Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic
Abstract
1. Introduction
2. SARS-CoV-2: Structure and Replication
3. Polysaccharides in Biological Diversity: Structure, Sources, and Potential Applications against SARS-CoV-2
3.1. Overview of Polysaccharides: Structure, Sources and Classification
3.2. Polysaccharides as Guardians: Exploring Their Potential against SARS-CoV-2
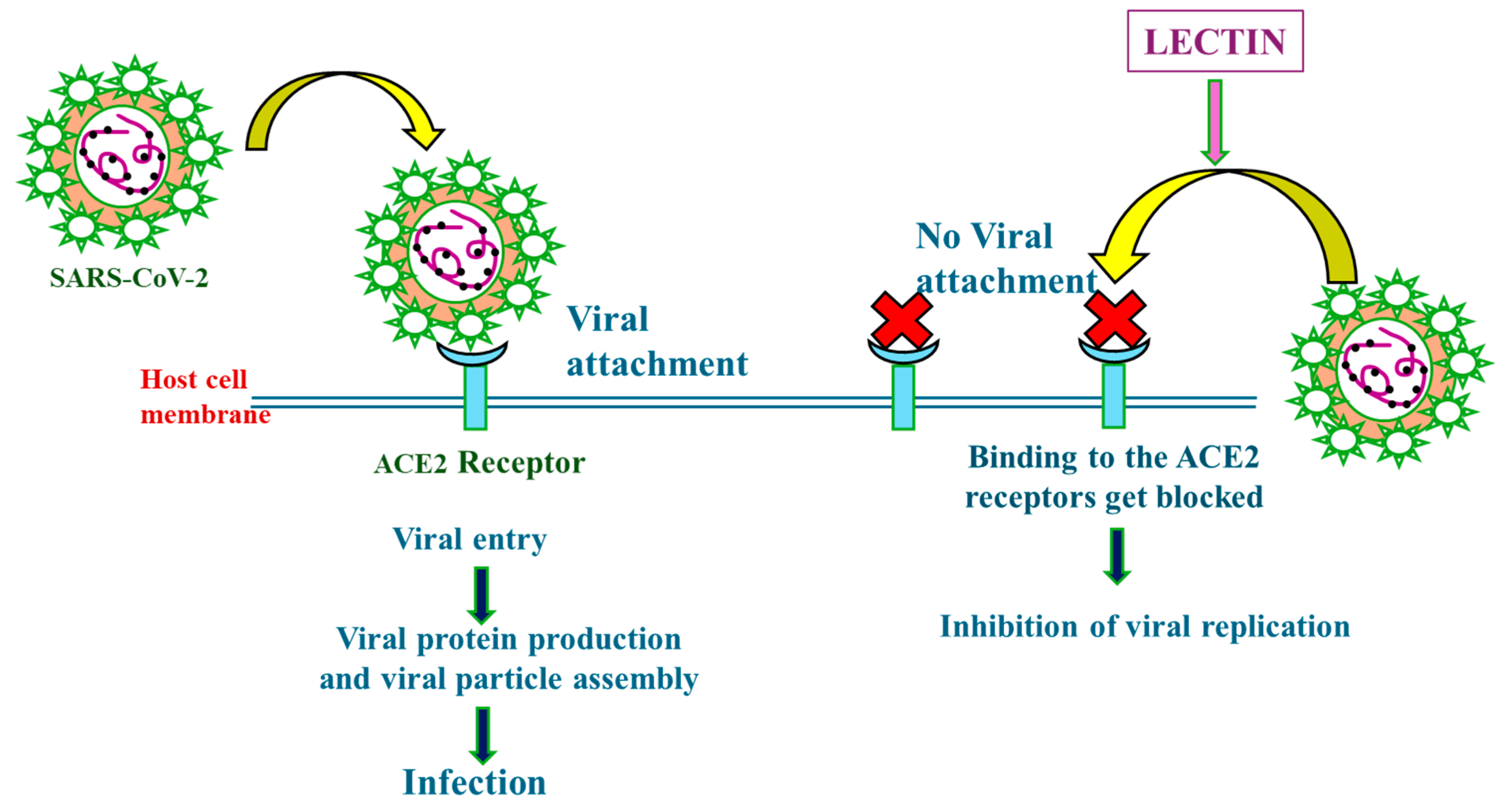
4. Lectin and SARS-CoV-2 Virus
4.1. Lectins: Molecular Recognition and Functional Diversity
4.2. Mechanisms of Action of Lectins in Combating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
5. Unveiling the Interplay: Complementary and Competitive Actions of Lectins and Polysaccharides in Combating SARS-CoV-2
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karami, H.; Karimi, Z.; Karami, N. SARS-CoV-2 in Brief: From Virus to Prevention. Osong Public Health Res. Perspect. 2022, 13, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, I.; Alabdulkarim, I.M.; Alhumimidi, A.S.; Albabtain, M.A.; Temsah, M.-H. Navigating Novel Uncertainties of COVID-19: The Rise of the JN.1 Variant. Cureus 2024, 16, e51497. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, L.; Zhang, S.; Su, S.; Liang, X. The Application of Biomaterials for the Vaccine, Treatment, and Detection of SARS-CoV-2. ACS Omega 2024, 9, 5175–5192. [Google Scholar] [CrossRef] [PubMed]
- Amit, A.M.L.; Pepito, V.C.F.; Sumpaico-Tanchanco, L.; Dayrit, M.M. COVID-19 vaccine brand hesitancy and other challenges to vaccination in the Philippines. PLoS Glob. Public Health 2022, 2, e0000165. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, W.; Alomary, F.; Mokni, R. COVID-19 Vaccine Rejection Causes Based on Twitter People’s Opinions Analysis Using Deep Learning. Soc. Netw. Anal. Min. 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A. A Review of SARS-CoV-2 Variants and Vaccines: Viral Properties, Mutations, Vaccine Efficacy, and Safety. Infect. Med. 2023, 2, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.F.; Fang, E.F.; Ng, T.B. Medicinal Applications of Plant Lectins. In Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds; Springer: Berlin/Heidelberg, Germany, 2013; pp. 55–74. [Google Scholar]
- Sharma, A.; Shahid, A.; Banerjee, R.; Kumar, K.J. Emerging Insights into the Structure-Activity Relationship of Water-Soluble Polysaccharides in Antiviral Therapy. Eur. J. Med. Chem. Rep. 2024, 10, 100122. [Google Scholar] [CrossRef]
- Usman, Τ.; Rasheed, A. Lectins and Polysaccharides against SARS-CoV-2; Elsevier: New York, NY, USA, 2023; pp. 223–252. [Google Scholar]
- Vijayan, M.; Chandra, N. Lectins. Curr. Opin. Struct. Biol. 1999, 9, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally Occurring Immune Modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Honein, M.A.; Christie, A.; Rose, D.A.; Brooks, J.T.; Meaney-Delman, D.; Cohn, A.; Sauber-Schatz, E.K.; Walker, A.; McDonald, L.C.; Liburd, L.C.; et al. Summary of Guidance for Public Health Strategies to Address High Levels of Community Transmission of SARS-CoV-2 and Related Deaths, December 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1860–1867. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Carretta, D.M.; De Nitto, E.; Lovero, R. The Human Coronaviruses (HCoVs) and the Molecular Mechanisms of SARS-CoV-2 Infection. J. Mol. Med. 2021, 99, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Alluwaimi, A.M.; Alshubaith, I.H.; Al-Ali, A.M.; Abohelaika, S. The Coronaviruses of Animals and Birds: Their Zoonosis, Vaccines, and Models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020, 7, 582287. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.R. Overview of Coronaviruses in Veterinary Medicine. Comp. Med. 2021, 71, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nyodu, R.; Maurya, V.K.; Saxena, S.K. Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). In Coronavirus Disease 2019 (COVID-19); Saxena, S.K., Ed.; Medical Virology: From Pathogenesis to Disease Control; Springer: Singapore, 2020; pp. 23–31. ISBN 9789811548130. [Google Scholar]
- Bhattacharyya, P.; Das, S.; Aich, S.; Sarkar. COVID-19: Morphology and Mechanism of the SARS-CoV-2, Global Outbreak, Medication, Vaccines and Future of the Virus. Front. Biosci. 2021, 13, 272. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Sriwilaijaroen, N.; Suzuki, Y. Host Receptors of Influenza Viruses and Coronaviruses—Molecular Mechanisms of Recognition. Vaccines 2020, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular Mechanism of Interaction between SARS-CoV-2 and Host Cells and Interventional Therapy. Sig Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Fotouh, S.; Mahmoud, A.N.; Elnahas, E.M.; Habib, M.Z.; Abdelraouf, S.M. What Are the Current Anti-COVID-19 Drugs? From Traditional to Smart Molecular Mechanisms. Virol. J. 2023, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Polysaccharides: Sources, Characteristics, Properties, and Their Application in Biodegradable Films. Polysaccharides 2022, 3, 480–501. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Painter, T.J. Algal polysaccharides. Polysaccharides 1983, 2, 195–285. [Google Scholar]
- Venugopal, V. Marine Polysaccharides; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4398-1527-4. [Google Scholar]
- Qiang, M.; Cai, P.; Ao, M.; Li, X.; Chen, Z.; Yu, L. Polysaccharides from Chinese Materia Medica: Perspective towards Cancer Management. Int. J. Biol. Macromol. 2023, 224, 496–509. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Spectroscopic Manifestation of Stretching Vibrations of Glycosidic Linkage in Polysaccharides. J. Mol. Struct. 2005, 752, 20–24. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Cosgrove, D.J. Diffuse Growth of Plant Cell Walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a Source, Starch as a Sink: The Bifunctional Role of Starch in Carbon Allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef]
- Daghlas, S.A.; Mohiuddin, S.S. Biochemistry, Glycogen; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Song, E.-h.; Shang, J.; Ratner, D.M. Polysaccharides; Elsevier: New York, NY, USA, 2012; pp. 137–155. [Google Scholar]
- Climov, M.; Leavitt, T.; Molnár, J.; Orgill, D.P. Natural Biomaterials for Skin Tissue Engineering; Elsevier: New York, NY, USA, 2016; pp. 145–161. [Google Scholar]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Niyigaba, T.; Liu, D.; de Dieu Habimana, J. The Extraction, Functionalities and Applications of Plant Polysaccharides in Fermented Foods: A Review. Foods 2021, 10, 3004. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Polysaccharides. In Encyclopedia of Life Sciences; Wiley: New York, NY, USA, 2014; ISBN 978-0-470-01617-6. [Google Scholar]
- Dwivedi, R.; Samanta, P.; Sharma, P.; Zhang, F.; Mishra, S.K.; Kucheryavy, P.; Kim, S.B.; Aderibigbe, A.O.; Linhardt, R.J.; Tandon, R.; et al. Structural and Kinetic Analyses of Holothurian Sulfated Glycans Suggest Potential Treatment for SARS-CoV-2 Infection. J. Biol. Chem. 2021, 297, 101207. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.R.; Rodrigues, J.L.; Rodrigues, L.R. Heterologous Production of Chondroitin. Biotechnol. Rep. 2022, 33, e00710. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application Prospect of Polysaccharides in the Development of Anti-Novel Coronavirus Drugs and Vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef]
- Claus-Desbonnet, H.; Nikly, E.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S.; Pierre, G.; Benbassat, N.; Katsarov, P.; Michaud, P.; Lukova, P.; et al. Polysaccharides and Their Derivatives as Potential Antiviral Molecules. Viruses 2022, 14, 426. [Google Scholar] [CrossRef]
- Onishi, A.; St Ange, K.; Dordick, J.S.; Linhardt, R.J. Heparin and anticoagulation. Front. Biosci. 2016, 21, 1372–1392. [Google Scholar]
- Hippensteel, J.A.; LaRiviere, W.B.; Colbert, J.F.; Langouët-Astrié, C.J.; Schmidt, E.P. Heparin as a Therapy for COVID-19: Current Evidence and Future Possibilities. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L211–L217. [Google Scholar] [CrossRef]
- Mikhailov, D.; Young, H.C.; Linhardt, R.J.; Mayo, K.H. Heparin Dodecasaccharide Binding to Platelet Factor-4 and Growth-Related Protein-α. J. Biol. Chem. 1999, 274, 25317–25329. [Google Scholar] [CrossRef]
- Peysselon, F.; Ricard-Blum, S. Heparin–Protein Interactions: From Affinity and Kinetics to Biological Roles. Application to an Interaction Network Regulating Angiogenesis. Matrix Biol. 2014, 35, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of Coronavirus Cell Entry Mediated by the Viral Spike Protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef] [PubMed]
- Cheudjeu, A. The SARS-CoV-2 Entry Inhibition Mechanisms of Serine Protease Inhibitors, OM-85, Heparin and Soluble HS Might Be Linked to HS Attachment Sites. Molecules 2022, 27, 1947. [Google Scholar] [CrossRef] [PubMed]
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.; Zeglen, S.; Baster, Z.; et al. Entry of Human Coronavirus NL63 into the Cell. J. Virol. 2018, 92, e01933-17. [Google Scholar] [CrossRef] [PubMed]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The Spike Glycoprotein of the New Coronavirus 2019-NCoV Contains a Furin-like Cleavage Site Absent in CoV of the Same Clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of Heparin and Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Spike Glycoprotein Binding Interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Shi, D.; Bu, C.; He, P.; Song, Y.; Dordick, J.S.; Linhardt, R.J.; Chi, L.; Zhang, F. Structural Characteristics of Heparin Binding to SARS-CoV-2 Spike Protein RBD of Omicron Sub-Lineages BA.2.12.1, BA.4 and BA.5. Viruses 2022, 14, 2696. [Google Scholar] [CrossRef]
- Thachil, J. The Versatile Heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95, e01987-20. [Google Scholar] [CrossRef] [PubMed]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Tree, J.A.; Turnbull, J.E.; Buttigieg, K.R.; Elmore, M.J.; Coombes, N.; Hogwood, J.; Mycroft-West, C.J.; Lima, M.A.; Skidmore, M.A.; Karlsson, R.; et al. Unfractionated Heparin Inhibits Live Wild Type SARS-CoV-2 Cell Infectivity at Therapeutically Relevant Concentrations. Br. J. Pharmacol. 2021, 178, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Guimond, S.E.; Mycroft-West, C.J.; Gandhi, N.S.; Tree, J.A.; Le, T.T.; Spalluto, C.M.; Humbert, M.V.; Buttigieg, K.R.; Coombes, N.; Elmore, M.J.; et al. Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike–ACE2 Interaction. ACS Cent. Sci. 2022, 8, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Bertini, S.; Alekseeva, A.; Elli, S.; Pagani, I.; Zanzoni, S.; Eisele, G.; Krishnan, R.; Maag, K.P.; Reiter, C.; Lenhart, D.; et al. Pentosan polysulfate Inhibits Attachment and Infection by SARS-CoV-2 In Vitro: Insights into Structural Requirements for Binding. Thromb. Haemost. 2022, 122, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Buijsers, B.; Yanginlar, C.; Maciej-Hulme, M.L.; de Mast, Q.; van der Vlag, J. Beneficial Non-Anticoagulant Mechanisms Underlying Heparin Treatment of COVID-19 Patients. eBioMedicine 2020, 59, 102969. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-Risk Hospitalized Patients with COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612. [Google Scholar] [CrossRef] [PubMed]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of Therapeutic Heparin versus Prophylactic Heparin on Death, Mechanical Ventilation, or Intensive Care Unit Admission in Moderately Ill Patients with COVID-19 Admitted to Hospital: RAPID Randomised Clinical Trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, J.; Liu, X.; Wang, F. Analysis of Chondroitin Sulfate from Different Sources of Cartilage by Electrophoretically Mediated Microanalysis. RSC Adv. 2015, 5, 52314–52319. [Google Scholar] [CrossRef]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef]
- Mizumoto, S.; Kwok, J.C.F.; Whitelock, J.M.; Li, F.; Perris, R. Editorial: Roles of Chondroitin Sulfate and Dermatan Sulfate as Regulators for Cell and Tissue Development. Front. Cell Dev. Biol. 2022, 10, 941178. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin Sulfate in the Treatment of Osteoarthritis: From In Vitro Studies to Clinical Recommendations. Ther. Adv. Musculoskelet. 2010, 2, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Ali, I.; Jana, S.; Mukherjee, S.; Pal, S.; Ray, S.; Schütz, M.; Marschall, M. Antiviral Strategies Using Natural Source-Derived Sulfated Polysaccharides in the Light of the COVID-19 Pandemic and Major Human Pathogenic Viruses. Viruses 2021, 14, 35. [Google Scholar] [CrossRef]
- Abidine, Y.; Liu, L.; Wallén, O.; Trybala, E.; Olofsson, S.; Bergström, T.; Bally, M. Cellular Chondroitin Sulfate and the Mucin-like Domain of Viral Glycoprotein C Promote Diffusion of Herpes Simplex Virus 1 While Heparan Sulfate Restricts Mobility. Viruses 2022, 14, 1836. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Takeda, K.; Hiemori, K.; Minamisawa, T.; Tateno, H. A Glycosaminoglycan Microarray Identifies the Binding of SARS-CoV-2 Spike Protein to Chondroitin Sulfate E. FEBS Lett. 2021, 595, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory Activities of Marine Sulfated Polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Sharma, P.; Farrag, M.; Kim, S.B.; Fassero, L.A.; Tandon, R.; Pomin, V.H. Inhibition of SARS-CoV-2 Wild-Type (Wuhan-Hu-1) and Delta (B.1.617.2) Strains by Marine Sulfated Glycans. Glycobiology 2022, 32, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Theiß, J.; Deinert, T.I.L.; Golat, K.; Heinze, J.; Niemeyer, D.; Wyrwa, R.; Schnabelrauch, M.; Bogner, E. High-Sulfated Glycosaminoglycans Prevent Coronavirus Replication. Viruses 2022, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Albtoush, N.; Petrey, A.C. The Role of Hyaluronan Synthesis and Degradation in the Critical Respiratory Illness COVID-19. Am. J. Physiol.-Cell Physiol. 2022, 322, C1037–C1046. [Google Scholar] [CrossRef]
- Queisser, K.A.; Mellema, R.A.; Middleton, E.A.; Portier, I.; Manne, B.K.; Denorme, F.; Beswick, E.J.; Rondina, M.T.; Campbell, R.A.; Petrey, A.C. COVID-19 Generates Hyaluronan Fragments That Directly Induce Endothelial Barrier Dysfunction. JCI Insight 2021, 6, e147472. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tong, Y.; Chen, L.; Yu, W. Human Identical Sequences, Hyaluronan, and Hymecromone—The New Mechanism and Management of COVID-19. Mol. Biomed. 2022, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, Alginate, Hyaluronic Acid, Gums, and β-Glucan as Potent Adjuvants and Vaccine Delivery Systems for Viral Threats Including SARS-CoV-2: A Review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaisamy, R.; Aroulmoji, V.; Iqbal, M.N.; Deepa, M.; Sivasankar, C.; Khan, R.; Selvankumar, T. Molecular Insights of Hyaluronic Acid-Hydroxychloroquine Conjugate as a Promising Drug in Targeting SARS-CoV-2 Viral Proteins. J. Mol. Struct. 2021, 1238, 130457. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.; Matulewicz, M.; Cerezo, A. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.E.M.; Thissera, B.; Yaseen, M.; Hassane, A.S.I.; El-Seedi, H.R.; Sayed, A.M.; Rateb, M.E. Marine Sulfated Polysaccharides as Promising Antiviral Agents: A Comprehensive Report and Modeling Study Focusing on SARS CoV-2. Mar. Drugs 2021, 19, 406. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Patel, N.B.; Gacem, A.; Alsufyani, T.; Reece, L.M.; Yadav, V.K.; Awwad, N.S.; Ibrahium, H.A.; Ahn, Y.; Yadav, K.K.; et al. Marine Alga Ulva fasciata-Derived Molecules for the Potential Treatment of SARS-CoV-2: An In Silico Approach. Mar. Drugs 2022, 20, 586. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Koenighofer, M.; Lion, T.; Bodenteich, A.; Prieschl-Grassauer, E.; Grassauer, A.; Unger, H.; Mueller, C.A.; Fazekas, T. Carrageenan Nasal Spray in Virus Confirmed Common Cold: Individual Patient Data Analysis of Two Randomized Controlled Trials. Multidiscip. Respir. Med. 2014, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Bernkop-Schnürch, A.; Egyed, A.; Koller, C.; Prieschl-Grassauer, E.; Morokutti-Kurz, M. Development of a Nasal Spray Containing Xylometazoline Hydrochloride and Iota-Carrageenan for the Symptomatic Relief of Nasal Congestion Caused by Rhinitis and Sinusitis. Int. J. Gen. Med. 2018, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-Carrageenan Neutralizes SARS-CoV-2 and Inhibits Viral Replication In Vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Jonsson, C.B.; Taylor, S.L.; Figueroa, J.M.; Dugour, A.V.; Palacios, C.; Vega, J.C. Iota-Carrageenan and Xylitol Inhibit SARS-CoV-2 in Vero Cell Culture. PLoS ONE 2021, 16, e0259943. [Google Scholar] [CrossRef] [PubMed]
- Schütz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan-Containing over-the-Counter Nasal and Oral Sprays Inhibit SARS-CoV-2 Infection of Airway Epithelial Cultures. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.; Kim, C.W.; Lee, H.-R.; Kim, M. Antiviral Activity of Lambda-Carrageenan against Influenza Viruses and Severe Acute Respiratory Syndrome Coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Fröba, M.; Große, M.; Setz, C.; Rauch, P.; Auth, J.; Spanaus, L.; Münch, J.; Ruetalo, N.; Schindler, M.; Morokutti-Kurz, M.; et al. Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta. Int. J. Mol. Sci. 2021, 22, 13202. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R.; Meier, C.; Jawad, M.; Weinmüllner, R.; Grassauer, A.; Prieschl-Grassauer, E. Efficacy and Safety of an Antiviral Iota-Carrageenan Nasal Spray: A Randomized, Double-Blind, Placebo-Controlled Exploratory Study in Volunteers with Early Symptoms of the Common Cold. Respir. Res. 2010, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R.; Winther, B.; Johnston, S.L.; Robinson, P.; Trampisch, M.; Koelsch, S. Efficacy and Safety of Iota-Carrageenan Nasal Spray versus Placebo in Early Treatment of the Common Cold in Adults: The ICICC Trial. Respir. Res. 2015, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Bichiri, D.; Rente, A.R.; Jesus, Â. Safety and Efficacy of a Iota-Carrageenan Nasal Spray in Treatment and Prevention of the Common Cold. Med. Pharm. Rep. 2021, 94, 28–34. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Carrageenan Nasal Spray May Double the Rate of Recovery from Coronavirus and Influenza Virus Infections: Re-analysis of Randomized Trial Data. Pharmacol. Res. Perspec. 2021, 9, e00810. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Nezafat, Z.; Shafiei, N. Polysaccharide Biopolymer Chemistry. In Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications; Elsevier: New York, NY, USA, 2021; pp. 45–105. [Google Scholar]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, Chemical Investigation and Antiviral Activity of Polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, K.; Ghosh, T.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Polysaccharides from Gracilaria corticata: Sulfation, Chemical Characterization and Anti-HSV Activities. Int. J. Biol. Macromol. 2008, 43, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.R.; Cauduro, J.P.; Noseda, D.G.; Noseda, M.D.; Gonçalves, A.G.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S. The Structure of the Agaran Sulfate from Acanthophora spicifera (Rhodomelaceae, Ceramiales) and Its Antiviral Activity. Relation between Structure and Antiviral Activity in Agarans. Carbohydr. Res. 2004, 339, 335–347. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Noseda, D.G.; Noseda, M.D.; Tulio, S.; Pujol, C.A.; Damonte, E.B. Inhibitory Effect of Sulfated Galactans from the Marine Alga Bostrychia Montagnei on Herpes Simplex Virus Replication In Vitro. Phytomedicine 2001, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Panggabean, J.A.; Adiguna, S.P.; Rahmawati, S.I.; Ahmadi, P.; Zainuddin, E.N.; Bayu, A.; Putra, M.Y. Antiviral Activities of Algal-Based Sulfated Polysaccharides. Molecules 2022, 27, 1178. [Google Scholar] [CrossRef] [PubMed]
- Geetha Bai, R.; Tuvikene, R. Potential Antiviral Properties of Industrially Important Marine Algal Polysaccharides and Their Significance in Fighting a Future Viral Pandemic. Viruses 2021, 13, 1817. [Google Scholar] [CrossRef] [PubMed]
- Farfour, E.; Lebourgeois, S.; Chenane, H.R.; Charpentier, C.; Pascreau, T.; Jolly, E.; Descamps, D.; Vasse, M.; Visseaux, B. SARS-CoV-2 Viability and Viral RNA Persistence on Microbiological Agar Plates. J. Hosp. Infect. 2023, 132, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O. Sulfated Fucans, Fresh Perspectives: Structures, Functions, and Biological Properties of Sulfated Fucans and an Overview of Enzymes Active toward This Class of Polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of Biomolecules from Seaweeds; Elsevier: New York, NY, USA, 2015; pp. 243–269. [Google Scholar]
- Zayed, A.; Ulber, R. Fucoidans: Downstream Processes and Recent Applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, M.; Imbs, T.; Shevchenko, N.; Spirin, P.; Horn, S.; Fehse, B.; Zvyagintseva, T.; Prassolov, V. Fucoidans as Potential Inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive Effects of a Fucoidan from Brown Alga Undaria Pinnatifida against Herpes Simplex Virus Infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Tang, Y.; Lin, L.; Xie, Z.; Zhou, J.; Zhang, L.; Zhang, X.; Zhao, X.; Chen, Z.; et al. Fucoidan from Fucus Vesiculosus Suppresses Hepatitis B Virus Replication by Enhancing Extracellular Signal-Regulated Kinase Activation. Virol. J. 2017, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.; Tai, W.; Yu, G. Inhibition of Influenza A Virus Infection by Fucoidan Targeting Viral Neuraminidase and Cellular EGFR Pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Ren, S.; Song, N.; Zhang, Q. Structural Analysis of a Heteropolysaccharide from Saccharina Japonica by Electrospray Mass Spectrometry in Tandem with Collision-Induced Dissociation Tandem Mass Spectrometry (ESI-CID-MS/MS). Mar. Drugs 2012, 10, 2138–2152. [Google Scholar] [CrossRef]
- Kwon, P.S.; Oh, H.; Kwon, S.-J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated Polysaccharides Effectively Inhibit SARS-CoV-2 In Vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, B.; Mourão, P.A.S. Structure and Anticoagulant Activity of Sulfated Fucans. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef]
- Kiselevskiy, M.V.; Anisimova, N.Y.; Bilan, M.I.; Usov, A.I.; Ustyuzhanina, N.E.; Petkevich, A.A.; Shubina, I.Z.; Morozevich, G.E.; Nifantiev, N.E. Prospects for the Use of Marine Sulfated Fucose-Rich Polysaccharides in Treatment and Prevention of COVID-19 and Post-COVID-19 Syndrome. Russ. J. Bioorg Chem. 2022, 48, 1109–1122. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Shah, M.; khan shuvo, S.; Khan, H.; Chowdhury, M.A.R.; Bulbul, I.J.; Hossain, M.S.; et al. Multifaceted Role of Natural Sources for COVID-19 Pandemic as Marine Drugs. Environ. Sci. Pollut. Res. 2022, 29, 46527–46550. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjåk-Bræk, G. Alginate as Immobilization Matrix for Cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Ficai, D.; Ficai, A.; Andronescu, E. From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19. Pharmaceuticals 2022, 15, 318. [Google Scholar] [CrossRef]
- Xin, X.; Geng, M.; Guan, H.; Li, Z. Study on the Mechanism of Inhibitory Action of 911 on Replication of HIV-1 In Vitro. Chin. J. Mar. Drugs 2000, 19, 15–18. [Google Scholar]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural Features and In Vitro Antiviral Activities of Sulfated Polysaccharides from Sphacelaria Indica. Phytochemistry 2011, 72, 276–283. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and Antioxidant Properties of Active Alginate Edible Films Containing Phenolic Extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Wang, S.; Xu, M.; Wang, D.; Li, X.; Xu, X.; Li, C. Inhibitory Activities of Alginate Phosphate and Sulfate Derivatives against SARS-CoV-2 In Vitro. Int. J. Biol. Macromol. 2023, 227, 316–328. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Yang, C.; Li, Q.; Wang, S.; Xu, X.; Hao, J.; Li, C. A Novel PTP1B Inhibitor-Phosphate of Polymannuronic Acid Ameliorates Insulin Resistance by Regulating IRS-1/Akt Signaling. Int. J. Mol. Sci. 2021, 22, 12693. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Hassan, S.S.u.; Bungau, S.; Si, Y.; Xu, H.; Rahman, M.H.; Behl, T.; Gitea, D.; Pavel, F.-M.; Corb Aron, R.A.; et al. Chemically Diverse and Biologically Active Secondary Metabolites from Marine Phylum chlorophyta. Mar. Drugs 2020, 18, 493. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Abdelrheem, D.A.; El-Mageed, H.R.A.; Mohamed, H.S.; Rahman, A.A.; Elsayed, K.N.M.; Ahmed, S.A. Destabilizing the Structural Integrity of COVID-19 by Caulerpin and Its Derivatives along with Some Antiviral Drugs: An In Silico Approaches for a Combination Therapy. Struct. Chem. 2020, 31, 2391–2412. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Shefer, S.; Robin, A.; Chemodanov, A.; Lebendiker, M.; Bostwick, R.; Rasmussen, L.; Lishner, M.; Gozin, M.; Golberg, A. Fighting SARS-CoV-2 with Green Seaweed Ulva Sp. Extract: Extraction Protocol Predetermines Crude Ulvan Extract Anti-SARS-CoV-2 Inhibition Properties in In Vitro Vero-E6 Cells Assay. PeerJ 2021, 9, e12398. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Cegłowska, M.; Konkel, R.; Pyrć, K. Antiviral Cyanometabolites—A Review. Biomolecules 2021, 11, 474. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, G.; Wang, K.; Yang, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Advances in Research on Antiviral Activities of Sulfated Polysaccharides from Seaweeds. Pharmaceuticals 2022, 15, 581. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, T.; Kojima, I. A Natural Sulfated Polysaccharide, Calcium Spirulan, Isolated from Spirulina platensis: In Vitro and Ex Vivo Evaluation of Anti-Herpes Simplex Virus and Anti-Human Immunodeficiency Virus Activities. AIDS Res. Hum. Retroviruses 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ratha, S.K.; Renuka, N.; Rawat, I.; Bux, F. Prospective Options of Algae-Derived Nutraceuticals as Supplements to Combat COVID-19 and Human Coronavirus Diseases. Nutrition 2021, 83, 111089. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, D.; Villalobos-Sánchez, E.; Alam-Escamilla, D.; Elizondo-Quiroga, D. In Vitro Inhibition of SARS-CoV-2 Infection by Dry Algae Powders. Sci. Rep. 2022, 12, 17101. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide Extraction from Spirulina Sp. and Its Antioxidant Capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.K.; Chakraborty, I.; Mandal, A.K.; Bhanja, S.K.; Patra, S.; Maity, P. A Review on Antiviral and Immunomodulatory Polysaccharides from Indian Medicinal Plants, Which May Be Beneficial to COVID-19 Infected Patients. Int. J. Biol. Macromol. 2021, 181, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Efferth, T.; Das, B.; Kar, A.; Ghosh, S.; Singha, S.; Debnath, P.; Sharma, N.; Bhardwaj, P.K.; Haldar, P.K. Role of Medicinal Plants in Inhibiting SARS-CoV-2 and in the Management of Post-COVID-19 Complications. Phytomedicine 2022, 98, 153930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl. Sci. 2019, 143, 423–444. [Google Scholar]
- Yi, Y.-S. Potential Benefits of Ginseng against COVID-19 by Targeting Inflammasomes. J. Ginseng Res. 2022, 46, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, A.-H.; Wang, X.-J. Traditional Chinese Medicine for COVID-19 Treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Wu, S.; Wu, T.; Deng, Y.; Zhang, Q.; Wang, K.; Zhang, Y. The Important Role of Polysaccharides from a Traditional Chinese Medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 Pandemic. Carbohydr. Polym. 2020, 240, 116346. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, M.; Abdulmalek, S.; Fadel, H.H. Biobran/MGN-3, an Arabinoxylan Rice Bran, Protects against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An In Vitro and In Silico Study. Nutrients 2023, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- García-Castro, A.; Román-Gutiérrez, A.D.; Castañeda-Ovando, A.; Cariño-Cortés, R.; Acevedo-Sandoval, O.A.; López-Perea, P.; Guzmán-Ortiz, F.A. Cereals as a Source of Bioactive Compounds with Anti-Hypertensive Activity and Their Intake in Times of COVID-19. Foods 2022, 11, 3231. [Google Scholar] [CrossRef]
- Barbosa, J.R.; de Carvalho Junior, R.N. Polysaccharides Obtained from Natural Edible Sources and Their Role in Modulating the Immune System: Biologically Active Potential That Can Be Exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Sasidharan, S.P.; Yang, X. A Concise Review of Mushrooms Antiviral and Immunomodulatory Properties That May Combat against COVID-19. Food Chem. Adv. 2022, 1, 100023. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.-K.; Kim, Y.-S.; Lee, C.-K.; Han, S.-S. Possible Mode of Antiviral Activity of Acidic Protein Bound Polysaccharide Isolated from Ganoderma lucidum on Herpes Simplex Viruses. J. Ethnopharmacol. 2000, 72, 475–481. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, J.S.; Cho, J.Y.; Kim, Y.E.; Hong, E.K. Study on immunostimulating activity of macrophage treated with purified polysaccharides from liquid culture and fruiting body of Lentinus edodes. J.Microbiol.Biotechnol. 2009, 19, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Rincão, V.P.; Yamamoto, K.A.; Silva Ricardo, N.M.P.; Soares, S.A.; Paccola Meirelles, L.D.; Nozawa, C.; Carvalho Linhares, R.E. Polysaccharide and Extracts from Lentinula Edodes: Structural Features and Antiviral Activity. Virol. J. 2012, 9, 37. [Google Scholar] [CrossRef]
- Krupodorova, T.; Rybalko, S.; Barshteyn, V. Antiviral Activity of Basidiomycete Mycelia against Influenza Type A (Serotype H1N1) and Herpes Simplex Virus Type 2 in Cell Culture. Virol. Sin. 2014, 29, 284–290. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P. Classification, Structure and Mechanism of Antiviral Polysaccharides Derived from Edible and Medicinal Fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Chen, J.; Hu, Y.; Wang, D.; Fan, Y.; Wang, J.; Abula, S.; Zhang, J.; Qin, T.; Chen, X.; et al. In Vitro Antiviral Activity of Sulfated Auricularia Auricula Polysaccharides. Carbohydr. Polym. 2012, 90, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-Glucans from Fungi: Biological and Health-Promoting Potential in the COVID-19 Pandemic Era. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan Extracts from the Same Edible Shiitake Mushroom Lentinus edodes Produce Differential In-Vitro Immunomodulatory and Pulmonary Cytoprotective Effects—Implications for Coronavirus Disease (COVID-19) Immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Feng, Y.; Jiao, F.; Jia, L. Lentinus edodes Polysaccharides Alleviate Acute Lung Injury by Inhibiting Oxidative Stress and Inflammation. Molecules 2022, 27, 7328. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Ha, D.; Yoshitake, R. White Button Mushroom (Agaricus bisporus) Interrupts Tissue AR-TMPRSS2 Expression and Attenuates Pro-Inflammatory Cytokines in C57BL/6 Mice: Implication for COVID-19 Dietary Intervention; Review. 2021. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8010737/ (accessed on 5 April 2024).
- Sun, T.-K.; Huang, W.-C.; Sun, Y.-W.; Deng, J.-S.; Chien, L.-H.; Chou, Y.-N.; Jiang, W.-P.; Lin, J.-G.; Huang, G.-J. Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2. Int. J. Mol. Sci. 2022, 23, 14766. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Cationic Pullulan Nanogel as a Safe and Effective Nasal Vaccine Delivery System for Respiratory Infectious Diseases. Hum. Vaccines Immunother. 2018, 14, 2189–2193. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Wypij, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Golińska, P. Emerging Trends in Pullulan-Based Antimicrobial Systems for Various Applications. Int. J. Mol. Sci. 2021, 22, 13596. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and Practical Applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M. Lectins as Tools to Select for Glycosylated Proteins. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 289–297. [Google Scholar]
- Kilpatrick, D.C. Mechanisms and Assessment of Lectin-Mediated Mitogenesis. Mol. Biotechnol. 1999, 11, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van Ranst, M. Plant Lectins Are Potent Inhibitors of Coronaviruses by Interfering with Two Targets in the Viral Replication Cycle. Antivir. Res. 2007, 75, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Recent Developments in Mushrooms as Anti-Cancer Therapeutics: A Review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lakhtin, V.; Lakhtin, M.; Alyoshkin, V. Lectins of Living Organisms. The Overview. Anaerobe 2011, 17, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V.; Varki, A.; Aebi, M. Microbial Lectins: Hemagglutinins, Adhesins and Toxins. In Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Goldstein, I.J.; Hayes, C.E. The lectins: Carbohydrate-binding proteins of plants and animals. Adv. Carbohydr. Chem. Biochem. 1978, 35, 127–340. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.S.; Da Silva, M.D.C.; Napoleão, T.H.; Paiva, P.M.G.; Correia, M.T.S.; Coelho, L.C.B.B. Lectins: Function, structure, biological properties and potential applications. Curr. Top. Pept. Protein Res. 2014, 15, 41–62. [Google Scholar]
- Balzarini, J. Inhibition of HIV Entry by Carbohydrate-Binding Proteins. Antivir. Res. 2006, 71, 237–247. [Google Scholar] [CrossRef]
- Martínez-Alarcón, D.; Blanco-Labra, A.; García-Gasca, T. Expression of Lectins in Heterologous Systems. Int. J. Mol. Sci. 2018, 19, 616. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Calcabrini, C.; Bishayee, A.; Fimognari, C. Antitumor Potential of Marine and Freshwater Lectins. Mar. Drugs 2019, 18, 11. [Google Scholar] [CrossRef]
- Anderson, K.N.; Evers, D.L.; Rice, K.G. Structure and Function of Mammalian Carbohydrate-Lectin Interactions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 2445–2482. [Google Scholar]
- De Bolle, M.F.C.; David, K.M.M.; Rees, S.B.; Vanderleyden, J.; Cammue, B.P.A.; Broekaert, W.F. Cloning and Characterization of a CDNA Encoding an Antimicrobial Chitin-Binding Protein from Amaranth, Amaranthus caudatus. Plant Mol. Biol. 1993, 22, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E. Plant Lectins: A Composite of Several Distinct Families of Structurally and Evolutionary Related Proteins with Diverse Biological Roles. Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Roth, J. Lectins for Histochemical Demonstration of Glycans. Histochem. Cell Biol. 2011, 136, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East Respiratory Syndrome Coronavirus Infection Is Inhibited by Griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum In Vitro Activity and In Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, S.S.; Suhail, M.; Kamal, M.A.; Ahmad, F.; Azhar, E.I. The Emergence of Human Pathogenic Coronaviruses: Lectins as Antivirals for SARS-CoV-2. Curr. Pharm. Des. 2020, 26, 5286–5292. [Google Scholar] [CrossRef]
- Tian, Y.; Parsons, L.M.; Jankowska, E.; Cipollo, J.F. Site-Specific Glycosylation Patterns of the SARS-CoV-2 Spike Protein Derived From Recombinant Protein and Viral WA1 and D614G Strains. Front. Chem. 2021, 9, 767448. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A.; Ramessar, K.; O’Keefe, B.R. Antiviral Lectins: Selective Inhibitors of Viral Entry. Antivir. Res. 2017, 142, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Jahan, R.; Nissapatorn, V.; Wilairatana, P.; Rahmatullah, M. Plant Lectins as Prospective Antiviral Biomolecules in the Search for COVID-19 Eradication Strategies. Biomed. Pharmacother. 2022, 146, 112507. [Google Scholar] [CrossRef] [PubMed]
- Surya, P.H.; Deepti, M.; Elyas, K.K. Plant Lectins: Sugar-Binding Properties and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 401–439. [Google Scholar]
- Yang, Q.; Hughes, T.A.; Kelkar, A.; Yu, X.; Cheng, K.; Park, S.; Huang, W.-C.; Lovell, J.F.; Neelamegham, S. Inhibition of SARS-CoV-2 Viral Entry upon Blocking N- and O-Glycan Elaboration. eLife 2020, 9, e61552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Shahed-Al-Mahmud, M.; Chen, X.; Chen, T.-H.; Liao, K.-S.; Lo, J.M.; Wu, Y.-M.; Ho, M.-C.; Wu, C.-Y.; Wong, C.-H.; et al. A Carbohydrate-Binding Protein from the Edible Lablab Beans Effectively Blocks the Infections of Influenza Viruses and SARS-CoV-2. Cell Rep. 2020, 32, 108016. [Google Scholar] [CrossRef]
- Auth, J.; Fröba, M.; Große, M.; Rauch, P.; Ruetalo, N.; Schindler, M.; Morokutti-Kurz, M.; Graf, P.; Dolischka, A.; Prieschl-Grassauer, E.; et al. Lectin from Triticum vulgaris (WGA) Inhibits Infection with SARS-CoV-2 and Its Variants of Concern Alpha and Beta. Int. J. Mol. Sci. 2021, 22, 10205. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Q.; Wu, J.; Hu, Y.; Wu, G.; Yu, C.; Xu, K.; Liu, X.; Wang, Q.; Huang, W.; et al. Lentil Lectin Derived from Lens culinaris Exhibit Broad Antiviral Activities against SARS-CoV-2 Variants. Emerg. Microbes Infect. 2021, 10, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Saad, D.A. Recombinant Lectins as Pioneering Anti-Viral Agents against COVID-19. Hematol. Transfus. Int. J. 2021, 9, 77–79. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Status of Mannose-Binding Lectin (MBL) and Complement System in COVID-19 Patients and Therapeutic Applications of Antiviral Plant MBLs. Mol. Cell Biochem. 2021, 476, 2917–2942. [Google Scholar] [CrossRef] [PubMed]
- Ahan, R.E.; Hanifehnezhad, A.; Kehribar, E.Ş.; Oguzoglu, T.C.; Földes, K.; Özçelik, C.E.; Filazi, N.; Öztop, S.; Palaz, F.; Önder, S.; et al. A Highly Potent SARS-CoV-2 Blocking Lectin Protein. ACS Infect. Dis. 2022, 8, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Oh, Y.J.; Yuan, S.; Chu, H.; Yeung, M.-L.; Canena, D.; Chan, C.C.-S.; Poon, V.K.-M.; Chan, C.C.-Y.; Zhang, A.J.; et al. A Molecularly Engineered, Broad-Spectrum Anti-Coronavirus Lectin Inhibits SARS-CoV-2 and MERS-CoV Infection In Vivo. Cell Rep. Med. 2022, 3, 100774. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Paul, S.; Singh, C.; Chowdhury, N.; Nag, P.; Das, S.; Kumar, S.; Sharma, A.; Das, D.K.; Dutta, D.; et al. A Novel Plant Lectin, NTL-125, Interferes with SARS-CoV-2 Interaction with HACE2. Virus Res. 2022, 315, 198768. [Google Scholar] [CrossRef] [PubMed]
- Nabi-Afjadi, M.; Heydari, M.; Zalpoor, H.; Arman, I.; Sadoughi, A.; Sahami, P.; Aghazadeh, S. Lectins and Lectibodies: Potential Promising Antiviral Agents. Cell Mol. Biol. Lett. 2022, 27, 37. [Google Scholar] [CrossRef]
- Meiers, J.; Dastbaz, J.; Adam, S.; Rasheed, S.; Kirsch, S.H.; Meiser, P.; Gross, P.; Müller, R.; Titz, A. Pineapple Lectin AcmJRL Binds SARS-CoV-2 Spike Protein in a Carbohydrate-Dependent Fashion. ChemBioChem 2023, 24, e202200463. [Google Scholar] [CrossRef] [PubMed]
- Alsaidi, S.; Cornejal, N.; Mahoney, O.; Melo, C.; Verma, N.; Bonnaire, T.; Chang, T.; O’Keefe, B.R.; Sailer, J.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination Results in Antiviral Synergy against SARS-CoV-1 and 2 in a Pseudoviral Model. Mar. Drugs 2021, 19, 418. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Lectins as Promising Therapeutics for the Prevention and Treatment of HIV and Other Potential Coinfections. BioMed Res. Int. 2018, 2018, 3750646. [Google Scholar] [CrossRef]
- Lu, W.; Yang, Z.; Chen, J.; Wang, D.; Zhang, Y. Recent Advances in Antiviral Activities and Potential Mechanisms of Sulfated Polysaccharides. Carbohydr. Polym. 2021, 272, 118526. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Donia, A.; Sial, U.; Zhang, X.; Bokhari, H. Glycoprotein- and Lectin-Based Approaches for Detection of Pathogens. Pathogens 2020, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Vanet, A.; Francesconi, V.; Tagliazucchi, L.; Tassone, G.; Venturelli, A.; Spyrakis, F.; Mazzorana, M.; Costi, M.P.; Tonelli, M. Antitarget, Anti-SARS-CoV-2 Leads, Drugs, and the Drug Discovery–Genetics Alliance Perspective. J. Med. Chem. 2023, 66, 3664–3702. [Google Scholar] [CrossRef]
- Sendi, P.; Razonable, R.R.; Nelson, S.B.; Soriano, A.; Gandhi, R.T. First-Generation Oral Antivirals against SARS-CoV-2. Clin. Microbiol. Infect. 2022, 28, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Rabinovich, G.A. Protein-Glycan Interactions in the Control of Innate and Adaptive Immune Responses. Nat. Immunol. 2008, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.H.; Park, J.-Y.; Cho, E.H.; Nah, S.-Y.; Kang, Y.-S. Animal Lectins: Potential Receptors for Ginseng Polysaccharides. J. Ginseng Res. 2017, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Breuilh, L.; Vanhoutte, F.; Fontaine, J.; van Stijn, C.M.W.; Tillie-Leblond, I.; Capron, M.; Faveeuw, C.; Jouault, T.; van Die, I.; Gosset, P.; et al. Galectin-3 Modulates Immune and Inflammatory Responses during Helminthic Infection: Impact of Galectin-3 Deficiency on the Functions of Dendritic Cells. Infect. Immun. 2007, 75, 5148–5157. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, F.; Bruijns, S.C.; Rodriguez, E.; Li, R.E.; Molinaro, A.; Silipo, A.; Di Lorenzo, F.; Garcia-Rivera, D.; Valdes-Balbin, Y.; Verez-Bencomo, V. Novel ACE2-Independent Carbohydrate-Binding of SARS-CoV-2 Spike Protein to Host Lectins and Lung Microbiota. bioRxiv 2020. [Google Scholar] [CrossRef]
- Douma, M.; Boualy, B.; Manaut, N.; Hammal, R.; Byadi, S.; Lahlali, M.; Eddaoudi, F.-E.; Mallouk, S. Sulphated polysaccharides from seaweeds as potential entry inhibitors and vaccine adjuvants against SARS-CoV-2 RBD spike protein: A computational approach. J. Taibah Univ. Sci. 2021, 15, 649–655. [Google Scholar] [CrossRef]

| Polysaccharide | Source | Structure | Antiviral Activity against SARS-CoV-2 | References |
|---|---|---|---|---|
| Heparin | Animal tissues | Highly sulfated glycosaminoglycan | Binds to RBD protein, inhibits viral attachment, induces conformational changes in spike protein receptor-binding domain, reduces viral titers | [48,49,51,52,53,54,55,56,58,59,60,61,62,63,64,65,66] |
| Chondroitin Sulfates | Bovine, porcine, chicken cartilage, shark cartilage | Linear polysaccharide with varying sulfation patterns | Competitive inhibitor of S-protein RBD binding, inhibit viral replication | [45,67,68,69,70,71,72,73,74,75,76] |
| Hyaluronans | Non-sulfated GAG | Repeating D-glucuronic acid and D-N-acetylglucosamine residues | Bind to SARS-CoV-2 spike glycoprotein, promote ARDS, contribute to cytokine storm | [77,78,79,80,81] |
| Marine Polysaccharides | Algae | Varied structures with high degree of sulfation | Block replication phase, destabilize SARS-CoV-2 spike protein | [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128] |
| Galactans, Sulfated Galactans | Red seaweeds | Chains of alternating residues with sulfation | Inhibit viral binding and penetration, suppress viral replication | [129,130,131,132,133,134,135,136,137,138] |
| Alginate | Brown algae | Alternating α-L-guluronic acid and β-D-mannuronic acid residues | Inhibits ACE2-S-protein RBD binding, suppresses viral gene expression | [104,118,119,120,121,122,123,124,125,126] |
| Plant Polysaccharides | Medicinal plants | Diverse structures and derivatives | Inhibit S-protein binding, suppress viral replication | [139,140,141,142,143,144,145,146] |
| Mushroom Polysaccharides | Edible, medicinal mushrooms | Immunomodulatory, antioxidant, antiviral | Inhibit viral entry, replication, and protein expression | [147,148,149,150,151,152,153,154,155,156,157,158,159,160,161] |
| Method | Description | In Vitro/In Vivo | Advantages | Disadvantages |
|---|---|---|---|---|
| Plaque Inhibition Assay | Assesses antiviral activity of heparins against live SARS-CoV-2 by measuring plaque formation in Vero E6 cells. | In Vitro | Provides direct evidence of antiviral activity. | Limited to assessing activity in cell culture; may not fully replicate in vivo interactions. |
| Docking Models | Evaluate binding affinity of specific polysaccharides to S-protein of SARS-CoV-2 using docking models. | In Vitro | Enable screening of compounds for further testing. | Results may not always correlate with experimental data; simplifications in computational model. |
| Cytopathic Assay | Determines antiviral activity of ulvan extracts from Ulva sp. against SARS-CoV-2 in VERO E6 cells. | In Vitro | Provides quantitative data on antiviral activity. | Relies on cell culture systems; may not fully replicate in vivo conditions. |
| Binding Affinity Studies | Evaluate binding affinity of polysaccharides to COVID-19 main protease using in silico methods. | In Vitro | Offer insights into potential therapeutic targets. | Computational results may not fully represent biological reality. |
| Mouse Model Studies | Assess in vivo antiviral activity of synthetic mimetics and natural polysaccharides in K18-hACE2 mouse models. | In Vivo | Provide insights into in vivo efficacy and safety. | Results may not always translate to humans; ethical considerations limit use of animal models. |
| Randomized Clinical Trials | Evaluate therapeutic effects of heparin in hospitalized COVID-19 patients for thromboprophylaxis. | In Vivo | Provide crucial data on therapeutic efficacy. | Time-consuming and expensive; large sample sizes may be needed; ethical considerations; results may not generalize to all patient populations. |
| Basis of Classification | Categories | References |
|---|---|---|
| Based on their source | Plant lectins, animal lectins, microbial lectins, etc. | [167] |
| Based on sequence and evolutionary similarity | Galectins, C-type lectins, P-type lectins, etc. | [172,173] |
| Based on their structural characteristics | Helix-rich lectins, β-sheet-rich lectins, etc. | [174] |
| Based on their number of binding sites | Monovalent lectins, bivalent lectins, multivalent lectins, etc. | [175] |
| Based on cellular localization | Intracellular lectins, extracellular lectins, membrane-bound lectins, etc. | [176] |
| Based on lectin specificity for binding carbohydrates | Galactose-specific lectins, Mannose-specific lectins, etc. | [172,173] |
| Lectin | Source | Antiviral Activity | References |
|---|---|---|---|
| Mannose-specific/mannose-binding lectins (MBLs) | Various sources | Strongly complement cascade induction, anti-infectivity, DC-SIGN antagonists, immunoadjuvants, and glycomimetic approach efficacies useful against COVID-19 and SARS-CoV-2 infections | [183] |
| FRIL | Plant-derived | Directly binds to virus particles; demonstrates antiviral activity against a SARS-CoV-2 strain originating from Taiwan | [186] |
| Wheat germ agglutin/lectin | Plant-derived | Exhibits antiviral efficacy against SARS-CoV-2 and its variants of concern (VoCs), Alpha and Beta | [187] |
| Lentil lectin | Derived from Lens culinaris | Demonstrates highly potent and broad-spectrum antiviral activity against various SARS-CoV-2 mutant strains and variants, including epidemic variants such as B.1.1.7, B.1.351, and P.1 | [188] |
| Recombinant lectins | Synthetic | Serve as new anti-SARS-CoV-2 agents by targeting SARS-CoV-2-associated glycans | [189] |
| Plant lectins | Various sources | Varied antiviral activity spectrum against SARS-CoV-2; potent against viral entry targets | [171,185,190] |
| Griffithsin lectin (GRFT) | Source unspecified | Binds to the SARS-CoV-2 spike protein and prevents infection | [191] |
| H84T-banana lectin (H84T-BanLec) | Engineered | Inhibits SARS-CoV-2, MERS-CoV, and other human-pathogenic coronaviruses at nanomolar concentrations | [192] |
| NTL-125 | New plant lectin | Blocks SARS-CoV-2 interaction with hACE2 | [193] |
| AcmJRL | Pineapple-derived | Binds the SARS-CoV-2 spike protein in a carbohydrate-dependent manner | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefter, R.; Balyan, P.; Balmus, I.-M.; Ech-Chahad, A.; Ali, A.; Ciobica, A.; Petroaie, A.D.; Halitchi, G.; Novac, B.; Ionescu, C.; et al. Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic. Microbiol. Res. 2024, 15, 525-549. https://doi.org/10.3390/microbiolres15020035
Lefter R, Balyan P, Balmus I-M, Ech-Chahad A, Ali A, Ciobica A, Petroaie AD, Halitchi G, Novac B, Ionescu C, et al. Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic. Microbiology Research. 2024; 15(2):525-549. https://doi.org/10.3390/microbiolres15020035
Chicago/Turabian StyleLefter, Radu, Prairna Balyan, Ioana-Miruna Balmus, Abdellah Ech-Chahad, Ahmad Ali, Alin Ciobica, Antoneta Dacia Petroaie, Gabriela Halitchi, Bogdan Novac, Catalina Ionescu, and et al. 2024. "Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic" Microbiology Research 15, no. 2: 525-549. https://doi.org/10.3390/microbiolres15020035
APA StyleLefter, R., Balyan, P., Balmus, I.-M., Ech-Chahad, A., Ali, A., Ciobica, A., Petroaie, A. D., Halitchi, G., Novac, B., Ionescu, C., & Kamal, F. Z. (2024). Polysaccharides and Lectins: A Natural Complementary Approach against the SARS-CoV-2 Pandemic. Microbiology Research, 15(2), 525-549. https://doi.org/10.3390/microbiolres15020035









