. Rev. Soc. Venez. Microbiol. 2010, 30, 29–36. [Google Scholar]
- EPA. Guía del Ciudadano: Técnicas de Tratamiento Innovadoras para Suelos Contaminados, Fango Residual, Sedimentos y Detritos. National Service Center for Environmental Publications (NSCEP). 2001. Available online: https://nepis.epa.gov/Exe/ZyNET.EXE?ZyActionL=Register&User=anonymous&Password=anonymous&Client=EPA&Init=1 (accessed on 12 October 2023).
- Hidalgo, J.C. Efectos de los derrames de petróleo sobre los hábitats marinos. Cienc. Ahora 2009, 24, 22–30. [Google Scholar]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- García-Cruz, N.U.; Valdivia-Rivera, R.; Narciso Ortiz, O.; García-Maldonado, J.Q.; Uribe-Flores, M.M.; Aguirre-Macedo, M.L.; Lizardi-Jiménez, M.A. Diesel uptake by an indigenous microbial consortium isolated from sediments of the Southern Gulf of Mexico: Emulsion characterisation. Environ. Pollut. 2019, 250, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Chávez, M.R.; Isidoro-Pio, A.J.; Lango-Reynoso, F.; Lizardi, J.M.A. Bubble Column Bioreactor using native non-genetically modified organisms: A remediation alternative by hydrocarbon-polluted water from the Gulf of Mexico. Int. J. Chem. React. Eng. 2022, 21, 431–443. [Google Scholar] [CrossRef]
- Lizardi, J.M.A.; Gutiérrez, R.M. Contribución al estudio de la hidrodinámica y transferencia simultánea de masa en biorreactores airlift de tres fases: Producción de un consorcio microbiano degradador de petróleo. Rev. Mex. Ing. Química 2011, 10, 8–11. [Google Scholar]
- Pucci, G.N.; Acuña, A.; Tonin, N.; Tiedemann, C.; Pucci, O.H. Diversidad de bacterias cultivables con capacidad de degradar hidrocarburos de la playa de Caleta Córdova, Argentina. Rev. Peru. De Biol. 2010, 17, 237–244. [Google Scholar] [CrossRef]
- Medina, M.S.A.; Jiménez, G.A.; Gutiérrez, R.M.; Lizardi, J.M.A. Hexadecane aqueous emulsion characterization and uptake by an oil-degrading microbial consortium. Int. Biodeterior. Biodegrad. 2013, 84, 1–7. [Google Scholar] [CrossRef]
- Quiroga, G.N. Reactor con Consorcios Bacterianos Degradador de Plásticos. Ph.D. Thesis, Instituto Tecnológico de Tehuacán, Tehuacán, Mexico, 2021. [Google Scholar]
- Angeles, O.; Medina, M.S.; Jiménez, G.A.; Coreño, A.A.; Lizardi, J.M.A. Predominant mode of diesel uptake: Direct interfacial versus emulsification multiphase bioreactor. Chem. Eng. Sci. 2017, 165, 108–112. [Google Scholar] [CrossRef]
- Denis, B.; Pérez, O.A.; Lizardi, J.M.; Dutta, A. Numerical evaluation of direct interfacial uptake by a microbial consortium in an airlift bioreactor. Int. Biodeterior. Biodegrad. 2017, 119, 542–551. [Google Scholar] [CrossRef]
- Koneman, E.; Allen, S.; Dowell, V.; Sommers, H. Diagnóstico Microbiológico, 6th ed.; Médica Panamericana: Buenos Aires, Argentina, 2006. [Google Scholar]
- Gerhardt, R.; Murray, E.; Wood, W.; Krieg, L. Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Escobar, L.F.; Rojas, C.A.; Giraldo, G.A.; Padilla, S.L. Evaluación del crecimiento de Lactobacillus casei Y producción de ácido láctico usando como sustrato el suero de leche de vacuno. Rev. Investig. Univ. Quindío 2010, 20, 42–49. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Wu, P.; Li, J.; Lu, X.; Tang, Y.; Cai, Z. Release of tens of thousands of microfibers from discarded face masks under simulated environmental conditions. Sci. Total Environ. 2022, 806, 150458. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Patrício-Silva, A.L.; Soares, A.M.V.M.; Barceló, D.; Duarte, A.C.; Rocha-Santos, T. Current knowledge on the presence, biodegradation, and toxicity of discarded face masks in the environment. J. Environ. Chem. Eng. 2023, 11, 109308. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological Degradation of Plastics and Microplastics: A Recent Perspective on Associated Mechanisms and Influencing Factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Veronelli, M.M.; Raguso, C.; Barana, D.D.; Galli, P.; Lasagni, M. The release process of microfibers: From surgical face masks into the marine environment. Environ. Adv. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Zhao, H.; Hong, X.; Chai, J.; Wan, B.; Zhao, K.; Han, C.; Zhang, W.; Huan, H. Interaction between Microplastics and Pathogens in Subsurface System: WhatWe Know So Far. Water 2024, 16, 499. [Google Scholar] [CrossRef]
- Hermoza, R.A.M. Biodegradación Microbiana de Polietileno de Baja Densidad, Bajo Condiciones Térmicas Controladas en Biorreactor Air Lift, en Santa Clara-Lima. Environmental. Ph.D. Thesis, Universidad César Vallejo, Vallejo, Peru, 2019. [Google Scholar]
- Thakur, B.; Singh, J.; Singh, J.; Angmo, D.; Pal-Vig, A. Biodegradation of different types of microplastics: Molecular mechanism and degradation efficiency. Sci. Total Environ. 2023, 877, 162912. [Google Scholar] [CrossRef]
- Maddela, N.R.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Additives of plastics: Entry into the environment and potential risks to human and ecological health. J. Environ. Manag. 2023, 348, 119364. [Google Scholar] [CrossRef]
- Narciso, O.L.; Coreño, A.A.; Mendoza, O.D.; Lucho, C.C.; Lizardi, J.M. Baseline for plastic and hydrocarbon pollution of rivers, reefs, and sediment on beaches in Veracruz State, México, and a proposal for bioremediation. Environ. Sci. Pollut. Res. 2020, 27, 23035–23047. [Google Scholar] [CrossRef] [PubMed]
- Tzintzun, C.O.; Loera, O.; Ramírez, S.H.; Gutiérrez, R.M. Comparison of mechanisms ofhexadecane uptake among pure and mixedcultures derived from a bacterial consortium. Int. Biodeterior. Biodegrad. 2012, 70, 1–7. [Google Scholar] [CrossRef]
- Valdivia, C.C. Generación y Caracterización de un Consorcio Bacteriano Aerobio que Degraden Polietileno y Polipropileno Como Alternativa de Manejo en la CDMX. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2023. [Google Scholar]
- Gutiérrez, P.J.G. Biodegradación de Polietileno de Baja Densidad por Consorcios Microbianos. Bachelor’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2013. [Google Scholar]
- Tirado, T.D.; Acevedo, S.O.; Romo, G.C.; Marmolejo, S.Y.; Gayosso, C.M. Participación de consorcios microbianos en la biodegradación de hidrocarburos aromáticos policíclicos. Rev. Iberoam. Cienc. 2015, 2, 77–86. [Google Scholar]
- Castañeda-Chávez, M.R.; López, S.B.; Reyes, V.C.; Lizardi, J.M.A. Identificación de especies dominantes en un consorcio microbiano eficiente en la degradación de diésel. Rev. Int. Contam. Ambient. 2022, 38, 155–167. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.Y. Bergey’s Manual of Determinative Bacterology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar] [CrossRef]
- Bou, G.; Fernández-Olmos, A.; García, C.; Sáez-Nieto, J.A.; Valdezate, S. Métodos de identificación bacteriana en el laboratorio de microbiología. Enferm. Infecc. Microbiol. Clin. 2011, 29, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kotova, I.B.; Taktarova, Y.V.; Tsavkelova, E.A.; Egorova, M.A.; Bubnov, I.A.; Malakhova, D.V.; Shirinkinaa, L.I.; Sokolovab, T.G.; Bonch-Osmolovskaya, E.A. Microbial Degradation of Plastics and Approaches to Make It More Efficient. Microbiology 2021, 90, 671–701. [Google Scholar] [CrossRef]
- Atlas, R.; Bartha, R. Ecología Microbiana y Microbiología Ambiental, 4th ed.; Pearson Educación: London, UK, 2008. [Google Scholar]
- Ruberto, L.; Vazquez, S.; Mac-Cormack, W. Effectiveness of the Natural Bacterial Flora, Biostimulation, and Bioaugmentation on the Bioremediation of a Hydrocarbon Contaminated Antarctic Soi. Int. Biodeterior. Biodegrad. 2003, 52, 115–125. [Google Scholar] [CrossRef]
- Narváez, M.; Gómez, M.; Martínez, M. Selección de bacterias con capacidad degradadora de hidrocarburos, aisladas a partir de sedimentos del Caribe Colombiano. Boletín Investig. Mar. Costeras 2008, 37, 61–75. [Google Scholar] [CrossRef]
- Ueno, A.; Hasanuzzaman, M.; Yumoto, I.; Okuyama, H. Verification of degradation of nalkanes in diesel oil by Pseudomonas aeruginosa strain WatG in soil microcosms. Curr. Microbiol. 2006, 52, 182–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaramillo, G.; Paba, G.; Ospino, M. Aislamiento de bacterias potencialmente degradadoras de petróleo en hábitats de ecosistemas costeros en la Bahía de Cartagena, Colombia. NOVA 2010, 8, 76–86. [Google Scholar]
- Crisafi, F.; Smedile, F.; Yakimov, M.M.; Aulenta, F.; Fazi, S.; La Cono, V.; Martinelli, A.; Di Lisio, V.; Denaro, R. Bacterial biofilms on medical masks disposed in the marine environment: A hotspot of biological and functional diversity. Sci. Total Environ. 2022, 837, 155731. [Google Scholar] [CrossRef] [PubMed]
- Medina-Moreno, S.A.; Jiménez-González, A.; Gutiérrez-Rojas, M.; Lizardi-Jiménez, M.A. Hydrocarbon pollution studies of underwater sinkholes along Quintana Roo as a function of tourism development in the Mexican Caribbean. Rev. Mex. Ing. Quim 2014, 13, 509–516. [Google Scholar]
- Dey, S.; Anand, U.; Kumar, V.; Kumar, S.; Ghorai, M.; Ghosh, A.; Kant, N.; Suresh, S.; Bhattacharya, S.; Bontempi, E.; et al. Microbial strategies for degradation of microplastics generated from COVID-19 healthcare waste. Environ. Res. 2023, 216, 114438. [Google Scholar] [CrossRef]
- Cacciari, I.; Quatrini, P.; Zirletta, G.; Mincione, E.; Vinciguerra, V.; Lupattelli, P.; Sermanni, G.G. Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 1993, 59, 3695–3700. [Google Scholar] [CrossRef]
- Arkatkar, A.; Juwarkar, A.A.; Bhaduri, S.; Uppara, P.V.; Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int. Biodeterior. Biodegrad. 2010, 64, 530–536. [Google Scholar] [CrossRef]
- Hamdan, P.A. Biomonitoreo: Seguimiento de Poblaciones Microbianas en Procesos de Biorremediación de Suelos Contaminados con. Master’s Thesis, Universidad Autónoma Metropolitana, Unidad Iztapalapa, Mexico City, Mexico, 2004. [Google Scholar]
- Cuellar, O.G.; Mesta, H.A.; Pineda, F.G.; Salgado, B.R. Degradación de parafinas por Pseudomonas aeruginosa MGP-1. Rev. Investig. Univ. Simón Bolívar 2004, 6, 41–44. [Google Scholar]
- Vijayalakshmi, S.; Gopalsamy, P.; Muthusamy, K.; Dinesh-Kumar, S.; Pulikondan-francis, S.; Ramesh, T.; Deog-Hwan, O.; Thi Thuy, D.L.; Anh-Truong, T.T.; Huu Tap, V.; et al. Environmental Hazard of Polypropylene from Disposable Face Masks Linked to the COVID-19 Pandemic and Its Possible Mitigation Techniques through a Green Approach. J. Chem. 2022, 1, 9402236. [Google Scholar] [CrossRef]
- Sridhar, S.; Murugesan, N.; Gopalakrishnan, M.; Janjoren, D.; Ganesan, S. Removal of microplastic for a sustainable strategy by microbial biodegradation. Sustain. Chem. Environ. 2024, 6, 100088. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, M.N. Isolation of a thermophilic bacterium capable of low molecular- weight polyethylene degradation. Biodegradation 2013, 24, 89–98. [Google Scholar] [CrossRef]
- Fontanella, S.; Bonhomme, S.; Brusson, J.M.; Pitteri, S.; Samuel, G.; Pichon, G.; Lacoste, J.; Fromageot, D.; Lemaire, J.; Delort, A.M. 2013. Comparison of biodegradability of various polypropylene films containing pro-oxidant additives based on Mn, Mn/Fe or Co. Polym. Degrad. Stab. 2013, 98, 875–884. [Google Scholar] [CrossRef]
- Ballesté, E.; Liang, H.; Migliorato, L.; Sala-Comorera, L.; Méndez, J.; Garcia-Aljaro, C. Exploring plastic biofilm formation and Escherichia coli colonisation in marine environments. Environ. Microbiol. Rep. 2024, 16, e13308. [Google Scholar] [CrossRef]
- Hernández-Sánchez, C.; Pestana-Ríos, Á.A.; Villanova-Solano, C.; Domínguez-Hernández, C.; Díaz-Peña, F.J.; Rodríguez-Álvarez, C.; Lecuona, M.; Arias, Á. Bacterial Colonization of Microplastics at the Beaches of an Oceanic Island, Tenerife, Canary Islands. Int. J. Environ. Res. Public Health 2023, 20, 3951. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Klavins, L.; Vaseashta, A. Microbial Life on the Surface of Microplastics in Natural Waters. Appl. Sci. 2021, 11, 11692. [Google Scholar] [CrossRef]
- Thangaraj-Uthra, K.; Chitra, V.; Damodharan, N.; Devadoss, A.; Kuehnel, M.; Exposito, A.J.; Nagarajan, S.; Pitchaimuthu, S.; Perumal-Pazhani, G. Microplastic emerging pollutants- impact on microbiological diversity, diarrhea, antibiotic resistance, and bioremediation. Environ. Sci. Adv. 2023, 2, 1469–1487. [Google Scholar] [CrossRef]
- Stoeck, T.; Kröncke, I.; Duineveld, G.; Palojärvi, A. Phospholipid fatty acid profiles at depositional and non-depositional sites in the North Sea. Mar. Ecol. Prog. Ser. 2002, 241, 57–70. [Google Scholar] [CrossRef][Green Version]
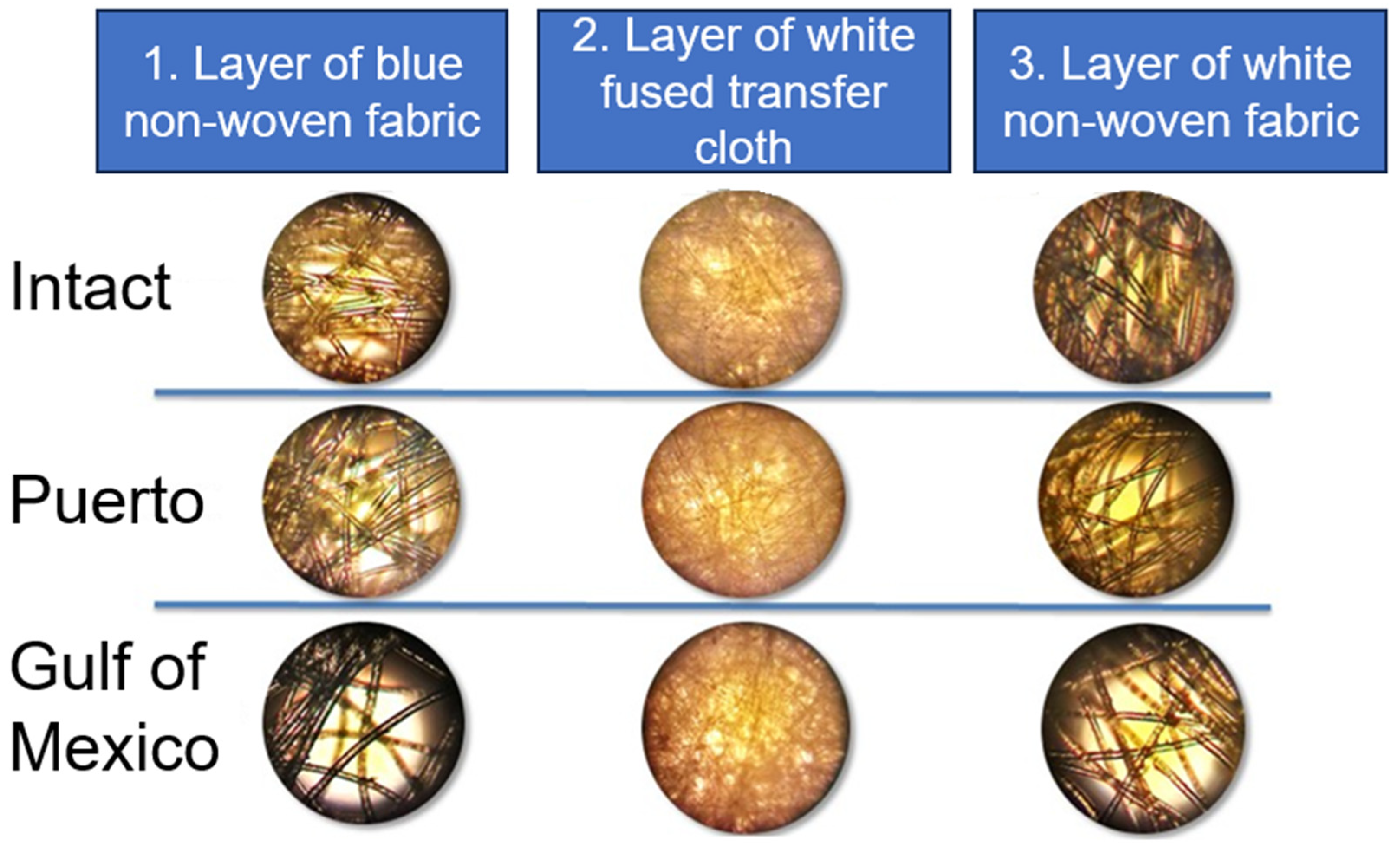
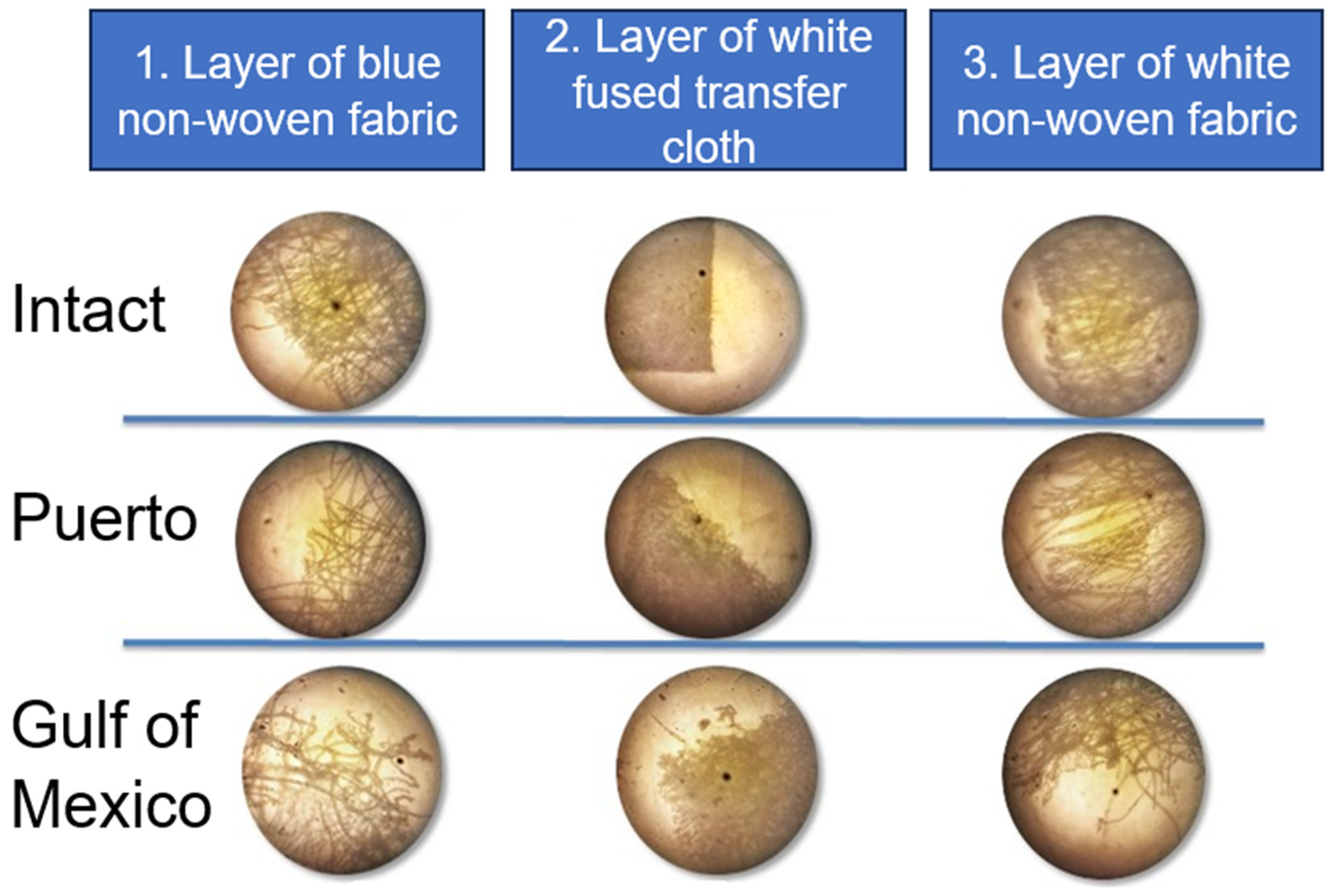
| Consortium | Face Masks | Value | % Biodegradation | ||
|---|---|---|---|---|---|
| P0 (g) | P1 (g) | PP (g) | |||
| Puerto | Three-layer | 4.0 | 3.8841 | 0.151 | 3.77 |
| Gulf of Mexico | Three-layer | 4.0 | 3.1884 | 0.7992 | 19.98 |
| Puerto Consortium | |||
|---|---|---|---|
| Bacteria | Catalase | Oxidase | Gram |
| Streptococcus pyogenes | Positive | Positive | Negative (Diplococci-coccobacilli) |
| Pseudomonas aeruginosa, Burkholderia cepacia, Escherichia coli, Stenotrophomonas maltophilia | Positive | Positive | Negative (Diplococci-coccobacilli) |
| Salmonella Typhimurium Shigella flexneri | Positive | Positive | Negative (Diplococci-bacilli-coccobacilli) |
| Vibrio parahaemolyticus | Positive | Positive | Negative (Diplococci-bacilli-coccobacilli) |
| Gulf of Mexico consortium | |||
| Enterococcus faecalis | Positive | Negative | Negative (Diplococci-bacilli-coccobacilli) |
| Pseudomonas aeruginosa Burkholderia cepacia Escherichia coli Stenotrophomonas maltophilia. | Positive | Positive | Negative (Diplococci-bacilli- curved bacilli-Vibrio) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda Chávez, M.d.R.; Campos García, L.M.; Reyes Velázquez, C.; Lango Reynoso, F.; Reynier Valdés, D.; Amaro Espejo, I.A.; Navarrete Rodríguez, G. Microbial Consortia in the Remediation of Single-Use Waste: The Case of Face Masks. Microbiol. Res. 2024, 15, 2070-2084. https://doi.org/10.3390/microbiolres15040139
Castañeda Chávez MdR, Campos García LM, Reyes Velázquez C, Lango Reynoso F, Reynier Valdés D, Amaro Espejo IA, Navarrete Rodríguez G. Microbial Consortia in the Remediation of Single-Use Waste: The Case of Face Masks. Microbiology Research. 2024; 15(4):2070-2084. https://doi.org/10.3390/microbiolres15040139
Chicago/Turabian StyleCastañeda Chávez, María del Refugio, Luz María Campos García, Christian Reyes Velázquez, Fabiola Lango Reynoso, David Reynier Valdés, Isabel Araceli Amaro Espejo, and Gabycarmen Navarrete Rodríguez. 2024. "Microbial Consortia in the Remediation of Single-Use Waste: The Case of Face Masks" Microbiology Research 15, no. 4: 2070-2084. https://doi.org/10.3390/microbiolres15040139
APA StyleCastañeda Chávez, M. d. R., Campos García, L. M., Reyes Velázquez, C., Lango Reynoso, F., Reynier Valdés, D., Amaro Espejo, I. A., & Navarrete Rodríguez, G. (2024). Microbial Consortia in the Remediation of Single-Use Waste: The Case of Face Masks. Microbiology Research, 15(4), 2070-2084. https://doi.org/10.3390/microbiolres15040139








