1. Introduction
Skin is the body’s largest organ that plays a crucial role in safeguarding internal organs and harboring colonizing microbiota. This normal flora prevents pathogenic bacteria from attaching to the healthy skin surface but, on the other hand, can become pathogenic if they gain entry into the body in the form of cuts or injuries [
1]. Wounds create a favorable environment for microbial colonization and infection due to their moist, warm, and nutritive nature. While the human body has defense mechanisms that prevent many harmless bacteria from causing disease, any breach in the skin, such as trauma or surgery, can lead to bacterial infections [
2]. The occurrence of pus within the wound site represents a classic sign of infection, along with systemic symptoms like fever, localized tenderness, and swelling [
3]. Patients with high-risk wounds, often associated with factors like old age or poor nutritional status, are more susceptible to pathogenic bacterial infiltration and infection [
4]. Bacterial infections in wounds can significantly impede the healing process by prolonging inflammation and producing virulence factors that promote bacterial growth and tissue destruction [
5].
Wounds can be classified into various types based on different criteria such as etiology, morphology, and healing process. Common types of wounds include diabetic foot ulcers, pressure sores, surgical site infections, and burns [
6,
7,
8]. Trauma, whether accidental or intentional, is the main cause of wounds. Hospital-acquired wounds, including surgical and device-related ones, are a distinct subgroup and pressure sores from immobility are another concern [
9]. Wound infection evolution is a complex process influenced by multiple factors. These factors include skin conditions, bacterial load and virulence, the nature of the surgical procedures, prior exposure to antibiotics, and the immune status of the affected individual [
10]. Infections in wounds can stem from a wide array of pathogens, including bacteria, fungi, protozoa, and viruses. Among the common bacterial culprits associated with wound infections are notorious species such as
Staphylococcus aureus,
Pseudomonas aeruginosa,
Escherichia coli,
Klebsiella pneumoniae,
Proteus species, as well as different
Enterococcus and
Streptococcus species [
11].
Annually, a significant number of individuals around the globe endure the pain and trauma of wounds, struggle with wounds that are slow to heal, or face the challenge of acute wounds that are further complicated due to infections. Significant progress has been made in infection control strategies, yet eradicating drug-resistant pathogens remains a challenge due to the overuse of antibiotics. These resistant strains contribute to higher morbidity and mortality, especially in wound infections. Healthcare settings are sources of these antibiotic-resistant pathogens like MRSA and VRE, leading to difficult-to-treat hospital-acquired wound infections [
12].
Wound infections are a significant concern in healthcare settings, including hospitals in Saudi Arabia. They pose a significant concern for healthcare practitioners due to the potential for escalating trauma to the patient, as well as the considerable impact they can have on both financial resources and the overall efficiency of the healthcare system [
13]. The prevalence of wound infections in the country is influenced by various factors such as the complexity of intensive care unit (ICU) environments, the increased number of patients with serious diseases, widespread gastrointestinal colonization, and extensive use of antimicrobial drugs [
14]. Effectively managing wound infections is essential not only for the well-being of the patient but also for the sustainability of healthcare services. Understanding the various causative agents responsible for wound infections plays a crucial role in guiding treatment decisions, implementing infection control protocols in healthcare facilities, and establishing effective antibiotic policies.
The high prevalence of multidrug-resistant bacteria accounts for a significant health issue in the region as inadequately managed wounds contribute significantly to heightened rates of patient suffering and prolonged hospitalization [
15]. This survey was conducted in central Riyadh at a tertiary care hospital to identify aerobic bacterial isolates from a group of patients with different types of wound infections. Furthermore, the study was designed to detect antimicrobial and MDR profiles of various bacterial isolates.
Consistent monitoring of evolving wound infection trends, including sensitivity and multidrug resistance profiles, is essential for effective treatment. Health providers can adapt therapies to combat antimicrobial resistance, improving patient care. This approach helps manage infections by adjusting treatments promptly and minimizing risks.
4. Discussion
This study evaluates the prevalence of different bacteria isolated from various types of wound infections among patients from a tertiary care hospital in central Riyadh. Out of 1186 specimens requested during the study period under pus and wound culture, 691 were positive for microbial growth with an isolation rate of 58.3%. This prevalence rate was similar to the findings of Alharbi, who reported a 56.1% isolation rate of bacterial growth from wound infections in Jeddah [
16].
The slight predominance of monomicrobial infection was evident in our study (53%) whereas 47% were polymicrobial infections. These findings were in line with those of Hassan et al., who reported 60% monomicrobial wound infection [
17]. Likewise, Alharbi also found a predominance of monomicrobial infections of 90.6% [
16]. The ratio of polymicrobial wound infections in this study is much higher as compared to those of Alharbi and Maharjan et al., who reported 9.4% and 2.5% of wound cultures yielding more than one type of bacteria, respectively [
16,
18]. This difference could be due to many factors, including wound characteristics and environmental conditions, that interact in complex ways to shape bacterial communities in wounds, impacting their composition, behavior, evolution, and response to treatment [
19].
Our survey revealed a slight predominance of Gram-positive bacteria (55.2%) over Gram-negative bacteria (44.8%). These findings are in contrast to other studies where Gram-negative predominance was reported [
16,
17,
19,
20]. Variations in participants’ demographic traits, reflecting diverse age, gender, and cultural backgrounds, could lead to these contrasting outcomes.
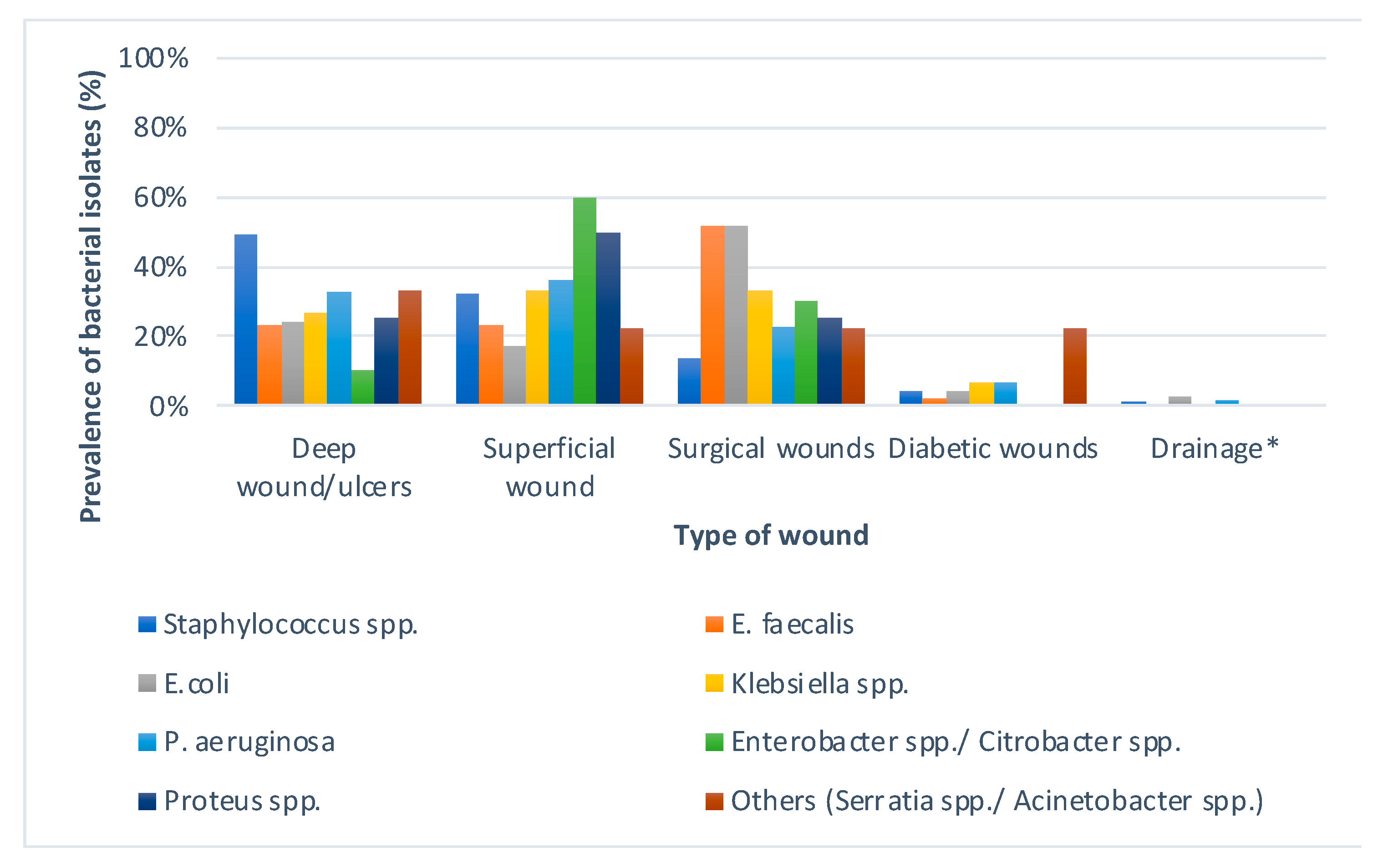
S. aureus was the most commonly isolated wound pathogen, followed by
E. coli and
P. aeruginosa. This was in parallel with the findings of El-Saed et al. [
21]. Another report by Puca et al. also supports our findings [
20]. Various studies confirm the predominance of
S. aureus in wound cultures, up to 65% reported by Mulu et al. [
22]. This predominance is not surprising as
S. aureus is normally present on human skin. Among Gram-negative isolates,
E. coli (13.6%) was the predominant isolate in our setting followed by
P. aeruginosa (12.9%). Various studies performed previously on wound infections reported
E. coli and
P. aeruginosa as the second most common cause of wound infection after
S. aureus [
16,
18,
22,
23].
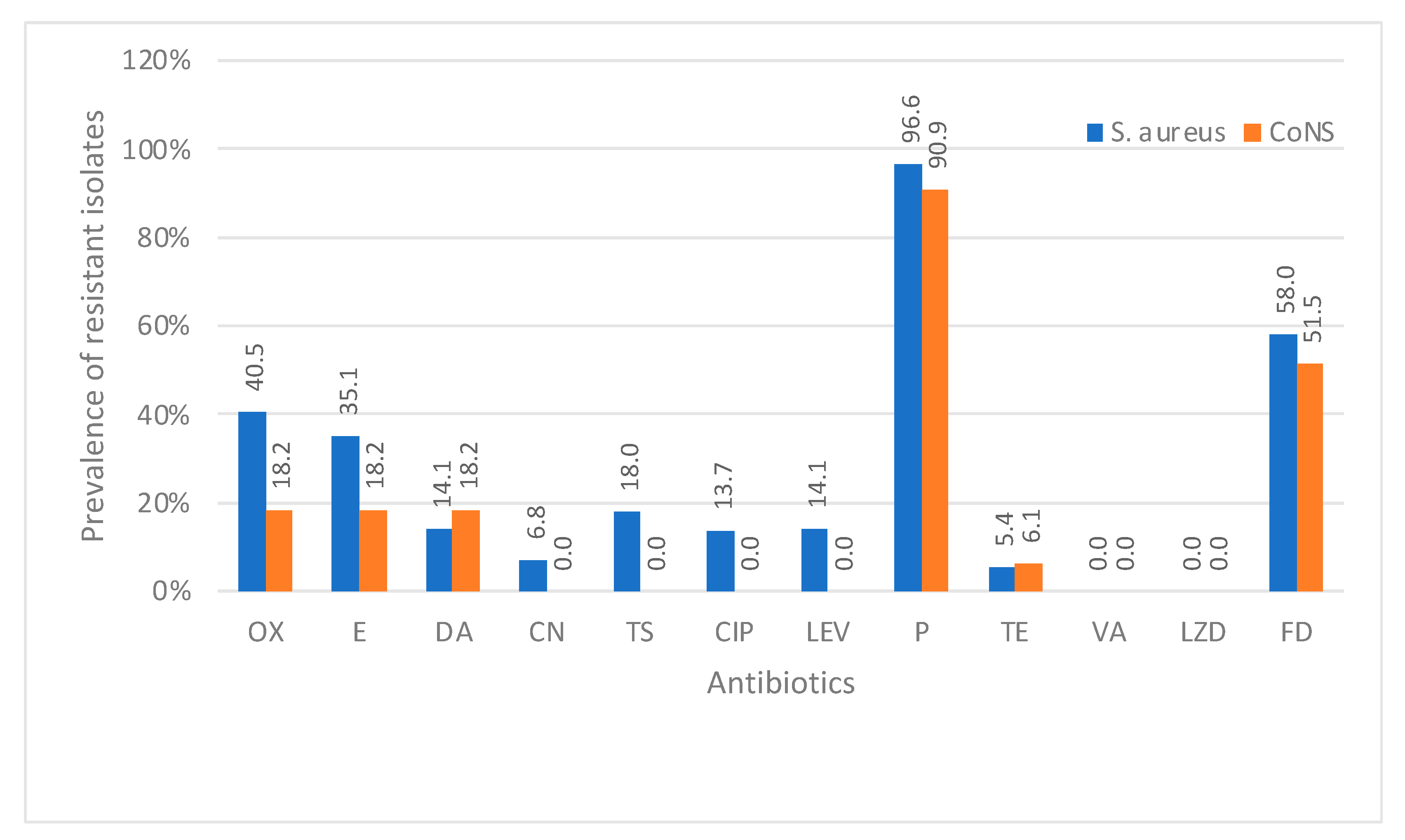
Staphylococci showed the highest resistance to penicillin with the resistance rate reaching up to 96.6%. Our results showed 40.5% MRSA strains among all
S. aureus isolates. The prevalence of MRSA in Saudi Arabia has been the subject of several studies. A review of the literature reveals varying prevalence rates across different regions and periods. El Amin et al. conducted a retrospective survey on 186
S. aureus isolates and reported almost the same rate (39.5%) of MRSA isolation [
24]. However, a remarkable increase in the prevalence of MRSA was revealed in our findings compared to a recent survey that was conducted by Alharbi et al. who reported a 29% resistance rate to oxacillin against
S. aureus [
16]. MRSA prevalence was reported to be 83% by Saba et al. [
25]. Reduced susceptibility to vancomycin has been reported in Saudi Arabia in previous studies by Al-Obeid et al. and Alzolibani et al. [
26,
27], although we found
S. aureus 100% susceptible to vancomycin and linezolid in our setting. However, vancomycin-resistant
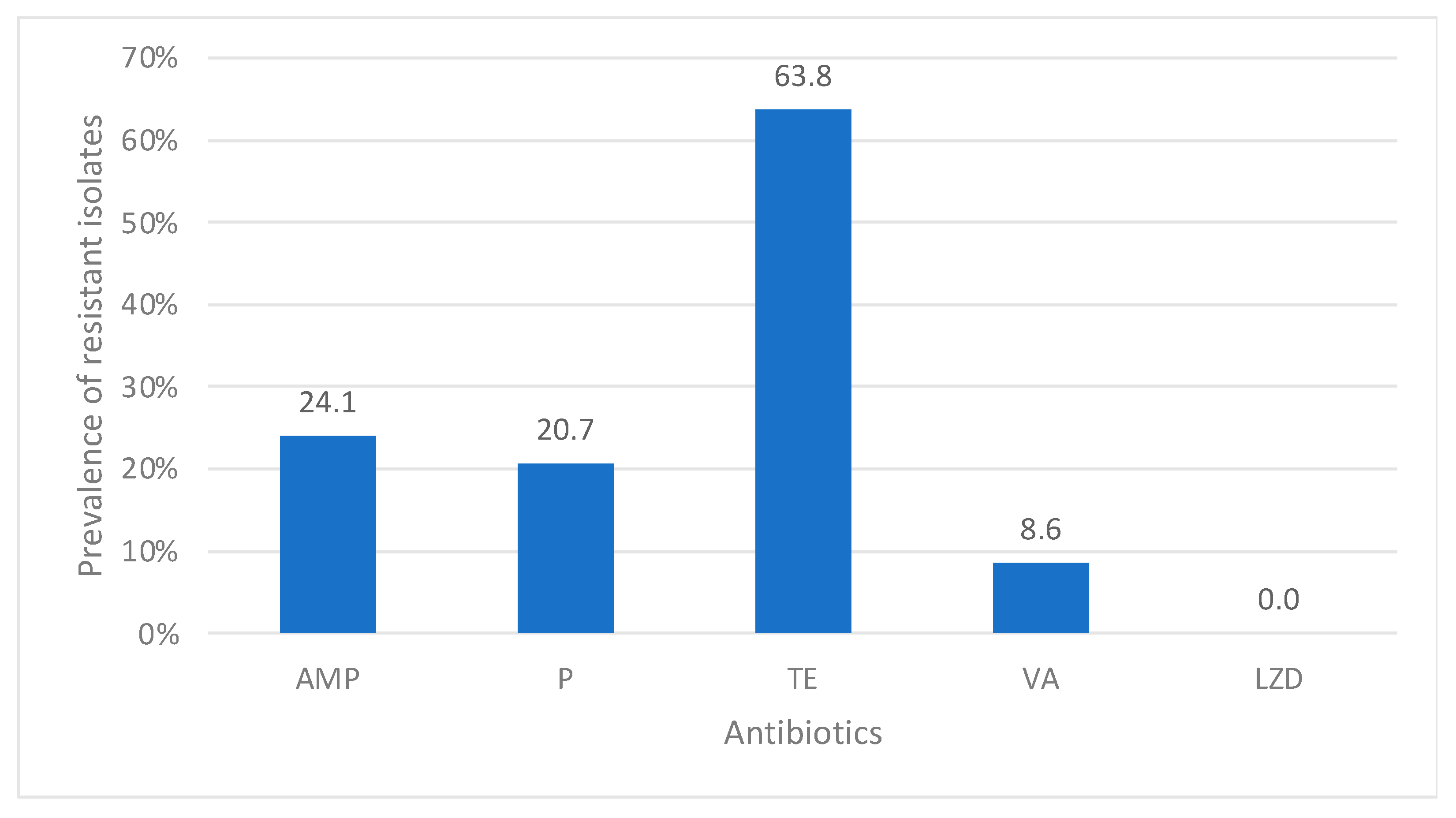
enterococci were observed at 8.6% in our study. This rate of resistance is also remarkably high in comparison to that of Somily et al., who reported vancomycin-resistant enterococci at 4.5% [
28]. These variations could be associated with the different patient settings and bacterial identification methods.
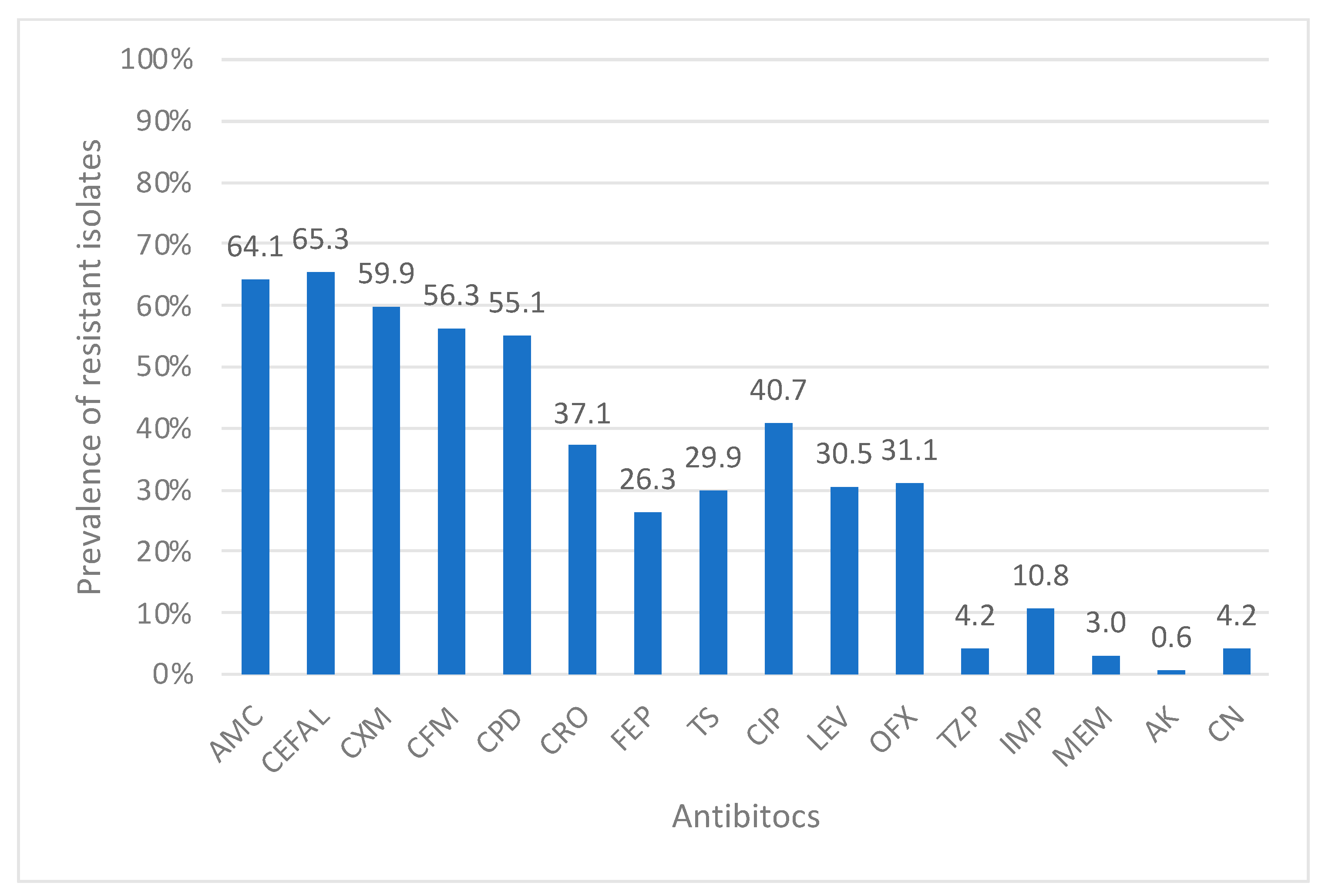
Regarding Enterobacteriaceae in Gram-negative sensitivity, amikacin was the most effective antibiotic tested with a resistance rate of 0.6%. High rates of resistance were noticed in all Enterobacteriaceae isolates against beta-lactam antibiotics except for carbapenems, imipenem (10.8%), and meropenem (3%). Resistance to meropenem was due to carbapenem-resistant
K. pneumoniae (3%). However,
Proteus spp. showed a resistance to imipenem but all these isolates were susceptible to meropenem. The rest of the Enterobacteriaceae members were 100% susceptible to these tested carbapenems in our survey. In the past decade, carbapenem-resistant Enterobacteriaceae (CRE), particularly carbapenem-resistant
K. pneumoniae with the New Delhi metallo-β-lactamase gene, has emerged as a global threat, spreading in regions like Asia, Europe, the UK, and the Arab countries [
29]. An abrupt increase in the prevalence of CRE was reported in a recent study from western Saudi Arabia from 8% in 2017 to 61% in 2019 [
30]. However, our low percentage could be due to the site of infection included in our study in comparison to the surveillance study for screening CRE only.
Likewise, amikacin was the most effective antibiotic against
P. aeruginosa as well. The highest resistance was noticed against aztreonam (15.9%), followed by quinolones (13–14.5%). Carbapenem-resistant
P. aeruginosa was found at 8.7% in our survey. Our results are not in agreement with those of Momenah et al., who reported a high resistance to cefepime (42.7%), followed by ciprofloxacin (34.3%), imipenem (30.5%), ceftazidime (29.2%), gentamicin (26%), and piperacillin/tazobactam (24.2%) [
31]. These variations in antibiotic susceptibility patterns stem from factors like number of isolates included in the study, screening duration, antibiotic use, selective pressure, and study conditions, reflecting diverse settings and environmental influences [
32].
Antimicrobial resistance is a significant global health concern affected by demographic changes, especially the increasing elderly population. Understanding the impact of demographic changes is crucial due to a lack of knowledge in this area. The relationship between age, gender, infections, and antibiotic resistance is complex, requiring a nuanced understanding to develop effective strategies against drug-resistant infections. In our study, the overall sample of male patients showed higher levels of antimicrobial resistance with statistically significant differences noticed in ampicillin, carbapenem, quinolones, and piperacillin/tazobactam. This male predominance for antimicrobial resistance was supported by Akhavizadegan et al. [
33]. Overall, antimicrobial resistance predominance was noticed in the age group of more than 60 years. Older patients with multiple comorbidities receive care in various healthcare settings and are more vulnerable to antibiotic-resistant pathogens, making careful antibiotic use crucial for effective infection management. The significance of age as a factor influencing the variation in antimicrobial resistance has not only been visually delineated for Europe in previous studies [
34] but has also been thoroughly investigated in studies focused on specific regions [
35,
36]. However, to our knowledge, this is the first report from the region highlighting the impact of gender and age on antimicrobial resistance from wound infections.
Our study highlights the urgent need for better infection control in healthcare settings due to high antibiotic resistance among inpatients, emphasizing monitoring, targeted interventions, and collaboration to combat resistance effectively. All five cases of vancomycin-resistant enterococci were inpatient. Two cases of colistin resistance were also isolated from inpatient samples. Carbapenem resistance was also significantly high among inpatients, indicating the overuse of these life-saving, last-resort drugs in our hospital setting. The prevalence of MDR Gram-negative bacteria was 51.9% in our study, which is supported by various other surveys [
37,
38]. However, our MDR prevalence is remarkably high as compared to that of Alharbi et al., who recorded a 22% isolation rate of MDR bacteria from wound infections [
16]. Variations in isolated pathogens, study population, medication access, treatment adherence, infection control, and antimicrobial use may contribute to these discrepancies.
As a retrospective study, in-depth details about patient profiles were not available, which was one of our study limitations. Missing data on pathologies (missing wound site, etc.) and limited numbers of bacterial isolates in this single-center study were other constraints. We could not retrieve data for anaerobic bacterial isolates and streptococci were also excluded from our survey as we were unable to have their antimicrobial susceptibility pattern recorded by the laboratory.
In conclusion, analysis of wound samples showed prevalent monomicrobial infections, with S. aureus, E. coli, and P. aeruginosa as the main pathogens. High MRSA incidence among S. aureus emphasized antibiotic resistance challenges. Vancomycin and linezolid were effective against S. aureus, while amikacin worked best against Gram-negative isolates. Many isolates were multidrug-resistant, escalating antibiotic resistance concerns and hindering treatment options. Urgent action is needed to develop new treatments and improve antimicrobial practices in healthcare settings. This knowledge enables local healthcare professionals to make informed choices regarding empirical therapy, enhances the effectiveness of infection prevention strategies within medical settings, and contributes to the development of rational approaches toward antibiotic usage. By staying abreast of the causative factors behind wound infections, healthcare providers can optimize patient care, minimize the risks associated with infections, and work towards achieving cost-effective and sustainable healthcare management strategies in our setting.