Antibacterial and Antioxidant Activities of Flavonoids, Phenolic and Flavonoid Glycosides from Gouania longispicata Leaves
Abstract
1. Introduction
2. Methods
2.1. Sampling and General Experimental Procedures
2.2. Extraction and Isolation Procedure
2.3. Antibacterial Assay
2.4. Free Radical Scavenging Activity of Compounds 1–5
2.5. Statistical Analysis
3. Results and Discussion
3.1. Compounds Isolated from Methanolic Extract of GLE Leaves
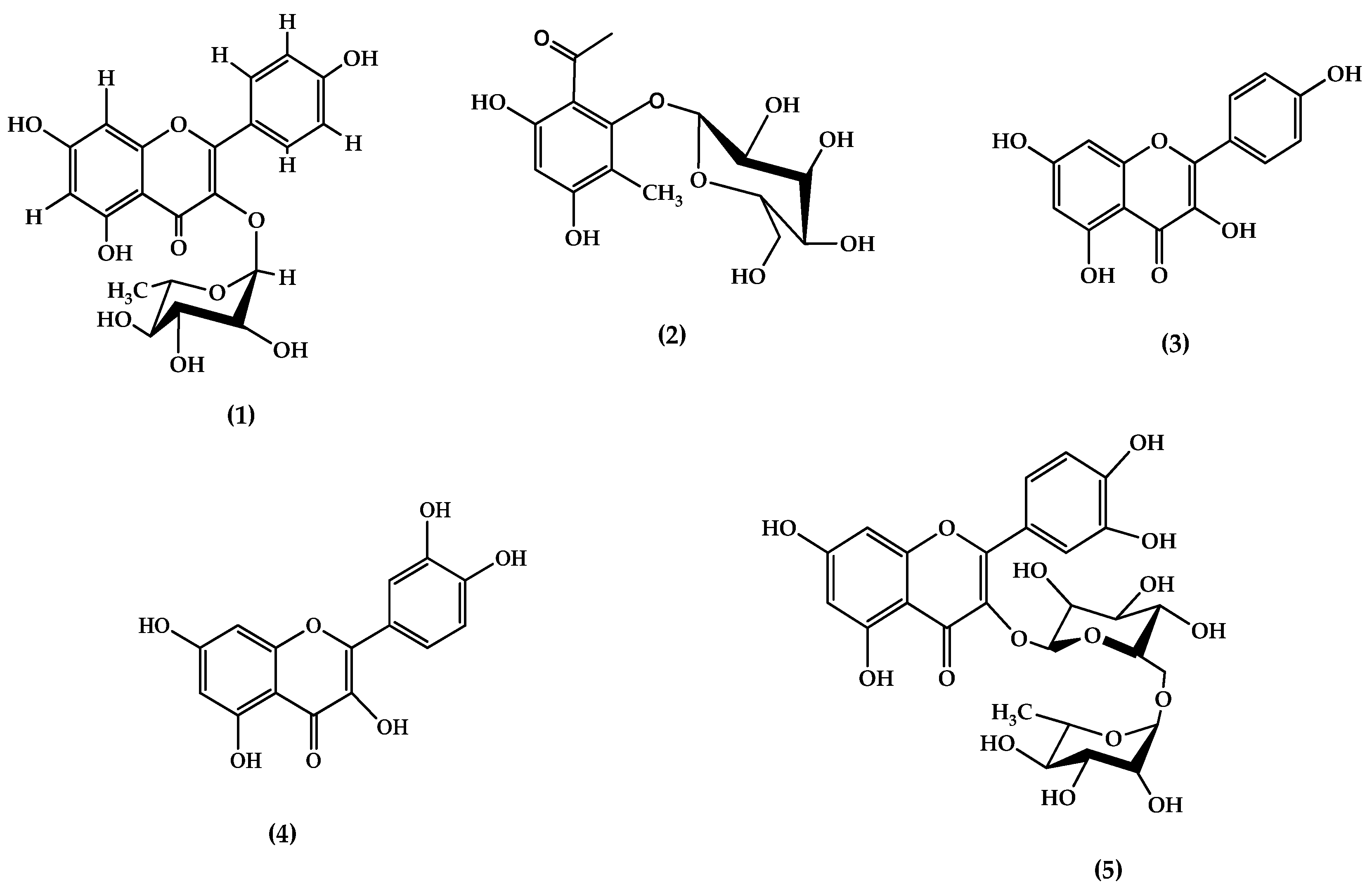
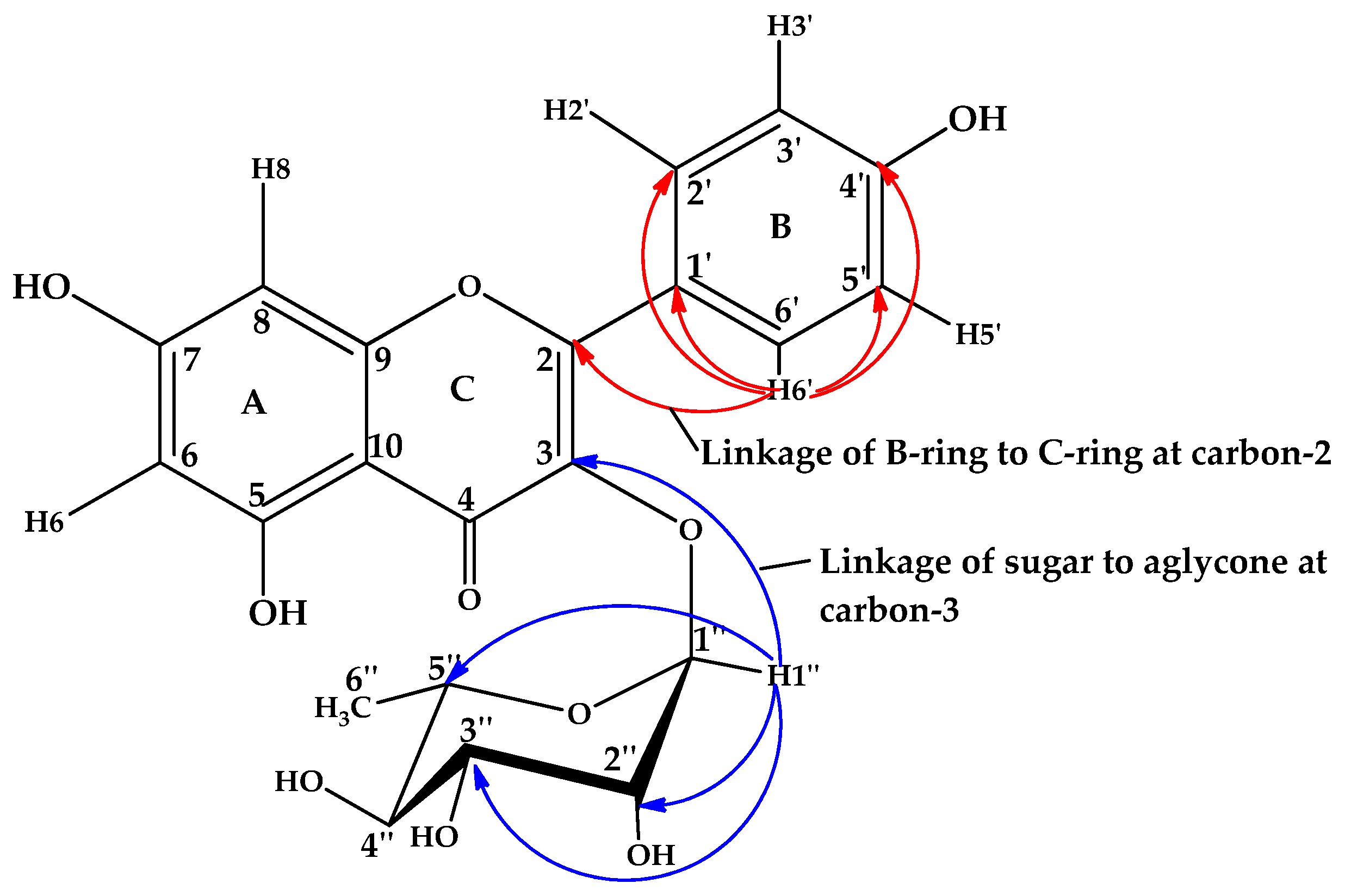
3.1.1. Compound 1
3.1.2. Compound 2
3.1.3. Compound 3
3.1.4. Compound 4
3.1.5. Compound 5
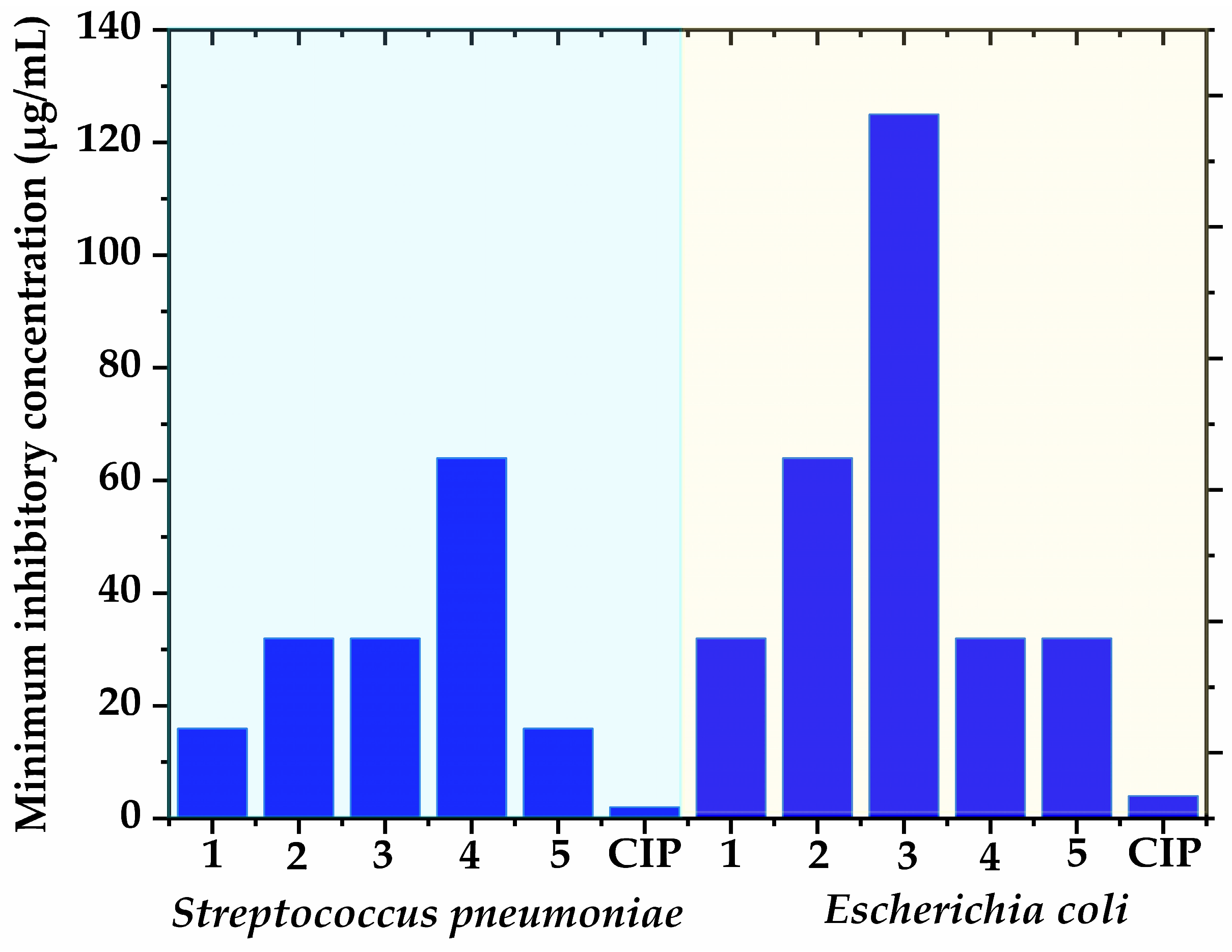
3.2. Antibacterial Potential of Isolated Polyphenols
3.3. Free Radical Scavenging Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2023, 400, 2221–2248. [Google Scholar]
- Pashkov, E.A.; Pak, A.V.; Pashkov, E.P.; Bykov, A.S.; Budanova, E.V.; Poddubikov, A.V.; Svitich, O.A.; Zverev, V.V. The prospects for the use of drugs based on the phenomenon of RNA interference against HIV infection. Probl. Virol. 2022, 67, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Tabuti, J.R.S.; Obakiro, S.B.; Nabatanzi, A.; Anywar, G.; Nambejja, C.; Mutyaba, M.R.; Omara, T.; Waako, P. Medicinal plants used for treatment of malaria by indigenous communities of Tororo District, Eastern Uganda. Trop. Med. Health 2023, 51, 34. [Google Scholar] [CrossRef] [PubMed]
- Ssenku, J.E.; Okurut, S.A.; Namuli, A.; Kudamba, A.; Tugume, P.; Matovu, P.; Wasige, G.; Kafeero, H.M.; Walusansa, A. Medicinal plant use, conservation, and the associated traditional knowledge in rural communities in Eastern Uganda. Trop. Med. Health 2022, 50, 39. [Google Scholar] [CrossRef] [PubMed]
- Omara, T. Antimalarial Plants Used across Kenyan Communities. Evid.-Based Complement. Altern. Med. 2020, 2020, 4538602. [Google Scholar] [CrossRef]
- Okello, D.; Kang, Y. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid.-Based Complement. Altern. Med. 2019, 2019, 3057180. [Google Scholar] [CrossRef]
- Gumisiriza, H.; Birungi, G.; Lejju, J.; Olet, E.; Kembabazi, O.; Duncan, C.; Sesaazi, C.D. Ethnobotany and Antimicrobial Activity of Gouania longispicata Engl. J. Complement. Med. Res. 2020, 11, 86–94. [Google Scholar] [CrossRef]
- Giday, M.; Asfaw, Z.; Woldu, Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J. Ethnopharmacol. 2010, 132, 75–85. [Google Scholar] [CrossRef]
- Irakiza, R.; Vedaste, M.; Elias, B.; Nyirambangutse, B.; Serge, N.; Marc, N. Assessment of traditional ecological knowledge and beliefs in the utilisationof important plant species: The case of Buhanga sacred forest, Rwanda. Koedoe 2016, 58, a1348. [Google Scholar] [CrossRef]
- Mollel, N.P.; Otieno, J.N.; Sitoni, D.K. Medicinal plants traded in Arusha city, Tanzania. J. Med. Plants Stud. 2022, 10, 175–182. [Google Scholar]
- Hamill, F.; Apio, S.; Mubiru, N.; Mosang, M.; Bukenya-Ziraba, R.; Maganyi, O.; Soejarto, D. Traditional herbal drugs of southern Uganda, I. J. Ethnopharmacol. 2000, 70, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Kudamba, A.; Kasolo, J.N.; Bbosa, G.S.; Lugaajju, A.; Wabinga, H.; Niyonzima, N.; Ocan, M.; Damani, A.M.; Kafeero, H.M.; Ssenku, J.E.; et al. Medicinal plants used in the management of cancers by residents in the Elgon Sub-Region, Uganda. BMC Complement. Med. Ther. 2023, 23, 450. [Google Scholar] [CrossRef] [PubMed]
- Gumisiriza, H.; Sesaazi, C.D.; Olet, E.A.; Kembabazi, O.; Birungi, G. Medicinal plants used to treat “African” diseases by the local communities of Bwambara sub-county in Rukungiri District, Western Uganda. J. Ethnopharmacol. 2021, 268, 113578. [Google Scholar] [CrossRef] [PubMed]
- Tabuti, J.R.; Kukunda, C.B.; Kaweesi, D.; Kasilo, O.M. Herbal medicine use in the districts of Nakapiripirit, Pallisa, Kanungu, and Mukono in Uganda. J. Ethnobiol. Ethnomed. 2012, 8, 35. [Google Scholar] [CrossRef]
- Olsthoorn, F.M.O. Determinants of Spatial Patterns of Human Activity in Bwindi Impenetrable National Park. Master’s Thesis, Imperial College London, London, UK, 2017. [Google Scholar]
- Kyoshabire, M.; Katuura, E.; Cunningham, A.B.; Hoeft, R. Medicinal plants and herbalist preferences around Bwindi Impenetrable National Park. J. Med. Plants Res. 2017, 11, 161–170. [Google Scholar]
- Tanno, T. Plant utilization of the Mbuti pygmies with Special Reference to their material culture and use of wild vegetable foods. Afr. Study Monogr. 1981, 1, 1–53. [Google Scholar]
- Chifundera, K. Livestock diseases and the traditional medicine in the bushi area, Kivu province, Democratic Republic of Congo. Afr. Stud. Monogr. 1998, 19, 13–33. [Google Scholar]
- Ganas, J.; Ortmann, S.; Robbins, M.M. Food preferences of wild mountain gorillas. Am. J. Primatol. 2008, 70, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.M.; Dierenfeld, E.S.; Molina, D.O.; Shaw, A.V.; Hintz, H.F.; Pell, A.N. Nutritional chemistry of foods eaten by gorillas in Bwindi Impenetrable National Park, Uganda. Am. J. Primatol. 2006, 68, 675–691. [Google Scholar] [CrossRef]
- Yamagiwa, J.; Basabose, A.K.; Kaleme, K.; Yumoto, T. Diet of Grauer’s Gorillas in the Montane Forest of Kahuzi, Democratic Republic of Congo. Int. J. Primatol. 2005, 26, 1345–1373. [Google Scholar] [CrossRef]
- Krief, S.; Hladik, C.M.; Haxaire, C. Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda. J. Ethnopharmacol. 2005, 101, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Freymann, E.; Carvalho, S.; Garbe, L.A.; Dwi Ghazhelia, D.; Hobaiter, C.; Huffman, M.A.; Muhumuza, G.; Schulz, L.; Sempebwa, D.; Wald, F.; et al. Pharmacological and behavioral investigation of putative self-medicative plants in Budongo chimpanzee diets. PLoS ONE 2024, 19, e0305219. [Google Scholar] [CrossRef] [PubMed]
- Demgne, O.M.F.; Tchinda, C.F.; Mbaveng, A.T.; Beng, V.P.; Kuete, V. Antibacterial and antibiotic-potentiating activities of nine Cameroonian medicinal plants against multidrug-resistant bacteria expressing active efflux pumps. Investig. Med. Chem. Pharmacol. 2022, 5, 58. [Google Scholar] [CrossRef]
- Migabo, H.; Izere, C.; Habyarimana, T.; Josiane, U.K.N.; Nsabayezu, E.; Niyonzima, F.N. Phytochemistry analysis and anti-bacterial properties of Rwandese medicinal plants. NeuroQuantology 2022, 20, 965–978. [Google Scholar]
- Gumisiriza, H.; Birungi, G.; Omara, T.; Lejju, J.B.; Sesaazi, C.D. Polyphenolic content, antioxidant activity and acute toxicity of Gouania longispicata Engl. leaves. LIANBS, 2025; in press. [Google Scholar]
- Ezeja, M.I.; Anaga, A.O.; Asuzu, I.U. Antidiabetic, antilipidemic, and antioxidant activities of Gouania longipetala methanol leaf extract in alloxan-induced diabetic rats. Pharm. Biol. 2015, 53, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.P.; Rao, J.M. Gouanic acid from the leaves of Gouania microcarpa. Phytochemistry 1993, 33, 711–712. [Google Scholar] [CrossRef]
- Giacomelli, S.R.; Maldaner, G.; Stücker, C.; Marasciulo, C.; Schmidt, J.; Wessjohann, L.; Dalcol, I.I.; Morel, A.F. Triterpenoids from Gouania ulmifolia. Planta Med. 2007, 73, 499–501. [Google Scholar] [CrossRef]
- Gossan, M.A.; Yao-kouassi, A.; Coffy, A.A.; Harakat, D.; Voutquenne-Nazabadioko, L. New acylated flavonol glycosides from the aerial parts of Gouania longipetala. Phytochem. Lett. 2015, 11, 306–310. [Google Scholar] [CrossRef]
- Gossan, D.P.; Alabdul Magid, A.; Yao-Kouassi, P.A.; Ahibo, C.A.; Josse, J.; Gangloff, S.C.; Morjani, H.; Voutquenne-Nazabadioko, L. Triterpene glycosides from the aerial parts of Gouania longipetala. Phytochemistry 2017, 134, 71–77. [Google Scholar] [CrossRef]
- Kennelly, E.J.; Lewis, W.H.; Winter, R.E.; Johnson, S.; Elvin-Lewis, M.; Gossling, J. Triterpenoid saponins from Gouania lupuloides. J. Nat. Prod. 1993, 56, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Désiré, S.; Ernestine, N.; Bruno, T.B.; Lazare, S.S.; Ulrich, D.D.; Lateef, M.; Schneider, B.; Ali, M.S.; Barthélemy, N. A new dammarane type triterpene glucoside from the aerial parts of Gouania longipetala (Rhamnaceae). Nat. Prod. Res. 2021, 35, 3192–3203. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ohtsuki, T.; Shindo, S.; Sato, M.; Koyano, T.; Preeprame, S.; Kowithayakorn, T.; Ishibashi, M. Mangiferin identified in a screening study guided by neuraminidase inhibitory activity. Planta Med. 2007, 73, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Hang, N.T.; Bich Thu, N.T.; Le Ba, V.; Van On, T.; Khoi, N.M.; Do, T.H. Characterisation of four new triterpenoid saponins with nitric oxide inhibitory activity from aerial parts of Gouania leptostachya. Nat. Prod. Res. 2022, 36, 5999–6005. [Google Scholar] [CrossRef] [PubMed]
- Hang, N.T.; Thu, N.T.B.; Vinh, L.B.; Phong, N.V.; On, T.V.; Lee, K.Y. A New Ceanothane-Type Triterpenoid Saponin Isolated from Gouania leptostachya DC. var. tonkinensis Pit. and Its Underlying Anti-Inflammatory Effects. J. Microbiol. Biotechnol. 2023, 33, 941–948. [Google Scholar] [CrossRef]
- Omara, T.; Kiprop, A.K.; Kosgei, V.J. Intraspecific variation of phytochemicals, antioxidant, and antibacterial activities of different solvent extracts of Albizia coriaria leaves from some agroecological zones of Uganda. Evid.-Based Complement. Altern. Med. 2021, 2021, 2335454. [Google Scholar] [CrossRef]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-qTOF-MS) Analysis and In Vitro Bioactivities of the Momordica balsamina Leaf Extract. Biochem. Res. Int. 2021, 2021, 2854217. [Google Scholar] [CrossRef]
- Guemari, F.; Laouini, S.E.; Rebiai, A.; Bouafia, A.; Meneceur, S.; Tliba, A.; Majrashi, K.A.; Alshareef, S.A.; Menaa, F.; Barhoum, A. UV-Visible Spectroscopic Technique-Data Mining Tool as a Reliable, Fast, and Cost-Effective Method for the Prediction of Total Polyphenol Contents: Validation in a Bunch of Medicinal Plant Extracts. Appl. Sci. 2022, 12, 9430. [Google Scholar] [CrossRef]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van der Westhuizen, J.H. Photochemistry of Flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.F.; Elkhateeb, A.; Marzouk, M.M.; Hussein, S.R.; Abdel-Hameed, E.S.; Kassem, M.E.S. Flavonoid investigation, LC–ESI-MS profile and cytotoxic activity of Raphanus raphanistrum L. (Brassicaceae). J. Chem. Pharm. Res. 2016, 8, 786–793. [Google Scholar]
- Nicolescu, T. Interpretation of Mass Spectra. In Mass Spectrometry; IntechOpen: London, UK, 2017; pp. 23–78. [Google Scholar]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass. Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Mair, C.E.; Grienke, U.; Wilhelm, A.; Urban, E.; Zehl, M.; Schmidtke, M.; Rollinger, J.M. Anti-Influenza Triterpene Saponins from the Bark of Burkea africana. J. Nat. Prod. 2018, 81, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Malee, T. Chemical Constituents from the Leaves and Stems of Citrus aurantifolia Swingle. Master’s Thesis, Prince of Songkla University, Hat Yai, Thailand, 2011. [Google Scholar]
- Hussain, J.; Ali, L.; Khan, A.L.; Rehman, N.U.; Jabeen, F.; Kim, J.-S.; Al-Harrasi, A. Isolation and Bioactivities of the Flavonoids Morin and Morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A Wild Arabian Medicinal Plant. Molecules 2014, 19, 17763–17772. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Ghaly, N.S.; Melek, F.R.; Abdelwahed, N.A.M. Flavonoids from Albizia chinensis of Egypt. Rev. Latinoam. Química 2010, 38, 153–158. [Google Scholar]
- Lee, S.Y.; So, Y.-J.; Shin, M.S.; Cho, J.Y.; Lee, J. Antibacterial Effects of Afzelin Isolated from Cornus macrophylla on Pseudomonas aeruginosa, A Leading Cause of Illness in Immunocompromised Individuals. Molecules 2014, 19, 3173–3180. [Google Scholar] [CrossRef]
- Adhikari-Devkota, A.; Dirar, A.I.; Kurizaki, A.; Tsushiro, K.; Devkota, H.P. Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations 2019, 6, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Cui, L.; Liu, Z.; Wei, J.; Kang, W. Antioxidant and α-glucosidase inhibitiory activity of Cercis chinensis flowers. Food Sci. Hum. Wellness 2020, 9, 313–319. [Google Scholar] [CrossRef]
- Söhretoğlu, D.; Sabuncuoğlu, S. Secondary metabolites, cytotoxic response by neutral red retention and protective effect against H2O2 induced cytotoxicity of Sedum caespitosum. Nat. Prod. Commun. 2012, 7, 39–40. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; George, M.Y.; Mostafa, N.M.; Eldahshan, O.A. In vivo hepatoprotective and nephroprotective effects of Stenocarpus sinuatus leaf extract against ifosfamide-induced toxicity in rats. Arch. Pharm. 2024, 357, e2300438. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Bauer, J.; Osborn, H.M.I.; Kuemmerle, R. A Solution NMR Approach To Determine the Chemical Structures of Carbohydrates Using the Hydroxyl Groups as Starting Points. ACS Omega 2018, 3, 17957–17975. [Google Scholar] [CrossRef] [PubMed]
- Ayräs, P.; Lötjönen, S.; Widén, C.J. NMR spectroscopy of naturally occurring phloroglucinol derivatives. Planta Med. 1981, 42, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.M.; Fossen, T. Characterization of anthocyanins by NMR. In Current Protocals in Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2003; pp. F.1.4.1–F.1.4.23. [Google Scholar]
- Ryu, B.; Kim, H.M.; Woo, J.H.; Choi, J.H.; Jang, D.S. A new acetophenone glycoside from the flower buds of Syzygium aromaticum (cloves). Fitoterapia 2016, 115, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Staerk, D.; Kongstad, K.T. Potential of Myrtus communis Linn. as a bifunctional food: Dual high-resolution PTP1B and α-glucosidase inhibition profiling combined with HPLC-HRMS and NMR for identification of antidiabetic triterpenoids and phloroglucinol derivatives. J. Funct. Foods 2020, 64, 103623. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, Y.; Chen, G.; Dong, Y.; Zhu, Y. Studies on Active Constituents of Root of Euphorbia ebracteolata Hayata Euphorbiaceae. J. Integr. Plant Biol. 1987, 29, 4. [Google Scholar]
- Zhang, N.; Cai, H.; Cai, B.; Yang, H.; Li, J.; Yang, G. Two new cytotoxic acetophenone derivatives from Euphorbia ebracteolata Hayata. Fitoterapia 2010, 81, 385–388. [Google Scholar] [CrossRef]
- Singh, A.K.; Pathak, V.; Agrawal, P.K. Annphenone, a phenolic acetophenone from Artemisia annua. Phytochemistry 1997, 44, 555–557. [Google Scholar] [CrossRef]
- Petroviciu, I.; Albu, F.; Medvedovici, A. LC/MS and LC/MS/MS based protocol for identification of dyes in historic textiles. Microchem. J. 2010, 95, 247–254. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants, 2nd ed.; Isolation, Purification and Extract Preparation; Academic Press: Cambridge, MA, USA, 2019; pp. 263–284. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.I. Interpretation of the Ultraviolet Spectra of Natural Products. In International Series of Monographs on Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1964; Volume 690, pp. 313–362. [Google Scholar]
- Satheeshkumar, N.; Shantikumar, S.; Komali, M. Identification and Quantification of Aldose Reductase Inhibitory Flavonoids in Herbal Formulation and Extract of Gymnema sylvestre Using HPLC-PDA and LC-MS/MS. Chromatogr. Res. Int. 2014, 2014, 518175. [Google Scholar] [CrossRef]
- Thuy, N.T.K.; Trang, D.T.; Thi, M.; Trang, N.; Bay, N.K.; Tai, B.H. Flavonol glycosides and dammarane saponin from Gouania leptostachya. Vietnam J. Chem. 2019, 57, 277–280. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Nwachukwu, K.C.; Aloh, G.S.; Egbachukwu, S.I.; Ijioma, S.N.; Alli, L.A.; Ugwuanyi, K.C.; Onwuchekwa, B.U.; Okoh, M.P. Phytochemical composition, GC-MS analysis and toxicological profiling of Gouania longipetala leaf extract in rats. Afr. J. Biotechnol. 2023, 22, 192–201. [Google Scholar]
- Omar, S.H. Discovery and Development of Neuroprotective Agents from Natural Products. In Natural Product Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–148. [Google Scholar]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Sandosh, T.A.; Peter, M.P.J.; Raj, J.Y. Phytochemical Analysis of Stylosanthes fruticosa using UV-VIS, FTIR and GC-MS. Res. J. Chem. Sci. 2013, 3, 14–23. [Google Scholar]
- Anand, D.A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Şengönül, H.; Demircan, O. Isolation and characterization of quercetin from Prunus Serrulata leaf extract in the synthesis of 708 Fe3O4 nanoparticles. Inorg. Chem. Commun. 2024, 159, 111688. [Google Scholar] [CrossRef]
- March, R.; Brodbelt, J. Analysis of flavonoids: Tandem mass spectrometry, computational methods, and NMR. J. Mass. Spectrom. 2008, 43, 1581–1617. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar]
- Gumisiriza, H.; Birungi, G.; Olet, E.A.; Sesaazi, C.D. Medicinal plant species used by local communities around Queen Elizabeth National Park, Maramagambo Central Forest Reserve and Ihimbo Central Forest Reserve, South western Uganda. J. Ethnopharmacol. 2019, 239, 111926. [Google Scholar] [CrossRef]
- Ahmadpourmir, H.; Attar, H.; Asili, J.; Soheili, V.; Taghizadeh, S.F.; Shakeri, A. Natural-derived acetophenones: Chemistry and pharmacological activities. Nat. Prod. Bioprospect. 2024, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.; Iniesta-Sanmartín, E.; Tomás-Lorente, F.; Rumbero, A. Antimicrobial phenolic compounds from three Spanish Helichrysum species. Phytochemistry 1990, 29, 1093–1095. [Google Scholar] [CrossRef]
- Teffo, L.S.; Aderogba, M.A.; Eloff, J.N. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. S. Afr. J. Bot. 2010, 76, 25–29. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Feng, J.; Lou, B.; Zhou, X.; Wu, H. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar]
- Almuhanna, Y.; Alshalani, A.; AlSudais, H.; Alanazi, F.; Alissa, M.; Asad, M.; Joseph, B. Antibacterial, Antibiofilm, and Wound Healing Activities of Rutin and Quercetin and Their Interaction with Gentamicin on Excision Wounds in Diabetic Mice. Biology 2024, 13, 676. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arh. Hig. Rada Toksikol. 2020, 71, 300–311. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [PubMed]
- Huang, Y.H.; Huang, C.C.; Chen, C.C.; Yang, K.J.; Huang, C.Y. Inhibition of Staphylococcus aureus PriA Helicase by Flavonol Kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Z.; Li, Z.; Ding, Y.; Jiang, F.; Liu, J. Antioxidant and antibacterial study of 10 flavonoids revealed rutin as a potential antibiofilm agent in Klebsiella pneumoniae strains isolated from hospitalized patients. Microb. Pathog. 2021, 159, 105121. [Google Scholar] [CrossRef]
- Czerkas, K.; Olchowik-Grabarek, E.; Łomanowska, M.; Abdulladjanova, N.; Sękowski, S. Antibacterial Activity of Plant Polyphenols Belonging to the Tannins against Streptococcus mutans—Potential against Dental Caries. Molecules 2024, 29, 879. [Google Scholar] [CrossRef]
- Tatsimo, S.J.; Tamokou, J.D.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef]
- Akter, M.; Parvin, M.S.; Hasan, M.M.; Rahman, M.A.A.; Islam, M.E. Anti-tumor and antioxidant activity of kaempferol-3-O-alpha-L-rhamnoside (Afzelin) isolated from Pithecellobium dulce leaves. BMC Complement. Med. Ther. 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Mohammed, M.J.; Yi-Chen, L.; Watson, D.G.; Lakhssassi, N.; Cacciola, F.; Ibrahim, S.A. Optimization of Ultrasonicated Kaempferol Extraction from Ocimum basilicum Using a Box–Behnken Design and Its Densitometric Validation. Foods 2020, 9, 1379. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]

| Position | δH (ppm), Multiplicity, J (Hz) 1 | δC (ppm) |
|---|---|---|
| Aglycone | ||
| 2 | 158.4 | |
| 3 | 136.7 | |
| 4 | 176.8 | |
| 5 | 162.4 | |
| 6 | 6.17, d, 2.0 | 97.8 |
| 7 | 164.8 | |
| 8 | 6.20, d, 2.0 | 94.9 |
| 9 | 158.4 | |
| 10 | 104.8 | |
| 1′ | 121.0 | |
| 2′ | 7.71, d, 8.7 | 128.1 |
| 3′ | 6.93, d, 8.7 | 112.5 |
| 4′ | 160.1 | |
| 5′ | 6.93, d, 8.7 | 112.5 |
| 6′ | 7.71, d, 8.7 | 128.1 |
| 3-O-α- rhamnopyranosyl | ||
| 1″ | 5.85, d, 1.3 | 101.1 |
| 2″ | 4.39, dd, 3.5, 1.2 | 82.9 |
| 3″ | 4.06, dd, 9.3, 3.5 | 71.8 |
| 4″ | 3.75, t, 9.2 | 76.8 |
| 5″ | 3.80, m | 74.0 |
| 6″ | 1.38, d, 6.2 | 17.8 |
| Carbon | δH (ppm), Multiplicity, J (Hz) 1 | δC |
|---|---|---|
| 1 | - | 61.07 |
| 2 | - | 139.40 |
| 3 | - | 97.57 |
| 4 | - | 147.85 |
| 5 | 6.42, s | 92.71 |
| 6 | - | 159.61 |
| β-D-Glucose | O-glycosylation | |
| 1′ | 5.03, d (7.8) | 72.12 |
| 2′ | 4.40, dd (7.8, 8.2) | 73.39 |
| 3′ | 3.54, dd (9.0, 9.1) | 76.55 |
| 4′ | 3.20, dd (9.1, 9.7) | 69.82 |
| 5′ | 3.35, ddd (2.3, 6.0, 9.7) | 70.14 |
| 6′ | 3.48, dd (2.2, 12.1) 3.17, dd (5.7, 12.5) | 65.61 |
| COCH3 | 208.57 | |
| COCH3 | 2.56, s | 29.34 |
| -CH3 | 2.14, s | 9.75 |
| Compound | Half Maximal Inhibitory Concentration (µg/mL) 1 |
|---|---|
| Kaempferol-3-O-α-rhamnopyranoside (compound 1) | 20.0 ± 0.45 a * |
| 4,6-dihydroxy-3-methylacetophenone-2-O-β-D-glucopyranoside (compound 2) | 26.3 ± 0.12 b * |
| Kaempferol (compound 3) | 19.8 ± 1.03 a,c,f |
| Quercetin (compound 4) | 18.6 ± 1.30 a,c,d,f |
| Rutin (compound 5) | 28.1 ± 0.09 e * |
| Ascorbic acid (positive control) | 14.6 ± 0.15 c,f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumisiriza, H.; Olet, E.A.; Mwikali, L.; Akatuhebwa, R.; Omara, T.; Lejju, J.B.; Sesaazi, D.C. Antibacterial and Antioxidant Activities of Flavonoids, Phenolic and Flavonoid Glycosides from Gouania longispicata Leaves. Microbiol. Res. 2024, 15, 2085-2101. https://doi.org/10.3390/microbiolres15040140
Gumisiriza H, Olet EA, Mwikali L, Akatuhebwa R, Omara T, Lejju JB, Sesaazi DC. Antibacterial and Antioxidant Activities of Flavonoids, Phenolic and Flavonoid Glycosides from Gouania longispicata Leaves. Microbiology Research. 2024; 15(4):2085-2101. https://doi.org/10.3390/microbiolres15040140
Chicago/Turabian StyleGumisiriza, Hannington, Eunice Apio Olet, Lydia Mwikali, Racheal Akatuhebwa, Timothy Omara, Julius Bunny Lejju, and Duncan Crispin Sesaazi. 2024. "Antibacterial and Antioxidant Activities of Flavonoids, Phenolic and Flavonoid Glycosides from Gouania longispicata Leaves" Microbiology Research 15, no. 4: 2085-2101. https://doi.org/10.3390/microbiolres15040140
APA StyleGumisiriza, H., Olet, E. A., Mwikali, L., Akatuhebwa, R., Omara, T., Lejju, J. B., & Sesaazi, D. C. (2024). Antibacterial and Antioxidant Activities of Flavonoids, Phenolic and Flavonoid Glycosides from Gouania longispicata Leaves. Microbiology Research, 15(4), 2085-2101. https://doi.org/10.3390/microbiolres15040140







