Abstract
Chlamydia trachomatis (CT) is a bacterium that causes one of the most common sexually transmitted infections (STIs) worldwide. In Panama, the prevalence of genital Chlamydia trachomatis (CT) among adolescents is 15.8%. However, no data describing circulating CT genotypes or evaluating molecular resistance are available. This study aims to determine the genotypes of genital CT infections and explore the macrolide resistance-associated mutations in this population to contribute to baseline information about CT circulating strains and antimicrobial resistance. Genomic analysis was performed on CT-positive, first-void urine specimens from school-going adolescents (14–19 years) in urban regions in Panama. The ompA gene was used for genotype and phylogenetic analysis, and the rplD, rplV, and 23S rRNA genes were used for molecular resistance analysis. Five genotypes were found: D, 15 (47%); F, 9 (28%); E, 4 (13%); Ia, 2 (6%); and Ja, 2 (6%) genotype Ja. A triple mutation (G52S, R65C, and V77A) was found in the rplV gene, though no mutations of interest were found for the rplD and 23S rRNA genes. The present study indicated CT genotype D had increased circulation within the population; mutations indicative of macrolide resistance were not found. Follow-up studies and implementation of active surveillance are necessary to understand the circulation of CT in Panama.
1. Introduction
Chlamydia trachomatis (CT) is a microorganism that causes one of the most prevalent bacterial sexually transmitted infections (STIs) reported, with a high prevalence among persons aged ≤24 years [1].
The prevalence of asymptomatic infection globally is around 3.1% (95% CI, 2.5–3.8%) in females and 2.6% (95% CI, 2.0–3.2%) in males [2]. However, Panama lacks a systematic laboratory diagnosis for CT in the public sector, making syndromic management and subsequent diagnosis difficult.
The World Health Organization (WHO) STI guidelines advocate for conducting research that describes the epidemiology and macrolide resistance of genital CT [3]. From 2015 to 2018, a large (>2000 adolescents 14–19 years) epidemiological study was conducted in urban (districts of Panama, San Miguelito, Colon, and Arraijan/La Chorrera [Panama Oeste]) and rural-Indigenous regions (La Comarca Ngäbe-Buglé) of Panama. This study found a CT prevalence of 15.8% across the country, with no significant difference in prevalence between regions [4]. Although some data exist on the prevalence in this population, the country lacks data on circulating genotypes and significant changes in the genome that could indicate macrolide resistance.
Currently, the feasibility and reliability of sequencing platforms allow the implementation of genotyping studies for infectious diseases to describe the presence of genetic associations among circulating strains and analyze their implications for clinical management. Research on genital CT genotyping has provided helpful information to understand the geographic and temporal dynamics in the United States [5] and to trace its circulation on transmission networks in Sweden [6].
Molecular surveillance of CT mutations associated with antibiotic resistance is not implemented worldwide. However, previous studies have suggested mutations in ribosomal proteins L4 (rplD gene) and L22 (rplV gene), together with two more mutations in domain V or II of the 23S rRNA gene, could generate resistance to macrolides [7] and therapeutic failure. Currently, azithromycin is used as first-line treatment for suspected and confirmed CT infections in Panama [8,9]; however, there are no available studies that evaluate its resistance level in the country.
Due to the lack of available data on CT genotyping and molecular surveillance of CT mutations associated with antibiotic resistance in Panama, this study aimed to describe circulating CT genotypes and sequence-level variations associated with macrolide resistance using urine storage samples from previous studies performed for our group.
2. Methods
2.1. Study Design
This descriptive cross-sectional study describes the circulating CT genotypes, and the sequence-level variations associated with macrolide resistance, in urine samples from a group of school-aged adolescents.
2.2. Sample Size Determination
This study was carried out using all 166 CT-positive archived urine samples collected from the large epidemiological study on STI prevalence among adolescents who attended urban public high schools during 2016–2018 in Panama (2016: San Miguelito, 2017: Colon, 2018: La Chorrera/Arraijan), as previously described [4,10]. Only samples for which permission was granted for further analysis were included in this study.
2.3. Amplification and Sequencing of Genes of Interest
According to the manufacturer’s instructions, C. trachomatis DNA/RNA was extracted using 1 mL of sample and the QIAamp Viral RNA Mini Kit (Qiagen, Germantown, TN, USA.
Based on the ompA gene, which encodes the major outer membrane protein (MOMP) and is widely used worldwide [11,12,13] to determine the genotypes of this microorganism, amplification was performed using specific primers for ompA, rplV, 23S rRNA [14], and the rplD gene [15] to identify the circulating genotypes and variations associated with macrolide resistance (Supplementary Table S1).
Reverse transcription of the 23S rRNA gene was performed using the AccessQuick RT-PCR System (Promega, Madison, WI, USA), following the reaction mixture: AccessQuick Master Mix 2X 25 µL, primer forward 1 µL, primer reverse 1 µL, AMV Reverse Transcriptase 1 µL, extracted RNA 10 µL, water 12 µL carries to a final volume of 50 µL with the following conditions: 45° for 45 min, 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR reaction for ompA, rplV, and rplD genes was performed using Taq Master Mix (Qiagen, Germantown, TN, USA ) 13 µL, primer forward 1 µL, primer reverse 1 µL, extracted DNA 12 µL, water 5 µL carries to a final volume of 32 µL with the following conditions: 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min. PCR products were stored at −20 °C until library preparation.
Library preparation was performed using the Nextera XT Library Prep Kit [16] and sequenced in a MiSeq [17] (Illumina Inc., Hayward, CA, USA) for 500 cycles.
2.4. Bioinformatic and Phylogenetic Analysis
The reads obtained were filtered by quality and mapped against reference (ompA: CTU78528; rplD, and rplV: NC_000117; and the 23S rRNA: NR_076160) using Quasitools-hydra algorithms [18].
For phylogenetic analysis, sequences were aligned with reference genomes (Figure 1). The ompA gene alignment was performed using Multiple Alignment using Fast Fourier Transform (MAFFT) v7.490 [19]. A phylogenetic tree was built with IQtree v.16.12 [20], applying the K2P + G4 model and maximum likelihood algorithm with statistical support of ultrafast bootstrap with 1000 replicates. The resulting tree was visualized in iTOL.
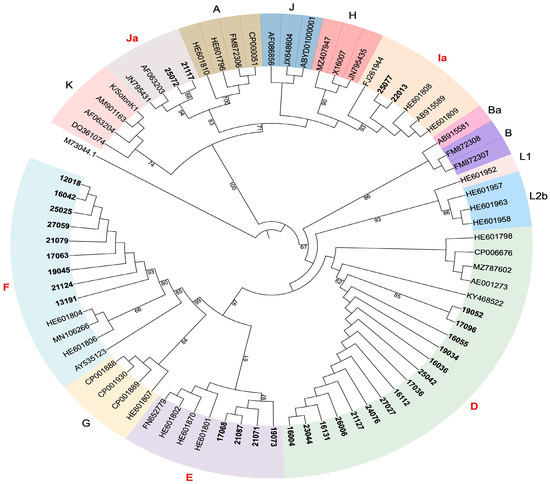
Figure 1.
Genotype analysis of Chlamydia trachomatis in a region of the ompA gene. A circular maximum likelihood phylogenetic tree using ompA genes reference from GeneBank is shown. Samples analyzed in this study are highlighted in bold, genotypes are color-coded and genotypes found in this study are highlighted in red. The tree image was generated using ITOL.
The following substitutions were quantified to evaluate mutations associated with antibiotic resistance: for the gene rplD (L4 protein) G66K; for gene rplV (L22 protein) G52S, R65C, V77A substitutions and 23S rRNA gene substitutions A2058C and T2611C (Escherichia coli numbering). Obtained sequences were compared after alignment against reference sequences indicated above.
All sequences presented in this study are available in GenBank database under the accession numbers: PQ177987–PQ178018 for rplD (L4); PQ178019–PQ178050 for rplV (L22); PQ178051–PQ178082 for ompA and PQ201841–PQ201855 for 23S rRNA.
2.5. Ethical Considerations
Underage students (14–17 years old) were given letters to bring to their parent or guardian, inviting them to a meeting with the study team to provide informed consent for their child. Each eligible minor student then provided their informed assent to participate. Students of legal age (18–19 years old) signed their own consent forms directly. Permission to use the sample in future studies was included in the informed consent.
3. Results
Of the 166 urine samples that tested positive for Chlamydia trachomatis (CT) via RT-PCR, 139 samples satisfied the volume requirement for the test (a minimum of 1 mL of urine) and were subsequently amplified. Among those, 32 were successfully amplified and sequenced for the ompA, rplD, and rplV genes. Of these 32, 15 samples were further amplified and sequenced for the 23S rRNA gene.
The greatest number of sequences were from participants collected in the district of Colon, with 46% (15/32), 11 from female participants, 4 males, followed by the districts of Panama Oeste with 28% (9/32), 6 females and 3 males and Panama district with 25% (8/32), belonging to 7 females and 1 male (Supplementary Table S2).
Five genotypes were identified based on sequence analysis of the ompA gene. Genotypes D (n = 15; 47%) and F (n = 9; 28%) were predominant, followed by E (n = 4; 13%), Ia (n = 2; 6%) and Ja (n = 2; 6%). D and F were found in all three regions among the genotypes identified; genotype Ia was found in Colon (1) and Panama Oeste (1) and genotype Ja was found in Colon (1) and Panama Oeste (1). Genotype E was only present in Colon (3). The diversity of the genotypes identified is shown in Figure 1 and Supplementary Table S2.
In all, 90% (29/32) of the samples had mutations in the rplV gene (L22) G52S, R65C and V77A. Additionally, we found 90% (29/32) samples with the double mutation R111Q and V132I in the rplD gene in the same samples with triple mutations in the rplV gene (See Table 1). In the 23S rRNA gene, we found a variation located in position 2107; on the contrary, the variation in the 2660 position was not found (Figure 2).

Table 1.
Result of mutations found in the rplD and rplV genes.
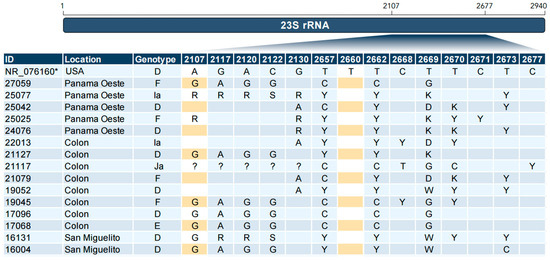
Figure 2.
Sequence alignment of the 23S rRNA gene. Changes at the nucleotide level relative to the reference sequence are shown. In the search for the A2058C and T2611C mutations, the nucleotides have been highlighted in yellow to indicate 2107 and 2660 positions homologous to position 2058 and 2611, respectively, to the numbering in the Escherichia coli 23S rRNA gene. Reference sequence (*); Identical ( ); Missing (?).
4. Discussion
This study was the first in Panama to evaluate Chlamydia trachomatis (CT) genotypes and molecular resistance from first-flow urine samples associated with them. Several genotypes were detected, and no genetically associated antibiotic resistance was found in the analyzed sequences.
Based on the genotyping of the ompA gene of CT, it has been classified depending on the anatomic affected place: Genotypes A to C are associated with trachoma, genotypes D to K are associated with urogenital and neonatal infections and genotypes L1, L2, L2b and L3 associated with lymphogranuloma venereum (LGV) causing genital ulcers [21]. Five Chlamydia trachomatis (CT) genotypes (D, E, F, Ia, Ja) were identified. Previous reports associated these as common in asymptomatic urogenital infections [11]. In our study, all of them were detected in asymptomatic students, reflecting an immune adaptation that permits increased transmission of CT within this population [22,23], accompanied by spontaneous clearance, as reported in other studies [24].
The prevalence of these genotypes is in line with reports from Chile, Brazil, and Argentina, where genotypes E, F, and D are the most common strains [23,25,26]. Genotypes Ia and Ja have been reported frequently in the USA [27] and, to a lesser extent, in European countries such as Sweden and Slovenia [28]; their presence in Panama suggests a very diverse circulation in the country. Genotype F has been reported as the most prevalent in Mexico followed by genotype E [29]. Moreover, in our study, genotype E was only found in the Colon district, possibly due to the high migration influx in this region. The diversity of genotypes found in the analyzed samples indicates ongoing asymptomatic transmission of CT in the studied population, highlighting the need to improve diagnosis availability and genotype surveillance in Panama.
In our study, the rplV gene was found to have triple mutation G52S, R65C and V77A in 29 (90%) samples. These triple mutations are of interest in macrolide resistance. Some reports suggested the prevalence of this triple mutation in our samples may be considered high [30]. However, for the resistant phenotype to be expressed, it must be accompanied by mutations at positions 2058 and 2611 (Escherichia coli numbering) of the 23S rRNA gene [7]. On the other hand, in the rplD and 23S rRNA genes, despite presenting substitutions, the accessory mutations necessary for CT to acquire genotypic resistance to macrolides were not found. However, avoiding macrolides in these cases would reduce the probability of developing the mutations described in the 23S rRNA to generate resistance to macrolides [7].
Among the samples analyzed, we did not find mutations associated with resistance to the antibiotics currently used to treat CT in Panama, as those described in CT and other species, such as C. psittaci, with mutations in the 23S rRNA gene and the Gln 66 Lys mutation, which made it eight times less susceptible to azithromycin [31]. Currently, typing and molecular surveillance based on sexually transmitted infections is not a widely used method in the region, especially in Panama. The results obtained in this study emphasize the need to use molecular diagnostic methods to diagnose CT across the country and undertake surveillance of antimicrobial resistance on CT circulating variants. By collecting this information, the health system could focus on available resources to control CT and guide therapies precisely, reducing the public health impact of this infection [32].
This investigation encountered certain limitations. Due to the use of urine samples rather than swabs containing genital discharge, many specimens exhibited a lower bacterial load, as several studies suggested [33], thus impeding the ability to perform this type of molecular analysis. Although most of the CT-positive subjects came from females, this demonstrates that it is still possible to detect and perform molecular characterization using first-catch urine samples. We could amplify only 23% (n = 32) of positive samples; another contributing factor was the available sample volume was limited to 1 mL of urine that had been archived. This precluded the use of concentration methods to increase the number of nucleic acids for amplification steps. The small sample size, coupled with the limited representation by region analyzed, along with the results obtained, may restrict the generalizability of the genotype description and macrolide resistance testing of the present study.
Program and Policy Implications
Our findings encourage the implementation of diagnosis and macrolide resistance programs across Panama. This could provide a better picture of the implications of the continuous transmission of the genotypes circulating in the country and even globally.
This study provides the first data on the genetics of CT in Panama and indicates that no genotypes of clinical significance for macrolide resistance were found, national-scale genomic surveillance is needed to anticipate the appearance of variants with pathogenic and epidemiological importance and capture the evolution of CT.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres15040134/s1. Table S1: Primers used for PCR amplification; Table S2: Distribution of sequenced samples by region of origin.
Author Contributions
Formal analysis, J.G. and C.A.; Investigation, J.G., J.C., C.G., A.M. and O.C.; Methodology, A.G., J.M.P. and A.A.M.; Supervision, A.A.M.; Writing—original draft, J.G. and A.A.M.; Writing—review and editing, C.A., J.C., C.G., A.M., O.C., A.G., J.M.P. and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was conducted at the Genomics Department laboratories, at the Gorgas Memorial Institute for Health Studies. This study was partially funded by the Ministerio de Economía y Finanzas de Panamá (MEF), grant number 1111301406, and SENACYT grant FID18-044.
Institutional Review Board Statement
The study was approved by the Instituto Conmemorativo Gorgas (N701/CBI/ICGES/15).
Informed Consent Statement
In the original study all participants provided informed consent (if ≥18 years old), parents/guardians provided consent, and permission was given by participants (if 14–17 years old), only samples in which permission was granted for posteriors used was included in this study.
Data Availability Statement
The original data presented in the study are available in GenBank database under the accession numbers: PQ177987–PQ178018 for rplD (L4), PQ178019–PQ178050 for rplV (L22), PQ178051–PQ178082 for ompA and PQ201841–PQ201855 for 23S rRNA.
Acknowledgments
The authors are grateful to the participants/guardians who consented to save their samples for future research and the sample processing unit for the facilities provided for handling samples, especially Migdalys Ortega and Kimberli Zamora.
Conflicts of Interest
The co-authors have no conflicts of interest to disclose.
References
- World Health Organization Sexually Transmitted Infections (STIs). 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 13 September 2024).
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Treatment of Chlamydia trachomatis. 2016. Available online: https://iris.who.int/bitstream/handle/10665/246165/9789241549714-eng.pdf?sequence=1 (accessed on 12 December 2023).
- Gabster, A.; Mayaud, P.; Ortiz, A.; Castillo, J.; Castillero, O.; Martínez, A.; López, A.; Aizprúa, B.; Pitano, S.; Murillo, A.; et al. Prevalence and determinants of genital Chlamydia trachomatis among school-going, sexually experienced adolescents in urban and rural Indigenous regions of Panama. Sex. Transm. Infect. 2021, 97, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.S.; Sanchez-Pescador, R.; Wagar, E.A.; Inouye, C.; Urdea, M.S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 1987, 169, 3879–3885. [Google Scholar] [CrossRef] [PubMed]
- Lysén, M.; Österlund, A.; Rubin, C.J.; Persson, T.; Persson, I.; Herrmann, B. Characterization of ompA Genotypes by Sequence Analysis of DNA from All Detected Cases of Chlamydia trachomatis Infections during 1 Year of Contact Tracing in a Swedish County. J. Clin. Microbiol. 2004, 42, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.T.; Brown, A.C.; Kundu, S.; Tutill, H.J.; Williams, R.; Brown, J.R.; Holdstock, J.; Holland, M.J.; Stevenson, S.; Dave, J.; et al. Whole-genome enrichment and sequencing of Chlamydia trachomatis directly from clinical samples. BMC Infect. Dis. 2014, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud; Caja de Seguro Social. Normativa Nacional para el Abordaje Integral de las Infecciones de Transmisión Sexual en Panamá. 2014. Available online: https://www.minsa.gob.pa/sites/default/files/programas/normas_its_panama.pdf (accessed on 8 January 2024).
- Ministerio de Salud Republica de Panamá Guías De Manejo De Las Infecciones Ginecológicas. 2021. Available online: https://www.minsa.gob.pa/sites/default/files/programas/guias_de_manejo_de_las_infecciones_ginecologicas_doc_impreso_para_distribuir.pdf (accessed on 8 January 2024).
- Gabster, A.; Mohammed, D.Y.; Arteaga, G.B.; Castillero, O.; Mojica, N.; Dyamond, J.; Varela, M.; Pascale, J.M. Correlates of sexually transmitted infections among adolescents attending public high schools, Panama, 2015. PLoS ONE 2016, 11, e0163391. [Google Scholar] [CrossRef]
- dos Santos, L.M.; dos Santos Vieira, M.R.M.; Vieira, R.C.; da Luz Silva, L.B.; de Macêdo, G.M.M.; Miranda, A.E.; Brasiliense, D.M.; de Paula Souza e Guimarães, R.J.; Sousa, E.C.; Ferrari, S.F.; et al. Prevalence and circulant genotypes of Chlamydia trachomatis in university women from cities in the Brazilian Amazon. PLoS ONE 2024, 19, e0287119. [Google Scholar] [CrossRef]
- Rajabpour, M.; Emamie, A.D.; Pourmand, M.R. Evaluation of Chlamydia trachomatis Genotypes in Endocervical Specimens by Sequence Analysis of ompA Gene among Women in Tehran. J. Trop. Med. 2023, 2023, 8845565. [Google Scholar] [CrossRef]
- Casillas-Vega, N.; Morfín-Otero, R.; García, S.; Llaca-Díaz, J.; Rodríguez-Noriega, E.; Camacho-Ortiz, A.; de la Merced Ayala-Castellanos, M.; Maldonado-Garza, H.J.; Ancer-Rodríguez, J.; Gallegos-Ávila, G.; et al. Frequency and genotypes of Chlamydia trachomatis in patients attending the obstetrics and gynecology clinics in Jalisco, Mexico and correlation with sociodemographic, behavioral, and biological factors. BMC Women’s Health 2017, 17, 83. [Google Scholar] [CrossRef]
- Misyurina, O.Y.; Chipitsyna, E.V.; Finashutina, Y.P.; Lazarev, V.N.; Akopian, T.A.; Savicheva, A.M.; Govorun, V.M. Mutations in a 23S rRNA Gene of Chlamydia trachomatis Associated with Resistance to Macrolides. Antimicrob. Agents Chemother. 2004, 48, 1347–1349. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.P.; Jiang, Y.; Hou, S.P.; Liu, Y.J.; Liu, Q.Z. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in Chlamydia trachomatis strains selected in vitro by macrolide passage. Andrologia 2010, 42, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Illumina, Inc. Nextera XT DNA Library Prep Reference Guide; Instruction Manual; 2019; pp. 1–22. Available online: https://support-docs.illumina.com/LP/NexteraXTRef/Content/LP/Nextera/XT/Protocol.htm (accessed on 10 September 2024).
- Illumina, Inc. Denature and Dilute Libraries Guide. Available online: https://support-docs.illumina.com/IN/MiSeq_DnD/Content/MiSeq/DnD-MiSeq.htm?protocol=standard (accessed on 10 September 2024).
- Taylor, T.; Lee, E.R.; Nykoluk, M.; Enns, E.; Liang, B.; Capina, R.; Gauthier, M.K.; Van Domselaar, G.; Sandstrom, P.; Brooks, J.; et al. A MiSeq-HyDRA platform for enhanced HIV drug resistance genotyping and surveillance. Sci. Rep. 2019, 9, 8970. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Bénard, A.; Domman, D.; Thomson, N. The Hidden Genomics of Chlamydia Trachomatis; Springer International Publisher: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Lima, H.E.; Oliveira, M.B.; Valente, B.G.; Afonso, D.A.F.; Darocha, W.D.; Souza, M.C.M.; Alvim, T.C.; Barbosa-Stancioli, E.F.; Noronha, F.S.M. Genotyping of Chlamydia trachomatis from endocervical specimens in Brazil. Sex. Transm. Dis. 2007, 34, 709–717. [Google Scholar] [CrossRef]
- Machado, A.C.S.; Bandea, C.I.; Alves, M.F.C.; Joseph, K.; Igietseme, J.; Miranda, A.E.; Guimarães, E.M.B.; Turchi, M.D.; Black, C.M. Distribution of Chlamydia trachomatis genovars among youths and adults in Brazil. J. Med. Microbiol. 2011, 60, 472–476. [Google Scholar] [CrossRef][Green Version]
- Lyu, H.; Tang, H.; Feng, Y.; Hu, S.; Wang, Y.; Zhou, L.; Huang, S.; Li, J.; Zhu, H.; He, X.; et al. Incidence and spontaneous clearance of gonorrhea and chlamydia infections among men who have sex with men: A prospective cohort study in Zhuhai, China. Front. Public Health 2024, 12, 1348686. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ovalle, A.; Camponovo, R.; Vidal, R. Chlamydia trachomatis genovars causing urogenital infections in Santiago, Chile. Infect. Dis. 2015, 47, 156–160. [Google Scholar] [CrossRef]
- Kiguen, A.X.; Marramá, M.; Ruiz, S.; Estofan, P.; Venezuela, R.F.; Mosmann, J.P.; Monetti, M.S.; Rivero, V.; Cuffini, C.G. Prevalence, risk factors and molecular characterization of Chlamydia trachomatis in pregnant women from Córdoba, Argentina: A prospective study. PLoS ONE 2019, 14, e0217245. [Google Scholar] [CrossRef]
- Millman, K.; Black, C.M.; Johnson, R.E.; Stamm, W.E.; Jones, R.B.; Hook, E.W.; Martin, D.H.; Bolan, G.; Tavaré, S.; Dean, D. Population-Based Genetic and Evolutionary Analysis of Chlamydia trachomatis Urogenital Strain Variation in the United States. J. Bacteriol. 2004, 186, 2457–2465. [Google Scholar] [CrossRef]
- Kese, D.; Potocnik, M.; Maticic, M.; Kogoj, R. Genotyping of Chlamydia trachomatis directly from urogenital and conjunctiva samples using an ompA gene pyrosequencing-based assay. FEMS Immunol. Med. Microbiol. 2011, 63, 210–216. [Google Scholar] [CrossRef] [PubMed]
- De Jesús De Haro-Cruz, M.; Deleón-Rodriguez, I.; Escobedo-Guerra, M.R.; López-Hurtado, M.; Arteaga-Troncoso, G.; Ortiz-Ibarra, F.J.; Guerra-Infante, F.M. Genotyping of Chlamydia trachomatis from endocervical specimens of infertile Mexican women. Enferm. Infecc. Microbiol. Clin. 2011, 29, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Djoumessi Gomseu, B.E.; Dadwal, R.; Tamokou, J.D.D.; Yadav, R.; Takougoum Marbou, W.J.; Kuiate, J.-R.; Sethi, S. Quinolines and Macrolides Resistance-Associated Mutations in Chlamydia trachomatis in Women Endocervical Samples in the West Region of Cameroon. Eur. J. Med. Health Sci. 2021, 3, 83–87. [Google Scholar] [CrossRef]
- Binet, R.; Maurelli, A.T. Frequency of Development and associated physiological cost of azithromycin resistance in Chlamydia psittaci 6BC and C. trachomatis L2. Antimicrob. Agents Chemother. 2007, 51, 4267–4275. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines; MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommendations and Reports; 2021; Volume 70, pp. 1–187. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8344968/ (accessed on 25 January 2024).
- Aaron, K.J.; Griner, S.; Footman, A.; Pol, B. Van Der Vaginal Swab vs Urine for Detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis: A Meta-Analysis. Ann. Fam. Med. 2023, 21, 172–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).