Microalgal Metabolomes and Recent Biotechnological Advances for Their Industrial Application
Abstract
1. Introduction
2. Industrially Important Microalgal Compounds
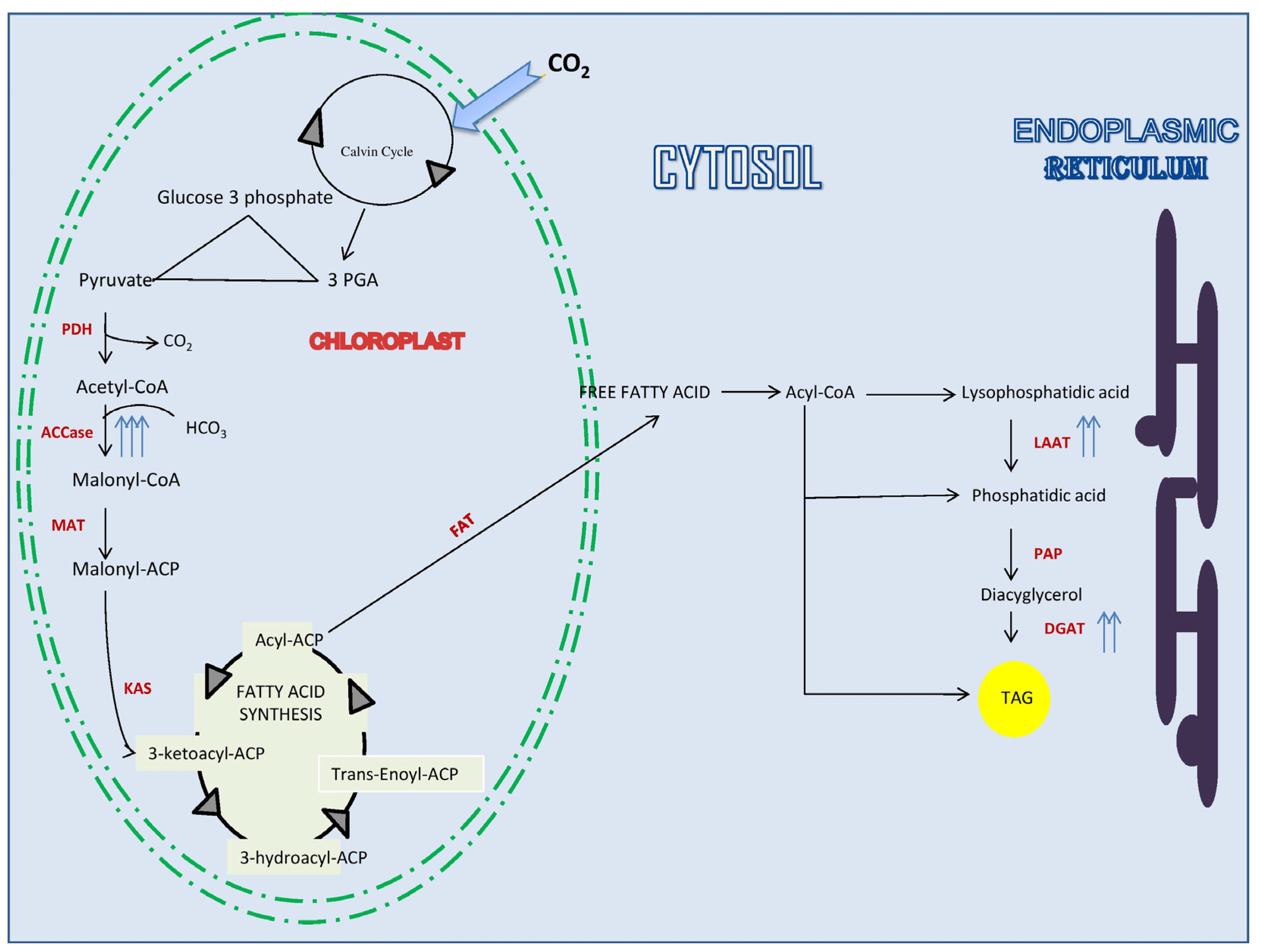
2.1. Biofuels
2.2. Carbohydrates
2.3. Biopigments
Astaxanthin
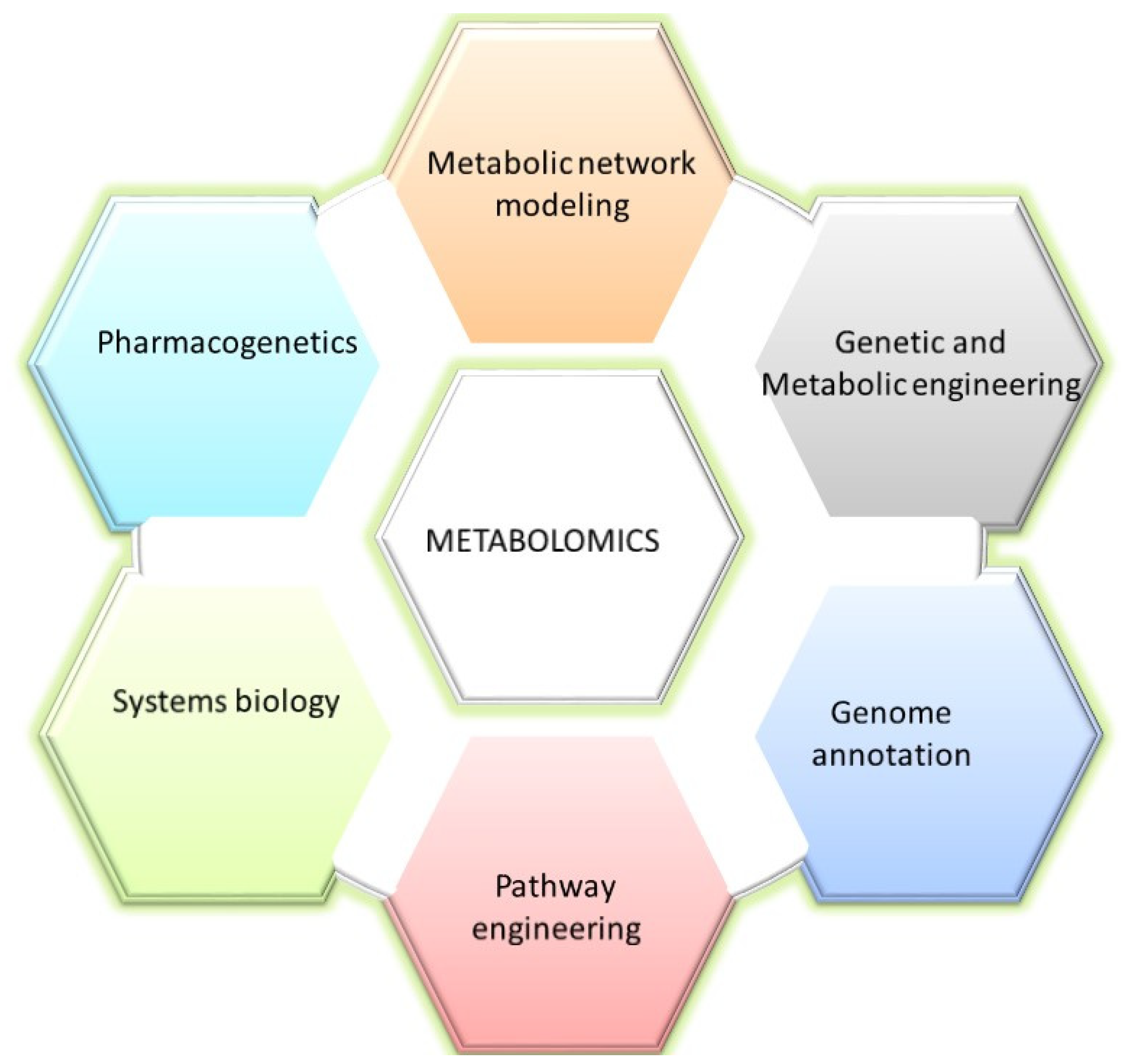
3. Applications of Metabolomics in Microalgal Studies
| S.No. | Organism | Tools | Type of Study | Insights | Ref. |
|---|---|---|---|---|---|
| 1. | Chlorella sorokiniana | GC-MS | Comparative metabolome profile analysis | A comparative study between a single culture of Chlorella sorokiniana and its consortium with bacteria for wastewater treatment. The results conclude with the differential metabolite synthesis of several classes, such as fatty acids, carbohydrates, amino acids, etc. | [57] |
| 2. | Chlorella vulgaris and Scenedesmus obliquus | GC–MS and LC-QTOF/MS | Untargeted metabolomics analysis | The toxicity and uptake mechanism of triphenyl phosphate by two microalgae was investigated in this study. The finding suggests an increase in membrane integrity, and a decrease in reactive oxygen species (ROS) was observed in Chlorella vulgaris, whereas there was damage to the cellular integrity and ROS reported in Scenedesmus obliquus. | [58] |
| 3. | Coccomyxa melkonianii SCCA 048 | GC–MS | Metabolomics analysis | The study revealed changes in metabolite synthesis under stress conditions and the effect of these metabolites in pathways like the ascorbate metabolism pathway, phytic acid biosynthesis, TCA cycle, etc. | [59] |
| 4. | Nannochloropsis oceanica CASA CC201 | LC-MS | In this study, the effect of various plant growth-promoting factors, such as gibberellic acid, malic acid, and salicylic acid, on lipid biosynthesis was investigated in Nannochloropsis. The results revealed an increase in the level of cofactor and amino acids for the up-regulation of lipid metabolism in the organisms. | [60] | |
| 5. | Haematococcus pluvialis | LC-MS | This study revealed an increase in astaxanthin and lipid production under melatonin stress. The metabolomics analysis shows the up-regulation of glucosamine-6-phosphate, maltose, gluconic acid, isocitric acid, etc., which are the precursors for TCA, astaxanthin, and fatty acid syntheses. | [61] | |
| 6. | Chlorella sp. | GC-MS | This study showed the effect of autotrophic cultivation and heterotrophic cultivation on lipid synthesis. | [62] | |
| 7. | Chlorella vulgaris | LC-QTOF | Metabolomics analysis | This study shows the copper nanoparticle with its microparticles and ions on metabolites of Chlorella vulgaris. The metabolic data conclude with alterations in various pathways such as chlorophyll synthesis and glutathione metabolism and the remodeling of membrane proteins. | [63] |
| 8 | Scenedesmus sp. IITRIND2 | NMR | Metabolomics analysis | This study identified an array of metabolites and provides brief insights regarding changes in metabolic pathways under arsenic stress. | [64] |
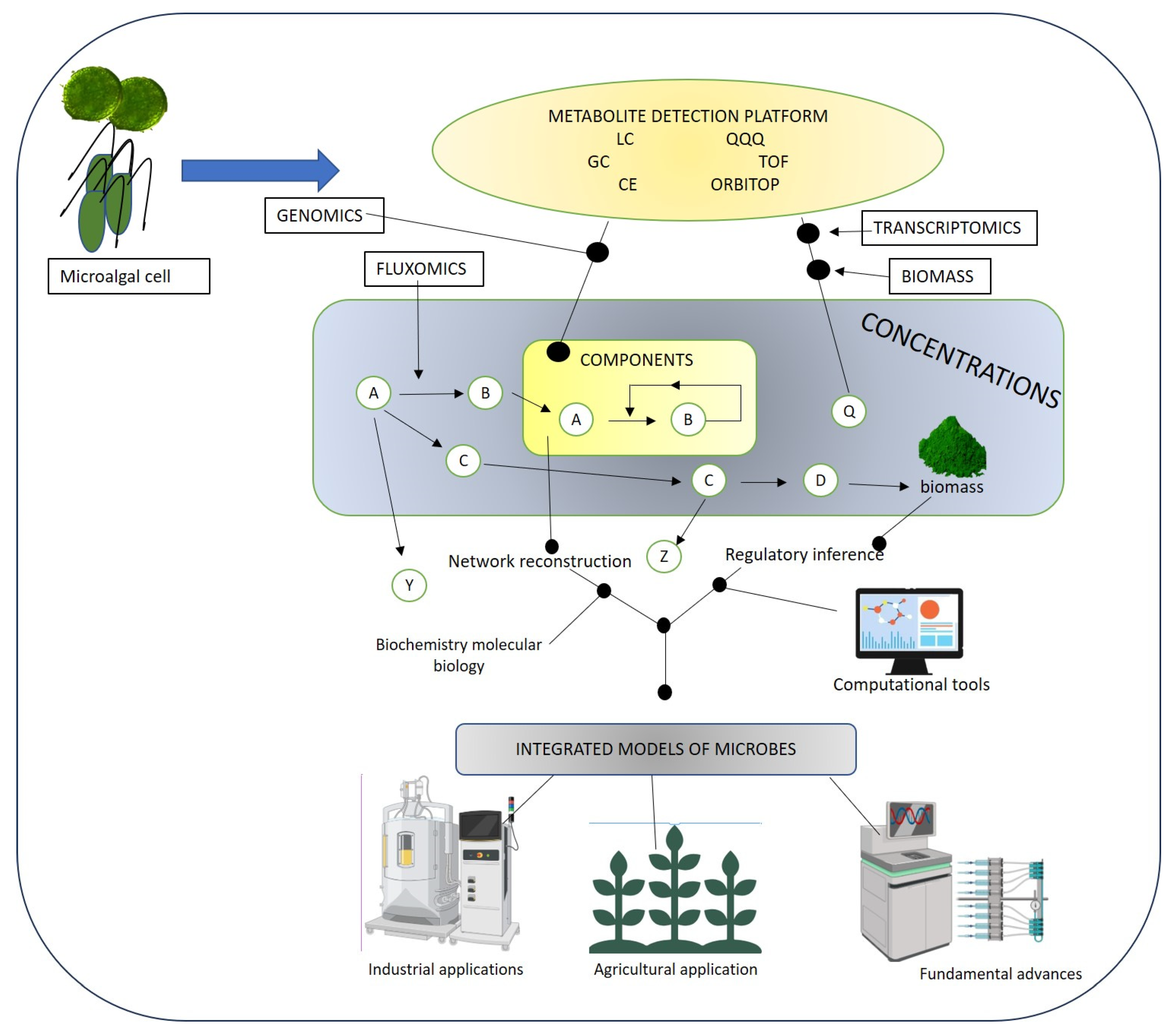
4. Integrating Metabolomics with Systems Biology Tools
Metabolomics and Microalgae
5. Bottlenecks and the Future Direction of Metabolomics with Microalgae
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of biodiesel from microalgae through biological carbon capture: A review. 3 Biotech 2017, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Archibald, J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Wang, Y.; Duanmu, D.; Spalding, M.H. Carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii: Inorganic carbon transport and CO2 recapture. Photosynth. Res. 2011, 109, 115–122. [Google Scholar] [CrossRef]
- Singh, S.; Datta, P. Outdoor evaluation of herbicide resistant strains of Anabaena variabilis as biofertilizer for rice plants. Plant Soil 2007, 296, 95–102. [Google Scholar] [CrossRef]
- Srivastava, A.; Shukla, P. Emerging tools and strategies in cyanobacterial omics. Trends Biotechnol. 2021, 40, 4–7. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H.M.; Sada, E.G. High-value compounds from microalgae with industrial exploitability—A review. Front. Biosci. 2017, 9, 319–342. [Google Scholar]
- Saini, D.K.; Pabbi, S.; Shukla, P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food Chem. Toxicol. 2018, 120, 616–624. [Google Scholar] [CrossRef]
- Pragya, N.; Pandey, K.K.; Sahoo, P.K. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.H. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar]
- Katiyar, R.; Gurjar, B.R.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sustain. Energy Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Lenka, S.K.; Carbonaro, N.; Park, R.; Miller, S.M.; Thorpe, I.; Li, Y. Current advances in molecular, biochemical, and computational modeling analysis of microalgal triacylglycerol biosynthesis. Biotechnol. Adv. 2016, 34, 1046–1063. [Google Scholar] [CrossRef]
- Wilhelm, C.; Büchel, C.; Fisahn, J.; Goss, R.; Jakob, T.; LaRoche, J.; Johann Lavaud, J.; Martin Lohr, M.; Riebesell, U.; Stehfest, K.; et al. The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 2006, 157, 91–124. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-generation biorefineries: A sustainable platform for food, clean energy, and nutraceuticals production. Biomass Convers. Biorefin. 2020, 12, 4215–4230. [Google Scholar]
- Willamme, R.; Alsafra, Z.; Arumugam, R.; Eppe, G.; Remacle, F.; Levine, R.D.; Remacle, C. Metabolomic analysis of the green microalga Chlamydomonas reinhardtii cultivated under day/night conditions. J. Biotechnol. 2015, 215, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Devendran, S.; Tsai, P.C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics integrated with transcriptomics and proteomics: Evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process Biochem. 2020, 91, 1–14. [Google Scholar]
- Chakdar, H.; Hasan, M.; Pabbi, S.; Nevalainen, H.; Shukla, P. High-throughput proteomics and metabolomic studies guide re-engineering of metabolic pathways in eukaryotic microalgae: A review. Bioresour. Technol. 2020, 321, 124495. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Parida, A.K.; Rangani, J. Advancement of metabolomics techniques and their applications in plant science: Current scenario and future prospective. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–36. [Google Scholar]
- Guo, B.; Chen, B.; Liu, A.; Zhu, W.; Yao, S. Liquid chromatography-mass spectrometric multiple reaction monitoring-based strategies for expanding targeted profiling towards quantitative metabolomics. Curr. Drug Metab. 2012, 13, 1226–1243. [Google Scholar] [PubMed]
- Mishra, A.; Medhi, K.; Malaviya, P.; Thakur, I.S. Omics approaches for microalgal applications: Prospects and challenges. Bioresour. Technol. 2019, 291, 121890. [Google Scholar]
- Guerrero-Lemus, R.; Martínez-Duart, J.M. Renewable Energies and CO2: Cost Analysis, Environmental Impacts and Technological Trends, 2012 ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 3. [Google Scholar]
- Lee, S.Y.; Khoiroh, I.; Vo, D.V.N.; Kumar, P.S.; Show, P.L. Techniques of lipid extraction from microalgae for biofuel production: A review. Environ. Chem. Lett. 2020, 19, 231–251. [Google Scholar]
- Nazir, M.S.; Mahdi, A.J.; Bilal, M.; Sohail, H.M.; Ali, N.; Iqbal, H.M. Environmental impact and pollution-related challenges of renewable wind energy paradigm—A review. Sci. Total Environ. 2019, 683, 436–444. [Google Scholar]
- Dhawane, S.H.; Kumar, T.; Halder, G. Recent advancement and prospective of heterogeneous carbonaceous catalysts in chemical and enzymatic transformation of biodiesel. Energy Convers. Manag. 2018, 167, 176–202. [Google Scholar]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels. 2018, 11, 185. [Google Scholar]
- Chowdhury, H.; Loganathan, B. Third-generation biofuels from microalgae: A review. Curr. Opin. Green Sustain. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Singh, R.; Manchanda, G.; Maurya, I.; Wei, Y. Microbial Versatility in Varied Environments; Springer: Singapore, 2020. [Google Scholar]
- Rahman, A.; Putman, R.J.; Inan, K.; Sal, F.A.; Sathish, A.; Smith, T.; Nielsen, C.; Sims, R.C.; Miller, C.D. Polyhydroxybutyrate production using a wastewater microalgae based media. Algal Res. 2015, 8, 95–98. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2013, 96, 631–645. [Google Scholar]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar]
- Ho, S.H.; Ye, X.; Hasunuma, T.; Chang, J.S.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar]
- Al Abdallah, Q.; Nixon, B.T.; Fortwendel, J.R. The enzymatic conversion of major algal and cyanobacterial carbohydrates to bioethanol. Front. Energy Res. 2016, 4, 36. [Google Scholar]
- Constantino, A.; Rodrigues, B.; Leon, R.; Barros, R.; Raposo, S. Alternative chemo-enzymatic hydrolysis strategy applied to different microalgae species for bioethanol production. Algal Res. 2021, 56, 102329. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Salama, E.S.; Kurade, M.B.; Hassan, S.H.; Oh, S.E.; Kim, S.; Jeon, B.H. Utilization of microalgal biofractions for bioethanol, higher alcohols, and biodiesel production: A review. Energies 2017, 10, 2110. [Google Scholar] [CrossRef]
- Deng, M.-D.; Coleman, J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Nwoba, E.G.; Ogbonna, C.N.; Ishika, T.; Vadiveloo, A. Microalgal pigments: A source of natural food colors. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 81–123. [Google Scholar]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Chakdar, H.; Pabbi, S.; Shukla, P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit. Rev. Food Sci. Nutr. 2020, 60, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Willette, S.; Gill, S.S.; Dungan, B.; Schaub, T.M.; Jarvis, J.M.; Hilaire, R.S.; Holguin, F.O. Alterations in lipidome and metabolome profiles of Nannochloropsis salina in response to reduced culture temperature during sinusoidal temperature and light. Algal Res. 2018, 32, 79–92. [Google Scholar] [CrossRef]
- Veyel, D.; Erban, A.; Fehrle, I.; Kopka, J.; Schroda, M. Rationales and approaches for studying metabolism in eukaryotic microalgae. Metabolites 2014, 4, 184–217. [Google Scholar] [CrossRef]
- Vendruscolo, R.G.; Fagundes, M.B.; Maroneze, M.M.; Nascimento, T.C.D.; de Menezes, C.R.; Barin, J.S.; Zepka, L.Q.; Jacob-Lopes, E.; Wagner, R. Scenedesmus obliquus metabolomics: Effect of photoperiods and cell growth phases. Bioprocess Biosyst. Eng. 2019, 42, 727–739. [Google Scholar] [CrossRef]
- Lv, H.; Xia, F.; Liu, M.; Cui, X.; Wahid, F.; Jia, S. Metabolomic profiling of the astaxanthin accumulation process induced by high light in Haematococcus pluvialis. Algal Res. 2016, 20, 35–43. [Google Scholar] [CrossRef]
- Vello, V.; Chu, W.-L.; Lim, P.-E.; Majid, N.A.; Phang, S.-M. Metabolomic profiles of tropical Chlorella species in response to physiological changes during nitrogen deprivation. J. Appl. Phycol. 2018, 30, 3131–3151. [Google Scholar] [CrossRef]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT-Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef]
- Altenhofen da Silva, M.; Barbosa, G.H.; Brito Codato, C.; Arjonilla de Mattos, L.F.; Gaspar Bastos, R.; Kieckbusch, T.G. Heterotrophic growth of green microalgae Desmodesmus subspicatus in ethanol distillation wastewater (vinasse) and lipid extraction with supercritical CO2. J. Chem. Technol. Biotech. 2017, 92, 573–579. [Google Scholar] [CrossRef]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical fluid extraction of lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef] [PubMed]
- Hollywood, K.A.; Schmidt, K.; Takano, E.; Breitling, R. Metabolomics tools for the synthetic biology of natural products. Curr. Opin. Biotechnol. 2018, 54, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhao, Q.; Wang, L.; Xu, Y.; Wei, W. Comparative metabolomic analysis of the green microalga Chlorella sorokiniana cultivated in the single culture and a consortium with bacteria for wastewater remediation. Appl. Biochem. Biotechnol. 2017, 183, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Lim, D.J.; Laserna, A.K.; Li, S.F. Uptake and toxic effects of triphenyl phosphate on freshwater microalgae Chlorella vulgaris and Scenedesmus obliquus: Insights from untargeted metabolomics. Sci. Total Environ. 2019, 650, 1239–1249. [Google Scholar] [CrossRef]
- Fais, G.; Malavasi, V.; Scano, P.; Soru, S.; Caboni, P.; Cao, G. Metabolomics and lipid profile analysis of Coccomyxa melkonianii SCCA 048. Extremophiles 2021, 25, 357–368. [Google Scholar] [CrossRef]
- Arumugam, M.; Udayan, A.; Sabapathy, H.; Abraham, B. Plant growth regulator triggered metabolomic profile leading to increased lipid accumulation in an edible marine microalga. J. Appl. Phycol. 2021, 33, 1353–1365. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Yu, C.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Integration of physiological and metabolomic profiles to elucidate the regulatory mechanisms underlying the stimulatory effect of melatonin on astaxanthin and lipids coproduction in Haematococcus pluvialis under inductive stress conditions. Bioresour. Technol. 2021, 319, 124150. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Sellstedt, A. Metabolomic Study of Heterotrophically Grown Chlorella sp. Isolated from Wastewater in Northern Sweden. Molecules 2021, 26, 2410. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Sun, W.; Too, H.Z.; Laserna, A.K.C.; Li, S.F.Y. A global metabolomic insight into the oxidative stress and membrane damage of copper oxide nanoparticles and microparticles on microalga Chlorella vulgaris. Environ. Pollut. 2020, 258, 113647. [Google Scholar] [CrossRef]
- Arora, N.; Dubey, D.; Sharma, M.; Patel, A.; Guleria, A.; Pruthi, P.A.; Poluri, K.M. NMR-based metabolomic approach to elucidate the differential cellular responses during mitigation of arsenic (III, V) in a green microalga. ACS Omega 2018, 3, 11847–11856. [Google Scholar] [CrossRef]
- Liu, G.N.; Zhu, Y.H.; Jiang, J.G. The metabolomics of carotenoids in engineered cell factory. Appl. Microbiol. Biotechnol. 2009, 83, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.L.; Ghamsari, L.; Manichaikul, A.; Hom, E.F.Y.; Balaji, S.; Fu, W.; Shen, Y.; Hao, T.; Palsson, B.; Salehi-Ashtiani, K.; et al. Metabolic network reconstruction of Chlamydomonas offers insight into light-driven algal metabolism. Mol. Syst. Biol. 2011, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kumar, N.; Varjani, S.J.; Guria, C.; Bandopadhyay, R.; Shukla, P.; Banerjee, C. Computational modelling and prediction of microalgae growth focused towards improved lipid production. In Biosynthetic Technology and Environmental Challenges; Varjani, S., Parameswaran, B., Kumar, S., Khare, S., Eds.; Springer: Singapore, 2018; pp. 223–232. [Google Scholar]
- Yizhak, K.; Benyamini, T.; Liebermeister, W.; Ruppin, E.; Shlomi, T. Integrating quantitative proteomics and metabolomics with a genome-scale metabolic network model. Bioinformatics 2010, 26, i255–i260. [Google Scholar] [CrossRef] [PubMed]
- Pries, C.; Razaghi-Moghadam, Z.; Kopka, J.; Nikoloski, Z. Integration of relative metabolomics and transcriptomics time-course data in a metabolic model pinpoints effects of ribosome biogenesis defects on Arabidopsis thaliana metabolism. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Kato, Y.; Inabe, K.; Hidese, R.; Kondo, A.; Hasunuma, T. Metabolomics-based engineering for biofuel and bio-based chemical production in microalgae and cyanobacteria: A review. Bioresour. Technol. 2022, 344, 126196. [Google Scholar] [CrossRef]
- Cagney, M.H.; O’Neill, E.C. Strategies for producing high value small molecules in microalgae. Plant Physiol. Biochem. 2024, 214, 108942. [Google Scholar] [CrossRef]
- Stavridou, E.; Karapetsi, L.; Nteve, G.M.; Tsintzou, G.; Chatzikonstantinou, M.; Tsaousi, M.; Martinez, A.; Flores, P.; Merino, M.; Dobrovic, L.; et al. Landscape of microalgae omics and metabolic engineering research for strain improvement: An overview. Aquaculture 2024, 587, 740803. [Google Scholar] [CrossRef]
- Rehman, H.; Saipriya, K.; Singh, A.K.; Singh, R.; Meena, G.S.; Khetra, Y.; Sharma, H. A metabolomics approach to establish the relationship between the techno-functional properties and metabolome of Indian goat yoghurt. Foods 2024, 13, 913. [Google Scholar] [CrossRef]
- Schwarz, D.; Orf, I.; Kopka, J.; Hagemann, M. Recent applications of metabolomics toward cyanobacteria. Metabolites 2013, 3, 72–100. [Google Scholar] [CrossRef]
- Pathania, R.; Srivastava, A.; Srivastava, S.; Shukla, P. Metabolic systems biology and multi-omics of cyanobacteria: Perspectives and future directions. Bioresour. Technol. 2022, 343, 126007. [Google Scholar] [CrossRef]
- Hegazi, N.; Khattab, A.R.; Saad, H.H.; Abib, B.; Farag, M.A. A multiplex metabolomic approach for quality control of Spirulina supplement and its allied microalgae (Amphora & Chlorella) assisted by chemometrics and molecular networking. Sci. Rep. 2024, 14, 2809. [Google Scholar]
- Chen, Q.; Chen, Y.; Hu, Q.; Han, D. Metabolomic analysis reveals astaxanthin biosynthesis in heterotrophic microalga Chromochloris zofingiensis. Bioresour. Technol. 2023, 374, 128811. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Manchanda, G.; Bhattacharjee, K.; Panosyan, H. (Eds.) Microbial Syntrophy-Mediated Eco-Enterprising; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Kapoore, R.V.; Vaidyanathan, S. Quenching for Microalgal Metabolomics: A Case Study on the Unicellular Eukaryotic Green Alga Chlamydomonas reinhardtii. Metabolites 2018, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Sáez, L.M.; Abreu, A.C.; Camacho-Rodríguez, J.; González-López, C.V.; del Carmen Cerón-García, M.; Fernández, I. NMR metabolomics as an effective tool to unravel the effect of light intensity and temperature on the composition of the marine microalgae Isochrysis galbana. J. Agric. Food Chem. 2019, 67, 3879–3889. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Tison-Rosebery, J.; Morin, S.; Mazzella, N. Metabolome response to anthropogenic contamination on microalgae: A review. Metabolomics 2020, 16, 8. [Google Scholar] [CrossRef]
- Azizan, A.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Mass spectrometry-based metabolomics combined with quantitative analysis of the microalgal diatom (Chaetoceros calcitrans). Mar. Drugs 2020, 18, 403. [Google Scholar] [CrossRef]
- Wen, M.; Zhu, M.; Han, Z.; Ho, C.T.; Granato, D.; Zhang, L. Comprehensive applications of metabolomics on tea science and technology: Opportunities, hurdles, and perspectives. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4890–4924. [Google Scholar] [CrossRef]
- Alothman, A.; Emwas, A.H.; Singh, U.; Jaremko, M.; Agusti, S. Metabolomics-based analysis of the diatom Cheatoceros tenuissimus combining NMR and GC–MS techniques. MethodsX 2024, 12, 102695. [Google Scholar] [CrossRef]
- Jeong, Y.; Cho, S.H.; Lee, H.; Choi, H.K.; Kim, D.M.; Lee, C.G.; Cho, S.; Cho, B.K. Current status and future strategies to increase secondary metabolite production from cyanobacteria. Microorganism 2020, 8, 1849. [Google Scholar] [CrossRef]
- Gomez-Casati, D.F.; Zanor, M.I.; Busi, M.V. Metabolomics in plants and humans: Applications in the prevention and diagnosis of diseases. BioMed Res. Int. 2013, 2013, 792527. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Carvalho, M.; Bastos, M.L.; Guedes de Pinho, P. Metabolomics analysis for biomarker discovery: Advances and challenges. Curr. Med. Chem. 2013, 20, 257–271. [Google Scholar] [CrossRef]
- Salem, M.A.; Perez de Souza, L.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef]
- Mishur, R.J.; Rea, S.L. Applications of mass spectrometry to metabolomics and metabonomics: Detection of biomarkers of aging and of age-related diseases. Mass Spectrom. Rev. 2012, 31, 70–95. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Cerofolini, L.; Silva, J.M.; Ravera, E.; Fragai, M.; Macedo, A.L.; Parigi, G.; Geraldes, C.F. Paramagnetic NMR of Transition Metal Derivatives of Human Carbonic Anhydrase: Implications for Enzyme Catalysis and Sustainability. In Synthesis and Applications in Chemistry and Materials: Volume 12: Enzymatic and Organic Systems; World Scientific: London, UK, 2024; pp. 39–78. [Google Scholar]
- Bule, M.H.; Ahmed, I.; Maqbool, F.; Bilal, M.; Iqbal, H.M. Microalgae as a source of high-value bioactive compounds. Front. Biosci. 2018, 10, 197–216. [Google Scholar]
- Bisht, B.; Kumar, V.; Gururani, P.; Tomar, M.S.; Nanda, M.; Vlaskin, M.S.; Kumar, S.; Kurbatova, A. The potential of nuclear magnetic resonance (NMR) in metabolomics and lipidomics of microalgae—A review. Arch. Biochem. Biophysics 2021, 710, 108987. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Q.; Zhou, H.; Kang, J.; Yu, X.; Qiu, G.; Shen, L. Physiological regulation of microalgae under cadmium stress and response mechanisms of time-series analysis using metabolomics. Sci. Total Environ. 2024, 916, 170278. [Google Scholar] [CrossRef]
- Fiehn, O.; Kind, T.; Barupal, D.K. Data processing, metabolomic databases and pathway analysis. In Annual Plant Reviews Volume 43: Biology of Plant Metabolomics; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; Volume 43, pp. 367–406. [Google Scholar]
- Sussman, E.M.; Oktem, B.; Isayeva, I.S.; Liu, J.; Wickramasekara, S.; Chandrasekar, V.; Nahan, K.; Shin, H.Y.; Zheng, J. Chemical characterization and non-targeted analysis of medical device extracts: A review of current approaches, gaps, and emerging practices. ACS Biomater. Sci. Eng. 2022, 8, 939–963. [Google Scholar] [CrossRef]
- Subhashini, D.V.; Singh, R.P.; Manchanda, G. OMICS Approaches: Tools to Unravel Microbial Systems; (OMICS Approaches: Tools to Unravel Microbial Systems—D. V. Subhashini, Raghvendra Pratap Singh (Writer on microbial biotechnology), Geetanjali Manchanda-Google Books); Directorate of Knowledge Management in Agriculture, Indian Council of Agricultural Research: New Delhi, India, 2017.
- Singh, R.P.; Manchanda, G.; Sarsan, S.; Kumar, A.; Panosyan, H. (Eds.) Microbial Essentialism: An Industrial Prospective; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, D.K.; Manchanda, G.; Chaudhary, R.G.; Singh, R.P. Microalgal Metabolomes and Recent Biotechnological Advances for Their Industrial Application. Microbiol. Res. 2024, 15, 2056-2069. https://doi.org/10.3390/microbiolres15040138
Saini DK, Manchanda G, Chaudhary RG, Singh RP. Microalgal Metabolomes and Recent Biotechnological Advances for Their Industrial Application. Microbiology Research. 2024; 15(4):2056-2069. https://doi.org/10.3390/microbiolres15040138
Chicago/Turabian StyleSaini, Dinesh Kumar, Geetanjali Manchanda, Ratiram Gomaji Chaudhary, and Raghvendra Pratap Singh. 2024. "Microalgal Metabolomes and Recent Biotechnological Advances for Their Industrial Application" Microbiology Research 15, no. 4: 2056-2069. https://doi.org/10.3390/microbiolres15040138
APA StyleSaini, D. K., Manchanda, G., Chaudhary, R. G., & Singh, R. P. (2024). Microalgal Metabolomes and Recent Biotechnological Advances for Their Industrial Application. Microbiology Research, 15(4), 2056-2069. https://doi.org/10.3390/microbiolres15040138






