Antimicrobial and Antioxidant Activities of 18β-Glycyrrhetinic Acid Biotransformed by Aspergillus niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Source of Plant
2.3. Microorganisms
2.4. Crude Glycyrrhizin Extraction
2.5. Preparation of the Fermentation Culture for the Biotransformation of Glycyrrhizin to Glycyrrhetinic Acid by Aspergilus niger
2.6. Multifactorial Experiments for Optimizing the Biotransformation of Glycyrrhizin to Glycyrrhetinic Acid
2.6.1. Screening of Factors Affecting the Biotransformation of Glycyrrhizin to Glycyrrhetinic Acid Using a Plackett–Burman Design
2.6.2. Optimization of Significant Variables Using a Box–Behnken Design
2.7. Extraction of of Glycyrrhizin (GL) and Glycyrrhetinic Acid (GA)
2.8. Determination of Glycyrrhizin (GL) and Glycyrrhetinic Acid (GA)
2.9. Antimicrobial Activity of the Biotransformed Glycyrrhetinic Acid
2.9.1. Paper Disc Diffusion Assay
2.9.2. Determination of Minimal Inhibitory Concentration
2.10. Antioxidant Activity of the Biotransformed Glycyrrhetinic Acid
2.11. Statistical Analysis
3. Results and Discussion
3.1. Multifactorial Designs for Optimizing the Biotransformation of Glycyrrhizin into Glycyrrhetinic Acid
3.1.1. Screening of Factors Affecting Biotransformation of Glycyrrhizin to Glycyrrhetinic Acid Using a Plackett–Burman Design
8.69 (GL%—0.75/0.25) + 13.084 (incubation time—5) + 0.477 (glucose%—2) + −3.71 (yeast
extract %—0.3/0.1) + −2.93 (CSL%—0.75/0.25).
| pH | Temperature | Glycyrrhizin Conc. (g/100 mL) | Incubation Time (days) | Glucose Conc. (g/100 mL) | Yeast Extract Conc. (g/100 mL) | CSL Conc. (g/100 mL) | Glycyrrhetinic Acid (mg/g) | |
|---|---|---|---|---|---|---|---|---|
| −−+−−+− | 5 | 25 | 1 | 4 | 1 | 0.4 | 0.5 | 28.98 |
| −+−+++− | 5 | 30 | 0.5 | 6 | 3 | 0.4 | 0.5 | 23.1 |
| −+++−−− | 5 | 30 | 1 | 6 | 1 | 0.2 | 0.5 | 48.31 |
| +−−+−++ | 7 | 25 | 0.5 | 6 | 1 | 0.4 | 1 | 50.17 |
| +−−−+−− | 7 | 25 | 0.5 | 4 | 3 | 0.2 | 0.5 | 31.77 |
| −+−−+−+ | 5 | 30 | 0.5 | 4 | 3 | 0.2 | 1 | 9.3 |
| −−−+−−+ | 5 | 25 | 0.5 | 6 | 1 | 0.2 | 1 | 28.34 |
| −−+−+++ | 5 | 25 | 1 | 4 | 3 | 0.4 | 1 | 10.11 |
| +++−−−+ | 7 | 30 | 1 | 4 | 1 | 0.2 | 1 | 36.21 |
| +++++++ | 7 | 30 | 1 | 6 | 3 | 0.4 | 1 | 60.21 |
| +−+++−− | 7 | 25 | 1 | 6 | 3 | 0.2 | 0.5 | 80.3 |
| ++−−−+− | 7 | 30 | 0.5 | 4 | 1 | 0.4 | 0.5 | 17.0.5 |
3.1.2. Optimization of Significant Variables Using a Box–Behnken Design
(pH) + −31.268 (GL%) + 2.42(pH) (incubation time) + 1.55 (GL%) (incubation time) +
0.115 (GL%) (pH) + 0.755 (incubation time) (incubation time) + −4.45 (pH) (pH) + 3.67 (GL%) (GL%)
3.1.3. Localization of the Optimum Condition
3.1.4. Model Validation
3.2. Antimicrobial Activity of Glycyrrhizin and the Biotransformed Glycyrrhetinic Acid
3.3. Antioxidant Activity of Glycyrrhizin and the Biotransformed Glycyrrhetinic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdallah, E.M.; Alhatlani, B.Y.; Menezes, R.d.P.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- AlDehlawi, H.; Jazzar, A. The Power of Licorice (Radix glycyrrhizae) to Improve Oral Health: A Comprehensive Review of Its Pharmacological Properties and Clinical Implications. Healthcare 2023, 11, 2887. [Google Scholar] [CrossRef]
- Tuli, H.S.; Garg, V.K.; Mehta, J.K.; Kaur, G.; Mohapatra, R.K.; Dhama, K.; Sak, K.; Kumar, A.; Varol, M.; Aggarwal, D.; et al. Licorice (Glycyrrhiza glabra L.)-Derived Phytochemicals Target Multiple Signaling Pathways to Confer Oncopreventive and Oncotherapeutic Effects. OncoTargets Ther. 2022, 15, 1419–1448. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Shinu, P.; Gupta, G.L.; Sharma, M.; Khan, S.; Goyal, M.; Nair, A.B.; Kumar, M.; Soliman, W.E.; Rahman, A.; Attimarad, M.; et al. Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential. Plants 2023, 12, 1086. [Google Scholar] [CrossRef]
- Sabbioni, C.; Mandrioli, R.; Ferranti, A.; Bugamelli, F.; Saracino, M.A.; Forti, G.C.; Fanali, S.; Raggi, M.A. Separation and analysis of glycyrrhizin, 18beta-glycyrrhetic acid and 18alpha-glycyrrhetic acid in liquorice roots by means of capillary zone electrophoresis. J. Chromatogr. A 2005, 15, 65–71. [Google Scholar] [CrossRef]
- Chamoli, A.; Hasan, M.; Ahmad, M.; Rashid, M.; Ali, B.; Panda, B.P. Microbial Screening for Biotransformation of Glycyrrhizin into 18α-Glycyrrhetinic Acid and 18β-Glycyrrhetinic Acid and their HPLC Analysis. Acta Sci. Microbiol. 2019, 2, 62–66. [Google Scholar] [CrossRef]
- Feng, S.; Chun Li, C.; Xu, X.; Wang, X. Screening strains for directed biosynthesis of β-D-mono-glucuronide-glycyrrhizin and kinetics of enzyme production. J. Mol. Catal. B Enzym. 2022, 43, 63–67. [Google Scholar] [CrossRef]
- Chen, L.; Gong, J.; Yong, X.; Li, Y.; Wang, S. A review of typical biological activities of glycyrrhetinic acid and its derivatives. RSC Adv. 2024, 14, 6557–6597. [Google Scholar] [CrossRef]
- Melekoglu, R.; Cifitci, O.; Eraslan, S.; Alan, S.; Basak, N. The Protective Effects of Glycyrrhetinic Acid and Chrysin against Ischemia-Reperfusion Injury in Rat Ovaries. BioMed. Res. Int. 2018, 2018, 5421308. [Google Scholar]
- Lu, D.; Hui, L.; Yan, D.; Ping-Kai, O. Biocatalytic properties of a novel crude glycyrrhizin hydrolase from the liver of the domestic duck. J. Mol. Catal. B Enzym. 2006, 43, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, C.; Liu, J.; Yu, F.; Gao, X.P. Pressured microwave-assisted hydrolysis of crude glycyrrhizic acid for preparation of glycyrrhetinic acid. Chin. J. Chem. Eng. 2012, 20, 152–157. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, X.; Jia, J.; Chen, X.; Jiang, T.; Rasool, A.; Lv, B.; Qu, L.; Li, C. A novel βglucuronidase from Talaromyces pinophilus Li-93 precisely hydrolyzes glycyrrhizin into glycyrrhetinic acid 3-O-mono-β-D-glucuronide. Appl. Environ. Microbiol. 2018, 84, e00755-18. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, B.; Xiao, Y.; Yang, H.; Wang, Y.; Du, L.; Zhu, D. Purification and characterization of a novel β-glucuronidase precisely converts glycyrrhizin to glycyrrhetinic acid 3-O-mono-β-D-glucuronide from plant endophytic Chaetomium globosum DX-THS3. Int. J. Biol. Macromol. 2020, 159, 782–792. [Google Scholar] [CrossRef]
- Chellapandi, P.; Jani, H.M. Production of endoglucanase by the native strains of Streptomyces isolates in submerged fermentation. Braz. J. Microbiol. 2008, 39, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Siracusa, L.; Saija, A.; Cristani, M.; Climino, F.; Arrigo, M.D.; Trombetta, D.; Rao, F.; Ruberto, G. Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves–chemical characterization and evaluation of their antioxidant, anti-genotoxic and anti-inflammatory activity. Fitoterapia 2011, 82, 546–555. [Google Scholar] [CrossRef]
- Nocca, G.; Callà, C.; Santini, S.A.; Amalfitano, A.; Marigo, L.; Rossetti, D.V.; Spagnuolo, G.; Cordaro, M. Quantitative determination of 18-β-glycyrrhetinic Acid in HepG2 cell line by High Performance Liquid Chromatography method. Int. J. Anal. Chem. 2018, 2018, 5673186. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Kroh, L.W.; Moersel, J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Wang, L.; Liu, Y.; Cong, J.; Xie, S.; Wu, X. Optimization of Fermentation Medium for Glycyrrhizin Biotransformation to Monoglucuronyl-glycyrrhetinic Acid by Plackett-Burman and Box-Behnken Design. Korean Chem. Eng. Res. 2015, 53, 321–326. [Google Scholar] [CrossRef]
- Ahmad, M.; Jalaluddin, M.; Panda, B.P. Bioconversion of glycyrrhizin of Glycyrrhiza glabra root extract to 18β-glycyrrhetinic acid. Drug Devel Ther. 2016, 7, 683–688. [Google Scholar]
- Ahmad, M.; Jalaluddin, M.; Pand, B.P. Enrichment of biologically active 18-β glycyrrhetinic acid in Glycyrrhiza glabra root by solid state fermentation. Ann Microbiol. 2014, 64, 683–688. [Google Scholar] [CrossRef]
- Mahdavi, V.; Monajem, A. Statistical Optimization for Oxidation of Ethyl Benzene over Co- Mn/SBA-15 catalyst by Box-Behnken Design. Korean J. Chem. Eng. 2013, 33, 2178–2185. [Google Scholar] [CrossRef]
- Chakotiya, A.S.; Tanwar, A.; Narula, A.; Sharma, R.K. Alternative to antibiotics against Pseudomonas aeruginosa: Effects of Glycyrrhiza glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time-kill activity. Microb. Pathog. 2016, 98, 98–105. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Ekanayaka, S.A.; McClellan, S.A.; Francis, R. Glycyrrhizin Use for Multi-Drug Resistant Pseudomonas aeruginosa: In Vitro and In Vivo Studies. Investig. Opthalmology Vis. Sci. 2019, 60, 2978–2989. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18b-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, Y.; Kim, H.N.; Choi, B.H.; Jeong, H.G.; Lee, D.G.; Hahm, K.S. Antimicrobial mechanism of β-glycyrrhetinic acid isolated from licorice, Glycyrrhiza glabra Hyung Keun. Biotechnol. Lett. 2002, 24, 1899–1902. [Google Scholar] [CrossRef]
- Long, D.R.; Mead, J.; Hendricks, J.M.; Hardy, M.E.; Voyich, J.M. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob. Agents Chemother. 2013, 57, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Kawada-Matsuo, M.; Oogai, Y.; Hayashi, T.; Nakamura, N.; Komatsuzawa, H. Antibacterial Effects of Glycyrrhetinic Acid and Its Derivatives on Staphylococcus aureus. PLoS ONE 2016, 11, e0165831. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Catteau, L.; Boukricha, L.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes. Antibiotics 2021, 10, 1381. [Google Scholar] [CrossRef]
- Weaver, A.J., Jr.; Borgogna, T.R.; O’Shea-Stone, G.; Peters, T.R.; Copié, V.; Voyich, J.; Teintze, M. 18β-Glycyrrhetinic Acid Induces Metabolic Changes and Reduces Staphylococcus aureus Bacterial Cell-to-Cell Interactions. Antibiotics 2022, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Imai, P.; Takagi, Y.; Iwazaki, A.; Nakanishi, N. Radical scavenging ability of glycyrrhizin. Free Radic. Antioxid. 2013, 3, 40–42. [Google Scholar] [CrossRef]
- Ageeva, A.A.; Kruppa, A.I.; Magin, I.M.; Babenko, S.V.; Leshina, T.V.; Polyakov, N.E. New Aspects of the Antioxidant Activity of Glycyrrhizin Revealed by the CIDNP Technique. Antioxidants 2022, 11, 1591. [Google Scholar] [CrossRef]
- Polyakova, N.E.; Leshinaa, T.V. Physicochemical Approaches to the Study of the Antioxidant Activity of Glycyrrhizin. Russ. J. Phys. Chem. A 2023, 97, 828–835. [Google Scholar] [CrossRef]
- Lefaki, M.; Papaevgeniou, N.; Tur, J.A.; Vorgias, C.E.; Sykiotis, G.P.; Chondrogianni, N. The dietary triterpenoid 18α-Glycyrrhetinic acid protects from MMC-induced genotoxicity through the ERK/Nrf2 pathway. Redox Biol. 2020, 28, 101317. [Google Scholar] [CrossRef]
- Kong, S.Z.; Chen, H.M.; Yu, X.T.; Zhang, X.; Feng, X.X.; Kang, X.H.; Li, W.-J.; Huang, N.; Luo, H.; Su, Z.-R. The protective effect of 18b-Glycyrrhetinic acid against UV irradiation induced photoaging in mice. Exp. Gerontol. 2015, 61, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhou, A.G.; Zhang, L.; Chen, W.J. Antioxidant Status and Immune Activity of Glycyrrhizin in Allergic Rhinitis Mice. Int. J. Mol. Sci. 2011, 12, 905–916. [Google Scholar] [CrossRef] [PubMed]
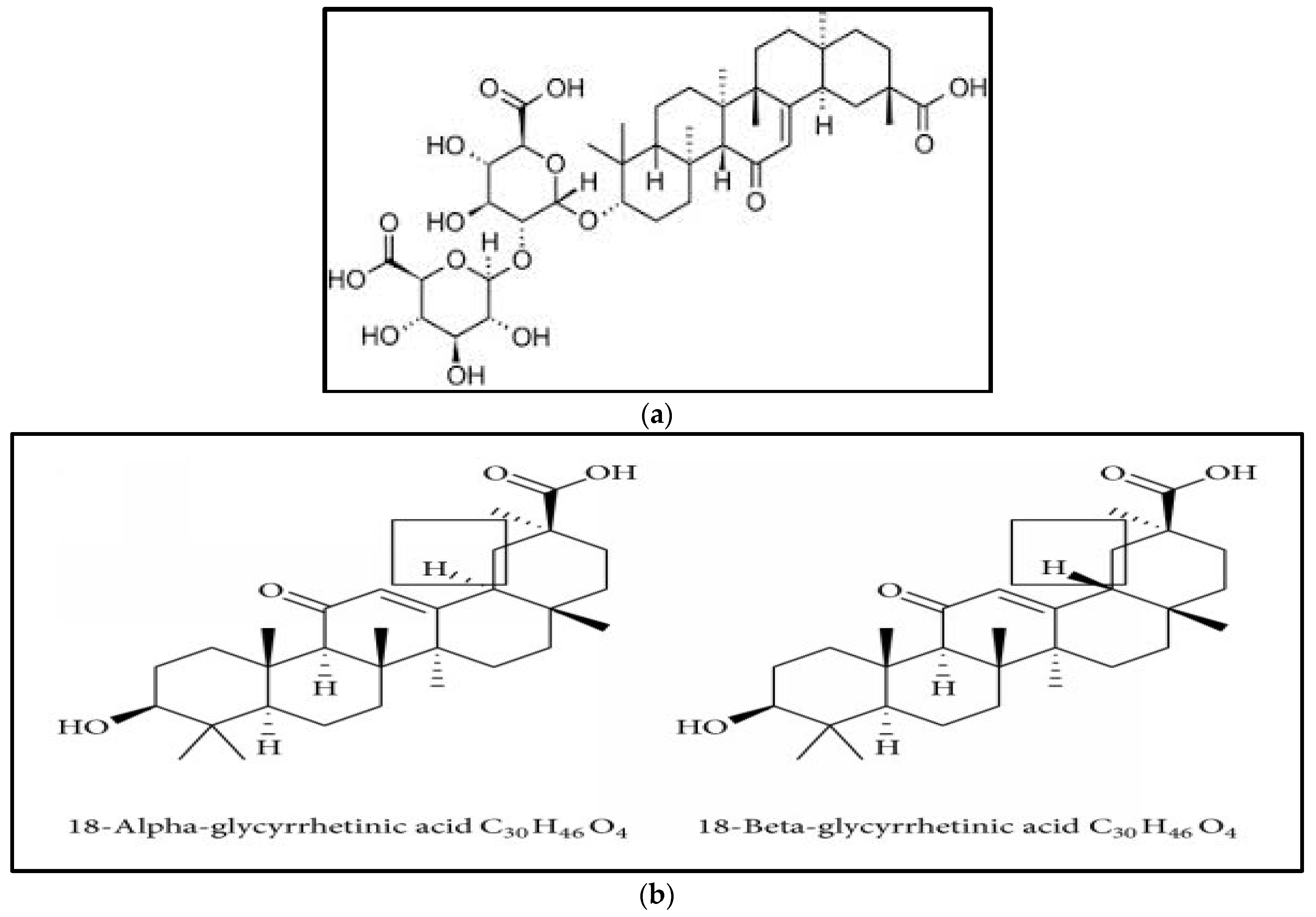
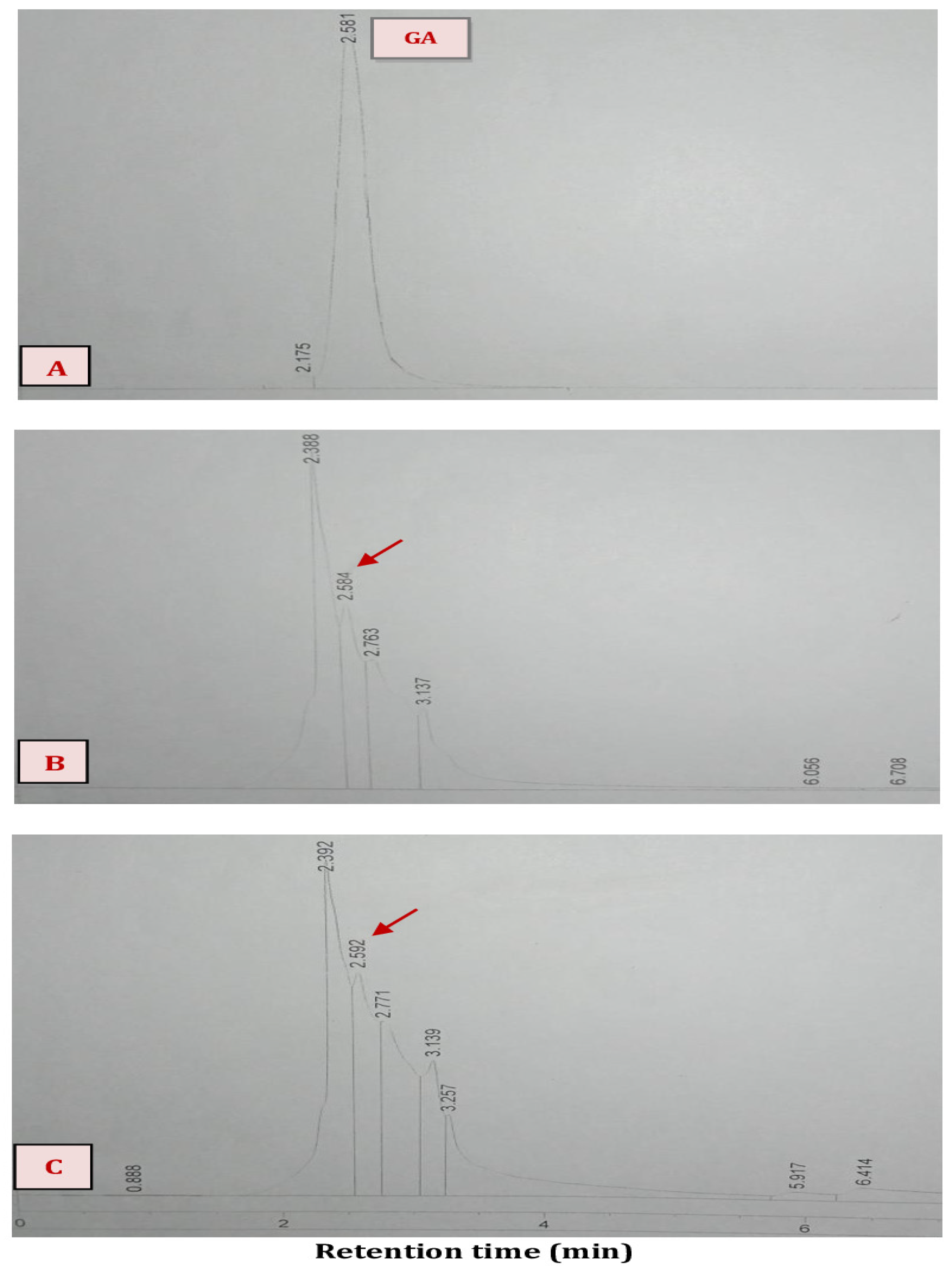
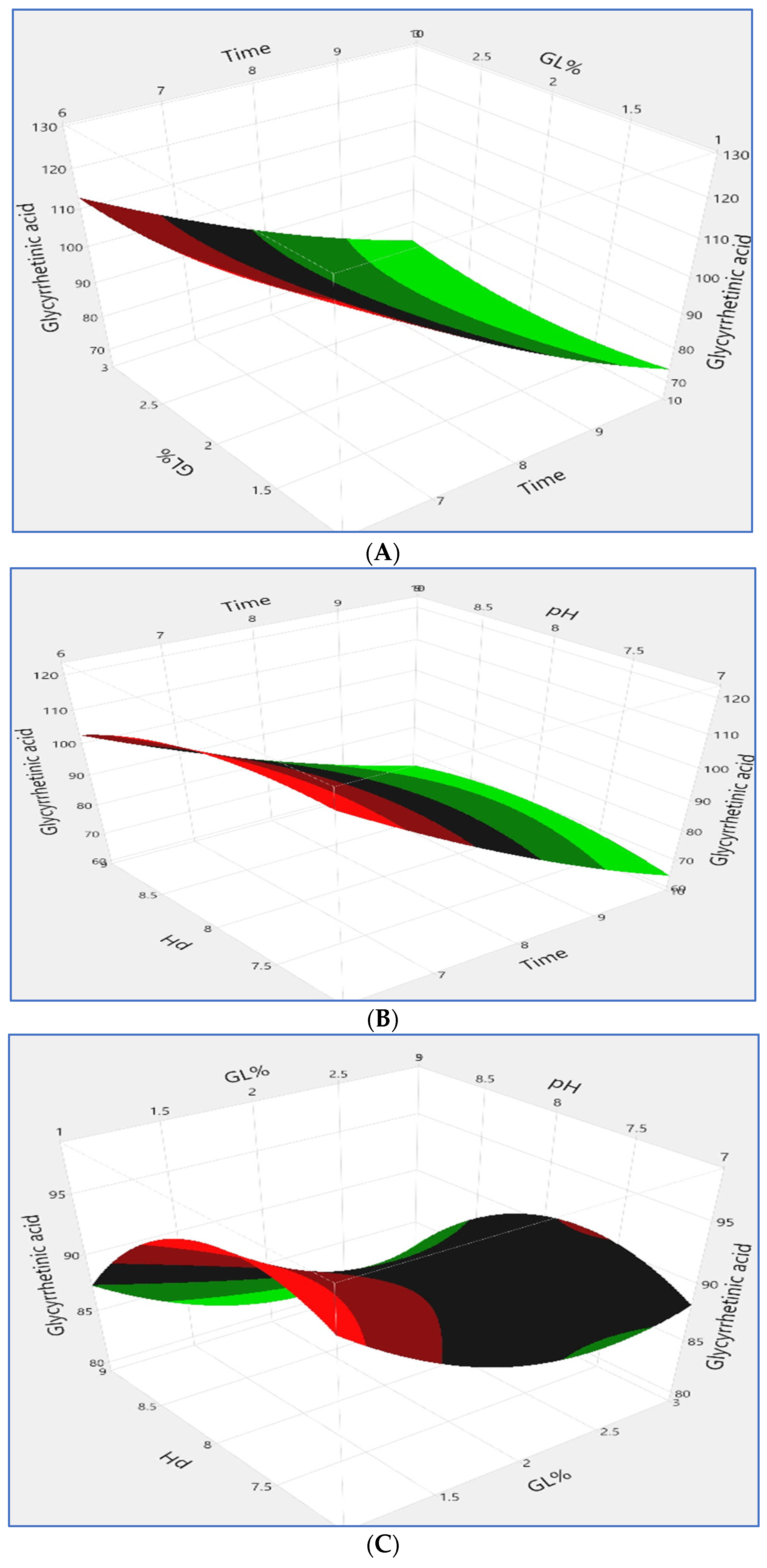
| Factor | Low Level (−1) | High Level (+1) |
|---|---|---|
| pH | 5 | 7 |
| Temperature (°C) | 25 | 30 |
| Glycyrrhizin (g/100 mL) | 0.5 | 1 |
| Incubation time (day) | 4 | 6 |
| Glucose (g/100 mL) | 1 | 3 |
| Yeast extract (g/100 mL) | 0.2 | 0.4 |
| CSL (g/100 mL) | 0.5 | 1 |
| Parameter Estimates | |||||
|---|---|---|---|---|---|
| Term | Estimate | Std Error | t Ratio | Prob > |t| | Uncoded Estimate |
| Intercept | 35.320833 | 2.211486 | 15.97 | <0.0001 * | −68.46 |
| pH (5, 7) | 10.630833 | 2.211486 | 4.81 | 0.0086 * | 10.630833 |
| Temperature (25, 30) | −2.9575 | 2.211486 | −1.34 | 0.2521 | −1.183 |
| GL% (0.5, 1) | 8.6991667 | 2.211486 | 3.93 | 0.0171 * | 34.796667 |
| Incubation time (4, 6) | 13.084167 | 2.211486 | 5.92 | 0.0041 * | 13.084167 |
| Glucose% (1, 3) | 0.4775 | 2.211486 | 0.22 | 0.8396 | 0.4775 |
| Yeast extract% (0.2, 0.4) | −3.7175 | 2.211486 | −1.68 | 0.1681 | −37.175 |
| CSL% (0.5, 1) | −2.930833 | 2.211486 | −1.33 | 0.2557 | −11.72333 |
| Pattern | Incubation Time | pH | GL% | Glycyrrhetinic Acid mg/g | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| ++0 | 10 | 9 | 2 | 66.39 | 66.67 |
| −0+ | 6 | 8 | 3 | 111.41 | 112.91 |
| 000 | 8 | 8 | 2 | 83.2 | 89.66 |
| 0++ | 8 | 9 | 3 | 85.19 | 82.25 |
| 0−− | 8 | 7 | 1 | 92.81 | 95.74 |
| 0−+ | 8 | 7 | 3 | 90.22 | 88.99 |
| +0+ | 10 | 8 | 3 | 70.67 | 73.32 |
| −0− | 6 | 8 | 1 | 128.25 | 125.59 |
| 0+− | 8 | 9 | 1 | 87.23 | 88.45 |
| 000 | 8 | 8 | 2 | 97.23 | 89.66 |
| +−0 | 10 | 7 | 2 | 65.44 | 64 |
| +0− | 10 | 8 | 1 | 75.11 | 73.6 |
| −−0 | 6 | 7 | 2 | 119.76 | 119.47 |
| 000 | 8 | 8 | 2 | 88.56 | 89.66 |
| −+0 | 6 | 9 | 2 | 101.34 | 102.77 |
| Parameter Estimates | ||||
|---|---|---|---|---|
| Term | Estimate | Std Error | t Ratio | Prob > |t| |
| Intercept | 175.39333 | 211.2406 | 0.83 | 0.4442 |
| Incubation time | −46.00521 | 15.70847 | −2.93 | 0.0327 * |
| pH | 48.147917 | 46.18208 | 1.04 | 0.3449 |
| Glycyrrhizin conc. | −31.26833 | 26.46856 | −1.18 | 0.2906 |
| Incubation time × pH | 2.42125 | 1.338002 | 1.81 | 0.1301 |
| Incubation time × glycyrrhizin conc. | 1.55 | 1.338002 | 1.16 | 0.2990 |
| pH × Glycyrrhizin conc. | 0.115 | 2.676004 | 0.04 | 0.9674 |
| Incubation time × incubation time | 0.7555208 | 0.696318 | 1.09 | 0.3274 |
| pH × pH | −4.452917 | 2.785274 | −1.60 | 0.1708 |
| Glycyrrhizin conc. × glycyrrhizin conc. | 3.6745833 | 2.785274 | 1.32 | 0.2443 |
| Bacterial Strain | Crude Glycyrrhizin | Bioconverted Glycyrrhetinic Acid | ||
|---|---|---|---|---|
| ZOI (mm) * | MIC (µg/mL) ** | ZOI (mm) | MIC (µg/mL) | |
| Bacillus subtilis | 17 ± 1.1 a | 60 | 32 ± 1.7 a | 20 |
| Escherichia coli | 12 ± 0.7 b | 200 | 22 ± 1.2 c | 80 |
| Pseudomonas aeruginosa | 10 ± 0.4 c | 400 | 18 ± 1.3 d | 140 |
| Salmonella typhimurium | 12 ± 0.8 b | 220 | 21 ± 1.1 c | 100 |
| Staphylococcus aureus | 15 ± 0.9 a | 100 | 29 ± 1.4 b | 40 |
| Radical Scavenging Activity * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Crude Glycyrrhizin | Biotransformed Glycyrrhetinic Acid | |||||||
| Incubation Time | Incubation Time | |||||||
| Conc. ** | 30 min | 60 min | 90 min | Conc. Mean ± SE | 30 min | 60 min | 90 min | Conc. Mean ± SE |
| 1000 | 45.92 ± 0.05 | 41.77 ± 0.05 | 35.73 ± 0.06 | 41.14 ± 4.18 | 80.58 ± 0.06 | 77.64 ± 0.05 | 72.33 ± 0.07 | 76.85 ± 3.41 |
| 500 | 36.71 ± 0.05 | 34.67 ± 0.05 | 30.61 ± 0.05 | 34.00 ± 2.53 | 71.84 ± 0.06 | 68.93 ± 0.05 | 62.59 ± 0.05 | 67.79 ± 3.86 |
| 250 | 29.59 ± 0.04 | 25.51 ± 0.05 | 21.44 ± 0.05 | 25.51 ± 3.33 | 58.74 ± 0.05 | 55.02 ± 0.04 | 49.00 ± 0.05 | 54.25 ± 4.01 |
| 125 | 22.49 ± 0.05 | 20.43 ± 0.06 | 16.37 ± 0.04 | 19.76 ± 2.50 | 49.51 ± 0.05 | 46.09 ± 0.04 | 41.03 ± 0.05 | 45.54 ± 3.48 |
| 63 | 15.97 ± 0.05 | 12.81 ± 0.04 | 09.66 ± 0.04 | 12.81 ± 2.58 | 39.32 ± 0.04 | 36.86 ± 0.03 | 30.33 ± 0.04 | 35.50 ± 3.80 |
| Incubation time mean ± SE | 30.14 ± 10.50 | 27.04 ± 10.23 | 22.76 ± 9.42 | 60.00 ± 14.85 | 56.91 ± 14.81 | 51.06 ± 14.97 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Far, S.W.; Al-Saman, M.A.; Abou-Elazm, F.I.; Ibrahim Shebl, R.; Abdella, A. Antimicrobial and Antioxidant Activities of 18β-Glycyrrhetinic Acid Biotransformed by Aspergillus niger. Microbiol. Res. 2024, 15, 1993-2006. https://doi.org/10.3390/microbiolres15040133
El-Far SW, Al-Saman MA, Abou-Elazm FI, Ibrahim Shebl R, Abdella A. Antimicrobial and Antioxidant Activities of 18β-Glycyrrhetinic Acid Biotransformed by Aspergillus niger. Microbiology Research. 2024; 15(4):1993-2006. https://doi.org/10.3390/microbiolres15040133
Chicago/Turabian StyleEl-Far, Shaymaa Wagdy, Mahmoud A. Al-Saman, Fatma I. Abou-Elazm, Rania Ibrahim Shebl, and Asmaa Abdella. 2024. "Antimicrobial and Antioxidant Activities of 18β-Glycyrrhetinic Acid Biotransformed by Aspergillus niger" Microbiology Research 15, no. 4: 1993-2006. https://doi.org/10.3390/microbiolres15040133
APA StyleEl-Far, S. W., Al-Saman, M. A., Abou-Elazm, F. I., Ibrahim Shebl, R., & Abdella, A. (2024). Antimicrobial and Antioxidant Activities of 18β-Glycyrrhetinic Acid Biotransformed by Aspergillus niger. Microbiology Research, 15(4), 1993-2006. https://doi.org/10.3390/microbiolres15040133






