Abstract
Biochar and arbuscular mycorrhizal fungi (AMF), a promising environmentally friendly soil enhancer and biostimulant, play a crucial role in sustainable agriculture by influencing soil properties and plant growth. This research investigates the chemical properties of three biochar types [bamboo (BB-char), corn cob (CC-char), and coffee grounds (CG-char)] derived from different biomass sources and their impact on soil quality and Chinese kale growth. The results reveal significant differences in chemical properties among different types of biochar. Particularly, CG-char showed the greatest pH value and phosphorus content, with an average of 10.05 and 0.44%, respectively. On the other hand, CC-char had the highest potassium content, with an average of 2.16%. Incorporating biochar into degraded soil enhances soil structure, promoting porosity and improved texture, as evidenced by scanning electron microscope images revealing distinct porous structures. Soil chemistry analyses in treatment T2–T14 after a 42-day cultivation demonstrate the impact of biochar on pH, electrical conductivity, organic matter, and organic carbon levels in comparison to the control treatment (T1). Furthermore, the research assesses the impact of biochar on Chinese kale growth and photosynthetic pigments. Biochar additions, especially 5% BB-char with AMF, positively influence plant growth, chlorophyll content, and photosynthetic pigment levels. Notably, lower biochar concentrations (5%) exhibit superior effects compared to higher concentrations (10%), emphasizing the importance of optimal biochar application rates. The study also delves into the total phenolic content in Chinese kale leaves, revealing that the synergistic effect of biochar and AMF enhances phenolic compound accumulation. The combination positively influences plant health, soil quality, and nutrient cycling mechanisms. Overall, the research indicates the multifaceted impact of biochar on soil and plant dynamics, emphasizing the need for tailored application strategies to optimize benefits in sustainable agriculture.
1. Introduction
Currently, the world’s population is increasing at a rapid rate and is expected to reach 8.5 billion by 2030. This increase in population stimulates demand for more agriculture and industrial activities, which in effect leads to an increase in agricultural waste. It is estimated that the amount of agricultural waste will reach 2.59 billion tons [1]. Simultaneously, one of the methods for enhancing agricultural crop production in modern-day agriculture is the use of chemical fertilizers; however, inappropriate excessive usage of such fertilizers has a negative impact on environmental pollution and eutrophication problems and contributes to a gradual reduction in soil fertility [2]. Therefore, the application of biochar to soil is considered to have potential for long-term soil carbon sequestration, as well as to improve plant growth and disease pathogens [3].
Biochar is a charcoal-like material that is rich in organic carbon, produced from biomass such as wood, leaf, seed/fruit, grass, or agricultural wastes through the process of pyrolysis (slow, intermediate, fast, and gasification) at high temperatures in a low-oxygen or oxygen-absent environment [4,5,6,7]. Biochar plays an important role in decreasing greenhouse gas emissions in the world and reducing atmospheric CO2 concentrations [8,9,10]. In terms of sustainable agriculture and the environment, biochar is receiving attention as an environmentally friendly alternative for sustainable agriculture and environmental improvement. It is used as a soil amendment to enhance soil fertility, structure, and biochemical quality while reducing the use of industrial fertilizer and the ecological impact of agriculture [11,12]. Biochar is well known for its unique properties, such as having a high surface area and porosity, low bulk density, high cation exchange capacity (CEC), neutral to alkaline pH, and high carbon content, and it also contains some essential plant nutrients, such as nitrogen (N), phosphorus (P), and basic cations such as calcium (Ca), magnesium (Mg), and potassium (K) that are necessary for crop growth and development [12]. Therefore, the addition of biochar to soil can improve soil organic carbon content, CEC, and soil porosity; stabilize soil pH; increase soil microbial activity; enhance moisture retention and availability; immobilize toxic elements like heavy metals; and increase the bioavailability of minerals, resulting in improved root development and nutrient uptake [11,12,13]. Previous studies reported that the application of biochar has been shown to increase soil microbial activity and improve the chemical and physical properties of the soil [14,15,16,17]. Fachini et al. [18] found that biochar produced from sewage sludge is an excellent alternative to traditional chemical fertilizers.
Moreover, combining biochar with soil microorganisms such as AMF has shown to be beneficial in agricultural applications, as AMF are considered a safe and efficient natural biofertilizer, while biochar has the potential to serve as an inoculum carrier for AMF, enhancing their abundance and infection rate by modifying soil properties and microbial activity [19,20].
AMF are a fundamental group of soil microorganisms belonging to the phylum Glomeromycota. They are among the most prominent microorganisms and are commonly used as biostimulants in sustainable agriculture [21,22]. In the natural system, AMF are involved in mutualistic symbiotic associations with plant species, are an essential component of rhizosphere microorganisms, and play an important role in nutrient cycling and enhancing plant nutrition [23,24,25]. The application of AMF as biostimulants has become known as a significant environmentally friendly approach to modern agriculture [26,27]. They are frequently used as biofertilizers in agriculture ecosystems because they provide several ecosystem benefits, such as nitrogen fixation, soil carbon cycling, plant nutrition, soil erosion control, soil pollutant remediation, biodiversity support, plant water regulation, and enhanced carbon sequestration [28]. Therefore, the combination of biochar and AMF application plays an important role in improving soil properties and plant growth. Several previous studies have reported that the combined application of biochar and AMF can improve soil nutrient content, enhance plant nutrient uptake, and increase antioxidant enzymes activity, as well as improve plant growth under saline stress [29,30,31,32]. Furthermore, it has been reported that the combination of biochar and AMF can not only improve soil properties but also improve plant growth and increase yields for a variety of plants, such as rice (Oryza sativa L.) [33], soybean (Glycine max) [34], spinach (Spinacia oleracea L.) [10], chili (Capsicum flutescens L.) [35,36], and corn (Zea mays L.) [29,31,37]. However, there is still a lack of information about the interaction of biochar and AMF in many economically important vegetable crops, particularly Chinese kale, which holds high economic value in numerous regions worldwide.
Chinese kale (Brassica oleracea var. alboglabra L.) is a traditional vegetable of Chinese origin belonging to the Brassicaceae family and is widely cultivated in southern China and Southeast Asia. Known for its nutritional richness, Chinese kale contains health-promoting phytochemicals such as carotenoids, glucosinolates, vitamin C, and phenolic compounds [38,39,40]. Despite its high demand and cultivation volume, intensive agricultural methods often result in soil degradation and nutrient loss. Therefore, adopting sustainable cultivation practices becomes imperative to preserving soil health and sustaining crop productivity. In Thailand, Chinese kale is one of the most popularly cultivated and consumed vegetables. Thus, this study was conducted on utilizing agricultural waste to produce biochar, and the effect of biochar and AMF on plant trials of Chinese kale growth and the surrounding soil properties was investigated. Specifically, it explores the potential benefits of biochar and AMF application in improving soil structure, nutrient cycling, and plant growth. Understanding these effects is crucial for developing sustainable cultivation practices that promote soil health and enhance crop productivity. Overall, this paper attempts to provide insights into sustainable agricultural practices for Chinese kale cultivation, with implications for enhancing soil health, crop yield, and environmental sustainability.
2. Materials and Methods
2.1. Source of Soil, Biochar, and Arbuscular Mycorrhiza Fungi
The soil sample was collected from an agricultural farm located in the province of Chiang Mai, Thailand. Before conducting the examination, the soil properties, including pH, total organic matter (OM), organic carbon (OC), were analyzed by the Central Laboratory, Faculty of Agriculture, Chiang Mai University, Thailand.
The biochar used in this study was produced from three types of biomasses, including bamboo (BB), corn cob (CC), and coffee grounds (CG), which were obtained from the Faculty of Engineering at Chiang Mai University, Thailand. The arbuscular mycorrhiza, which consists of Acaulospora foveate, Glomus etunicatum, G. geosporum, and G. mosseae (MYCOBTECHTM BIO STIMULANT), was obtained from SV BIOTECH, Ratchaburi, Thailand.
2.2. Soil and Biochar Preparation
Three different types of biomass were subjected to a drying process at a temperature of 80 °C for 24 h. Then, each dried biomass was subjected to a slow pyrolysis process at 500 °C for 2 h in a fixed-bed reactor. After preparation of the biochar, it was pulverized into a fine powder and sieved through a sieve with a mesh of 1 to 2 mm. The soil sample was air-dried until it became completely dry. After that, it was broken down to a small size and sieved through a 3 mm mesh sieve.
After preparing the biochar and soil, the soil sample and each biochar type were immediately transferred to plastic bags and sterilized by autoclaving two times at 121 °C for 20 min [41]. All soil and biochar bags were stored at room temperature for further use.
2.3. Biochar and Soil Analysis
Each biochar type and soil after the experiment were analyzed for different chemical characteristics. The moisture content was determined following the methods of the Association of Official Analytical Chemists (AOAC) [42]. To determine the electronic conductivity (EC) and pH, a 1:10 ratio of biochar:water was soaked in 50 mL distilled water for 30 min. Measurements were conducted using an Ecoscan COND 6+ Conductivity Meter (EUTECH Instruments, Malaysia) for EC and a pH meter (Sartorius PB-10, Sartorius, Germany) for pH determination. Organic carbon (OC) content was determined by the Walkley and Black method [43]. Total nitrogen (N) was determined by the Kjeldahl method [44]. Furthermore, vanadomolybdate and atomic absorption spectrophotometry (AAS) methods were used to determine the content of phosphorus (P) and potassium (K), respectively [45,46]. All samples were analyzed by the Central Laboratory, Faculty of Agriculture, Chiang Mai University, Thailand. Moreover, the biochar and soil samples were photographed with the scanning electron microscope (SEM) JEOL JSM-5910 LV SEM (JEOL, Tokyo, Japan) using an accelerating voltage of 15 kV at the Science and Technology Service Center, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand.
2.4. Experimental Design
The experimental setup for the different treatments was designed using a completely randomized design (CRD) with 3 treatment factors: (1) Biochar type: There were 3 types of biochar and no biochar or AMF soil as a control; (2) level of biochar (5% and 10% by weight); and (3) arbuscular mycorrhiza mixed with each biochar type and without any biochar, resulting in a total of 14 treatments. Each treatment provided 5 replicates. Therefore, 70 experimental units were used in the present study. The treatments are listed in Table 1.

Table 1.
Experimental details.
2.5. Planting and Pot Experiment for Chinese Kale Cultivation
In this study, Chinese kale (Brassica oleracea var. alboglabra L.) seed (Chia Tai Co., Ltd., Bangkok, Thailand) was used. Chinese kale seeds were surface sterilized with 0.5% sodium hypochlorite solution (v/v) for 5 min, washed 5 times with sterile distilled water, and then germinated on moist filter papers in sterile Petri dishes for 2 days at 25 °C [47]. The germinated seeds were grown in a nursery tray (54 cm in height × 26 cm in depth × 3.6 cm in width) using sterilized vermiculite: perlite: peat moss (1:1:2 w/w/w) as a substrate for 2 weeks in greenhouse conditions. After that, the healthy seedlings were transferred to plastic pots (6.3 cm in diameter and 4.5 cm in depth) containing 800 g of sterilized soil mixed with each type of biochar and each type of biochar combined with AMF (25 kg/g soil). The pot experiment was conducted in a greenhouse located in the Faculty of Science, Chiang Mai University, Thailand, for 42 days. Planting pots with each treatment were randomly arranged in the greenhouse. The plants were grown in greenhouses where the temperature was an average of 27 °C and the mean relative humidity was 62.5%. Each pot was watered every two days.
2.6. Plant Harvest and Analysis
After 42 days of cultivation, plant leaf number, plant height, and leaf chlorophyll content were measured with a SPAD-502Plus chlorophyll meter (KONICA MINOLTA, INC., Tokyo, Japan) before harvesting. Chinese kale plants were carefully separated from the soil, and the roots were washed under running water to remove soil and organic matter. The plants were placed in plastic containers containing a small amount of water to preserve their freshness and transported to the laboratory. Then, shoots and roots were cut from each plant. Total fresh shoot and root weight was measured using an analytical balance [48], and shoot and root length was measured using a vernier caliper. Shoot and root dry weight was measured by analytical balance after drying at 60 °C for at least 72 h or until they became totally dried [49].
2.7. Determination of Photosynthetic Pigment Content
In this experiment, fresh leaf tissue was used for the determination of photosynthetic pigments. The chlorophyll content was determined as previously described by Lichtenthaler et al. [50]. Five replicates of 0.2 g of fresh leaf tissue (fresh weight; FW) were extracted in a mixture of acetone and ethanol (1:1, v:v) solution and subsequently kept in the dark at room temperature for 24 h or until the color faded to white. The supernatant was collected after centrifugation at 3000 rpm at room temperature for 10 min, and photosynthetic pigment content was determined by measuring the absorbance at 663 nm, 645 nm, and 440 nm with a UV-spectrophotometer. The content of photosynthetic pigments was calculated as follows:
where V is the volume of the extract and W is the weight of the sample.
Chlorophyll a (mg g−1) = (12.7 × OD663 − 2.69 × OD645) × V/W × 1000
Chlorophyll b (mg g−1) = (22.9 × OD645 − 4.86 × OD663) × V/W × 1000
Total chlorophyll (mg g−1) = (8.02 × OD663 − 20.20 × OD645) × V/W × 1000
Carotenoids (mg g−1) = (4.7 × OD440 − 0.27 × Total chlorophyll content) × V/W × 1000
2.8. Determination of Total Phenolic Content
The total phenolic content was determined using the Folin–Ciocalteu assay [51]. The determination was performed according to the method described by Rahman [52]. Briefly, a 0.5 g ground fresh sample (fresh weight; FW) was subjected to extraction by mixing with 8 mL of alcohol for 30 min and centrifuged at 3000 rpm for 15 min. Then, the reaction mixture was prepared by mixing 1 mL of supernatant, 0.5 mL of Folin–Ciocalteu’s phenol, and 1.5 mL of a 26.7% Na2CO3 solution, followed by the addition of 7 mL of distilled water. The mixture was thoroughly mixed and incubated in the dark condition at room temperature for 2 h. The absorbance was measured at 760 nm using a spectrometer. Total phenolic content values were determined from a calibration curve, which was prepared with different concentrations of gallic acid solution. The total phenolic content was expressed as mg of gallic acid equivalents/g fresh weight (mg GAE/g FW).
2.9. Data Analysis
All the data were shown as means ± standard deviation (SD), which were analyzed by one-way analysis of variance (ANOVA) and carried out by IBM SPSS statistic software version 26.0. The significant differences (p ≤ 0.05) between the mean value of each treatment were considered statistically significant using Duncan’s multiple range test.
3. Results and Discussion
3.1. Biochar Analysis
Understanding the chemical properties of biochar is important to supporting sustainable agriculture, restoring soil quality, managing the environment, and improving soil fertility [53]. Several previous studies have been conducted on its chemical properties [54,55,56]. Before the start of our experiment, each biochar type was analyzed for chemical parameters such as moisture content, pH, EC, OC, and nutrient content (N, P, and K). The results of the chemical properties of biochar are presented in Table 2. It was found that the chemical properties of biochar can vary significantly depending on the type of biomass. The results indicate that BB-char had the highest moisture content (5.44%), followed by CC-char (2.49%) and CG-char (1.33%). The moisture content of each type of biochar investigated in this study was consistent with the findings of several previous studies, which had reported that the biochar moisture content ranged from 1.84 to 5.70% [56,57,58]. According to Ali et al. [56], the moisture content of biochar produced from corn cob pyrolyzed at 300 °C and 400 °C was found to be 3.21% and 1.84%, respectively. Schneider et al. [57] reported that the moisture content of biochar produced from bamboo was 5.70%. In the case of pH, it was found to be within a range of 8.83 to 10.05, with CG-char having the highest average pH value at 10.05 ± 0.03, followed by CC-char and BB-char with an average pH value of 9.94 ± 0.03 and 8.83 ± 0.03, respectively. However, a pH value of biochar ranging from 3.1 to 12.0 has been reported [59,60]. The results of this study are consistent with the findings of Prasad et al. [61], who also found that biochar produced from bamboo, cereal husk/paper fiber, forest wood, and wood screenings all showed alkaline pH values of 8.84, 9.56, 9.51, and 9.54, respectively. Moreover, it was found that most biochar with alkaline pH was used for soil amendment [62]. Sahoo et al. [63] investigated the pH of biochar derived from pigeon pea stalks and bamboo that had been pyrolyzed at different temperatures of 400 °C, 500 °C, and 600 °C. They revealed that the pH values of both biochars were in the alkaline range, with pH values of 7.90 to 10.14 for biochar derived from pigeon pea stalks and 7.25 to 10.07 for biochar derived from bamboo, respectively. Therefore, the findings from this experiment were supported by several previous studies that found that biochar’s chemical properties were influenced by the pyrolysis conditions and the type of raw materials [64,65,66,67].

Table 2.
Chemical properties of biochars used in the experiment.
Biochar, an environmentally friendly soil enhancement, serves as an essential source of important nutrients for plants [68]. One of the most important factors to be considered is the influence of biochar nutrient content. In this study, the nutrient content in biochar, including total N, P, and K, as well as total OC, was determined. The results reveal that the CC-char had the highest OC and K content, with a total OC and K content of 5.63% and 2.16%, respectively, while CG-char had the highest total P content of 0.44% (Table 2). Several earlier studies involving different types of biomasses (bamboo shoot shell, chicken feather waste, cow dung, cucumber plant waste, palm leaf waste, poultry litter, tomato plant waste, wood) as raw materials for the production of biochar and determined based on nutrient content have been reported [67,69,70]. Generally, the nutrient content of biochar products differs depending on the biomass type, pyrolysis temperature, and heating rates used during the production process [67,71,72]. For instance, the study conducted by Ye et al. [69] revealed that the nutrient content of biochar obtained from bamboo shoot shell at pyrolytic temperatures ranging from 300 to 500 °C had a value of total OC, N, P, and K of 64.0–69.3%, 2.80–3.10%, 0.35–0.56%, and 0.31–1.71%, respectively. Tsai et al. [73] found that the biochar produced from swine dung at a temperature of 400 °C had high levels of nitrogen (3.2%) and phosphorus (6.1%). On the other hand, biochar made from giant reed (Arundo donax) at the same temperature had low levels of N (0.69%) and P (0.13%) [74].
In scanning electron microscope images, Figure 1A–C displays three types of raw materials used for biochar production. The microporous structure of biochar derived from each biomass type was distinctly evident, featuring visible pores and pits (Figure 1D–F). Upon the addition of biochar and mycorrhiza to the deteriorated soil, the soil structure exhibited the incorporation of biochar and the colonization of mycorrhizal fungi. Although it remains uncertain whether this indicates inadvertent colonization by mycorrhizal fungi, there is unequivocal evidence of substantial alterations in soil structure attributed to biochar (Figure 1G,H). The soil structure, particularly in the presence of both biochar and AMF, displayed enhanced porosity and improved soil texture compared to soil without biochar. In contrast, the degraded soil exhibited a dense, smooth, and non-porous surface (Figure 1I), directly impacting the growth rate of the test plants.
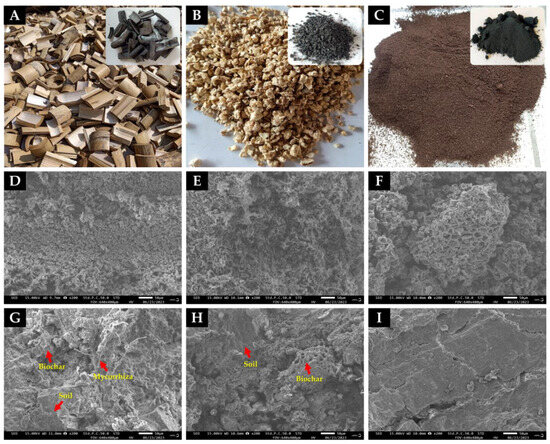
Figure 1.
The raw materials before pyrolysis for biochar production, including bamboo (A), corn cob (B), and coffee grounds (C), as well as the electron microscopy investigation of each biochar and soil structure. Microporous biochar structures derived from bamboo (D), corn cob (E), and coffee grounds (F). The soil structure with incorporated biochar and AMF colonization (G), deteriorated soil with biochar (H), and untreated deteriorated soil (I). The red arrows indicate the internal structure of the soil, comprising biochar components, soil particles, and AMF.
3.2. Effects of Biochar and Biochar with AMF on Soil Chemical Properties
The soil chemical parameters were determined after a cultivation duration of 42 days. The results of the investigation of the chemical properties of soils are shown in Table 3. The soil analysis results demonstrate a statistically significant difference in pH increase when biochar was added to the soil compared to the initial soil analysis conducted before the experiment, where no biochar was applied. The initial pH level of the soil sample was approximately 6.31 and exhibited an increase during the growth of Chinese kale in treatments T2–T14. These pH levels were slightly alkaline, ranging from 7.44 to 7.86, with variations attributed to the levels of addition and the type of biochar used. The highest pH value of the soil samples was obtained with the treatment where the soil was applied with 5% CG-char (T6) and 10% CG-char combined with AMF (T14). Furthermore, it was found that higher biochar application rates (10%) increased soil pH more than lower biochar rates (5%). The findings of this study align with numerous prior research studies exploring the incorporation of biochar into agricultural soil systems. These studies consistently report that the addition of biochar to the soil leads to changes in pH levels. In general, biochar typically exhibits alkaline properties that vary based on its type. When incorporated into the soil, biochar has the capacity to increase the overall pH of the soil [75]. Simultaneously, adding different concentrations of biochar to the soil induces changes in the pH value, with an increase corresponding to the applied level [76]. These findings are supported by the results of Hailegnaw et al. [77], who noted that the highest biochar application rate (8%) was more effective in altering soil properties than lower biochar rates, significantly increasing pH in all incubated soils. Furthermore, Kizito et al. [78] reported a similar trend, with the addition of corncobs and wood biochar leading to an increase in soil pH from 6.92 to a range of 7.7–8.1. Nevertheless, the results of this study highlight that the addition of biochar can improve soil properties, particularly in acidic soils, offering potential benefits for modern agricultural systems.

Table 3.
Chemical properties of soil after the application of treatments for 42 days.
In terms of EC, the addition of each type of biochar to the soil led to an increase in soil EC compared to the control treatment (T1). It was observed that the addition of either biochar without AMF or biochar combined with AMF resulted in a significant increase in soil EC. Therefore, the addition of biochar and the combination of biochar and AMF resulted in a significant improvement in soil EC. In addition, both the additional 5% and 10% rate applications of biochar resulted in increases in soil EC. Our results show that all biochar increased soil EC, which is consistent with many previous studies [79,80,81]. Furthermore, Mohawesh et al. [80] also indicated that using biochar as a soil amendment has the potential to increase both soil electrical conductivity (EC) and pH levels, potentially influencing the growth of plant shoots and roots. However, certain studies found that soil EC was either not associated with or not affected by the amount of biochar applied [82,83].
Biochar holds the potential to impact the composition of organic matter and organic carbon in the soil [84]. In our study, the results in Table 3 revealed that after 42 days of cultivation, adding 5 and 10% biochar to the soil significantly improved organic matter and organic carbon compared to the initial soil. The soil initially employed for planting exhibited organic matter and organic carbon levels of 1.12% and 0.65% biochar, respectively. Following the addition of biochar to the soil (T2–T14), a noticeable increase was observed in both OM and OC levels, ranging approximately between 2.58 and 3.52% and between 1.53 and 2.30%, respectively. The highest improvement in the planting soil’s organic matter and organic carbon values was observed with the addition of 10% CC-char (3.61 and 2.21, respectively). This was followed by the application of 10% BB-char and 10% BB-char combined with AMF. Conversely, the addition of 5% CG-char (T13) to the soil resulted in the lowest levels of organic matter and organic carbon among the treatments. Notably, these values did not exhibit statistical differences from the untreated planting soil (T1 and T8). Our findings largely align with numerous prior studies suggesting that the addition of biochar to soil can enhance organic matter and organic carbon levels through diverse mechanisms, like stable carbon sequestration, reduced decomposition, enhanced microbial activity, improved soil structure, promotion of plant growth, and chemical binding [3,85,86]. However, it is important to highlight that the specific effects of biochar on organic matter and organic carbon levels can vary, influenced by factors such as biochar type, feedstock, and application rate, along with the soil characteristics [87]. Despite these consistent trends, certain studies have suggested that some types of biochar may exhibit minimal or no impact on these properties [84,88]. Conducting long-term studies is imperative for comprehending the sustained impacts of biochar on soil carbon dynamics.
In addition to influencing other chemical properties, the incorporation of biochar into soil for crop cultivation enhances the levels of primary nutrients, namely, nitrogen, phosphorus, and potassium [89]. The results indicate that the application of both biochar and biochar mixed with AMF led to significant increases in total nitrogen content across most soils. Similarly, the addition of biochar to the soil showed a propensity to increase the levels of phosphorus and potassium in overall soils. The outcomes of this study demonstrated that the use of both 10% CG-char and 10% CG-char combined with AMF resulted in soil with substantial amounts of all three primary nutrients. Simultaneously, it was observed that the addition of certain types of biochar to the soil may have minimal or no impact on primary nutrients. These effects vary based on the specific type of biochar and the amount applied. Generally, the addition of biochar to soil can increase primary nutrients through various mechanisms related to cation exchange capacity (CEC) and nutrient retention [89]. Possessing a high CEC, biochar is adept at attracting, retaining, and exchanging positively charged ions, including essential nutrients such as nitrogen, phosphorus, and potassium. This inherent property enhances the availability of these nutrients for plant uptake. Simultaneously, biochar can retain nutrients within its porous structure, preventing them from leaching away due to rainfall or irrigation. This retention helps create a reservoir of nutrients in the soil, promoting sustained availability for plants [68,90]. Furthermore, the specific impact of biochar on primary nutrient levels is also associated with microbial activity and symbiotic relationships. Typically, biochar serves as a habitat for beneficial microorganisms that play a pivotal role in nutrient cycling and mineralization. These microbes contribute to breaking down organic matter, rendering nutrients like N, P, and K more abundant and accessible to plants [91]. Additionally, when used in combination with specific microorganisms such as AMF, biochar can enhance nutrient uptake. This enhancement occurs as AMF forms symbiotic relationships with plant roots, further facilitating the absorption of nutrients from the soil [33]. According to study conducted by Ndiate et al. [29], combining biochar with AMF effectively improved soil nutrient content, enhanced plant nutrient uptake, increased antioxidant enzyme activity, and improved some specific fatty acid content. Furthermore, Kotby et al. [31] demonstrated that when used in combination with AMF inoculation, maize plants exhibited considerably higher nutrient uptake and growth promotion compared to non-treated plants. When investigated under soil with salt stress conditions, Gunes et al. [36] revealed that biochar and AMF positively improved microbial activity.
3.3. Effect of Biochar and Biochar with AMF on Chinese Kale Growth Promoting
During the planting period, there were no observable symptoms of plant diseases in any treatments of biochar and AMF application, or in the control group. After a growth period of 42 days, the results indicate that there was not a statistically significant difference in the number of leaves per plant between the treatment groups (Figure 2A). The chlorophyll content of Chinese kale leaves, as measured using SPAD chlorophyll meters, ranged from a 51.22 ± 0.81 to a 53.80 ± 0.89 SPAD value. The highest chlorophyll content (53.80 ± 0.89 SPAD value) was observed in the T4 treatment, while the lowest content (51.22 ± 0.81 SPAD value) was found in the T2 treatment (Figure 2B). The plant height and root length of Chinese kale are shown in Figure 2C,D,F. The results indicate that the addition of 5% BB-char and AMF to the soil (T11) increased plant height, root length, plant and root dry weight of Chinese kale compared with the plant in other treatments, as well as the control treatment (T1) (Figure 2C). Likewise, it was found that the T11 treatment significantly improved the root length of Chinese kale compared with the other treatments (Figure 2D). After the drying of plants and roots at a temperature of 60 °C, the results indicate that all the treatments involving additional biochar and biochar with AMF (T2–T14) had a higher plant dry weight than the control treatment (T1). The highest value of dried plant weight was obtained in the Chinese kale plant that had been supplemented with 5% BB-char and AMF (T11), followed by Chinese kale supplemented with 5% CC-char and AMF (T9) and Chinese kale supplemented with 5% CG-char and AMF (T13). However, the lowest dry weight of Chinese kale plant was found in the control treatment (T1) (Figure 2E). Likewise, the T11 treatment had the highest dry weight of roots, while treatments T1, T3, and T7 had the lowest dry weight of roots (Figure 2E). Interestingly, the results of this study reveal that the addition of 5% biochar resulted in higher growth of Chinese kale compared to the addition of 10% biochar (Figure 2C–E).
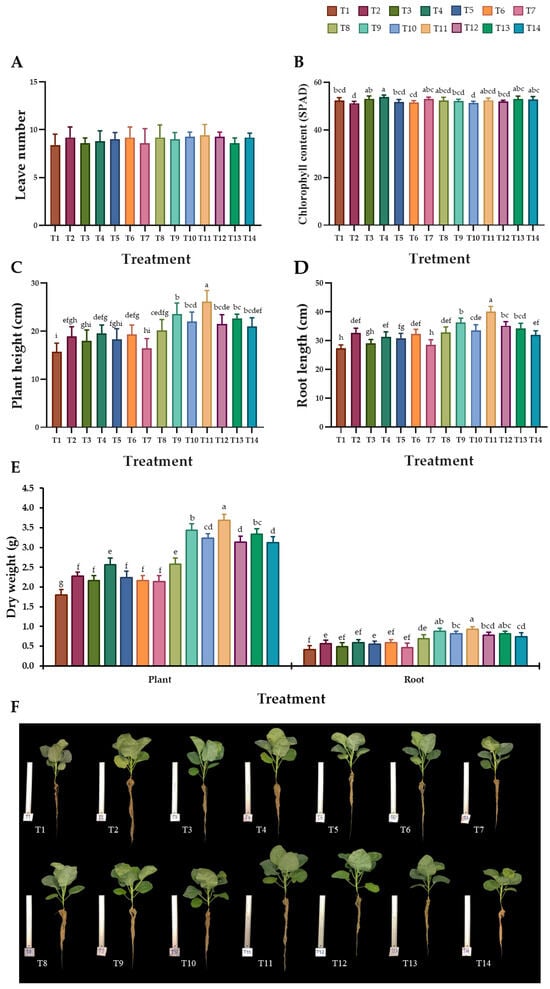
Figure 2.
Effect of biochar and arbuscular mycorrhizal fungi on the growth of Chinese kale. (A) Leaf number, (B) chlorophyll content, (C), plant height, (D) root length, (E) plant and root dry weight, and (F) Chinese kale growth in each treatment. The data are presented as means, with error bars at each point indicating the ± standard deviation. Differences within the same experiment are analyzed using Duncan’s multiple range test, with distinct letters indicating statistical significance (p ≤ 0.05).
This study aligns with several prior studies that indicate the overall benefits of combining biochar and mycorrhizae in a sustainable agriculture system. Prior research indicates that the co-application of biochar and AMF has significant effects on enhancing the growth of several plant species, such as maize, pepper, and tomato plants [20,29,30,31,36]. Sun et al. [20], Ndiate et al. [29], and Kotby et al. 2023 [31] revealed that the combined application of biochar and AMF can affect maize growth in comparison to the control treatments. Recently, Gunes et al. [36] also found that both AMF and biochar had significant effects on the pepper plant’s morphological growth factors. Therefore, synergistic interaction offers advantages for soil health and nutrient management, promoting crop productivity, resource efficiency, and long-term soil fertility [10,92,93]. Biochar enhances soil structure, which increasing porosity and water retention and leads to improved aeration, root penetration, and nutrient availability [12]. Similarly, mycorrhizal fungi establish a symbiotic relationship with plant roots, extending nutrient uptake, enhancing resistance to soil-borne pathogens, and boosting overall plant immunity [94]. However, the study notes that a high concentration of biochar may change microbial mechanisms, affecting the balance of beneficial and harmful microorganisms. Maintaining a lower percentage could support a more balanced microbial community, promoting optimal plant growth.
3.4. Photosynthetic Pigment Content
The content of photosynthetic pigment, including chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid in Chinese kale, is displayed in Figure 3. The chlorophyll and carotenoid content were dependent on each type of biochar; however, it was found that the addition of 5% BB-char and AMF (T11) to the soil revealed the highest chlorophyll and carotenoid content in Chinese kale leaves, with averages of 0.67 ± 0.02, 3.53 ± 0.14, and 4.24 ± 0.15 mg/g FW for chlorophyll a, b, and total chlorophyll, respectively (Figure 3A–C), and a carotenoid content of 3.25 ± 0.17 mg/g FW (Figure 3D). On the other hand, the lowest contents of chlorophyll and carotenoid were found in Chinese kale leaves of the control treatment (T1). Additionally, a comparison between the addition of 5% and 10% biochar revealed that the addition of 5% biochar resulted in higher levels of both chlorophyll and carotenoid content compared to the addition of 10% biochar (Figure 3A–D). According to the findings, these results suggest that the type and concentration rate of biochar have a significant impact on the levels of chlorophyll and carotenoids in Chinese kale plants. These results are supported by other previous studies, which revealed that the content of chlorophyll and carotenoids was related to biochar type, concentration rate, and plant species [95,96,97,98]. Kamran et al. [96] investigated the photosynthetic pigments in rapeseed (Brassica napus L.) at varying concentrations of wood chip (Acacia nilotica) biochar (0%, 1%, and 2%). Their study revealed that the addition of biochar at a concentration of 2% to the soil resulted in a higher content of photosynthetic pigments when compared with the addition of biochar at a concentration of 1%. Similarly, studies conducted by Ali et al. [95] found that adding 2% of biochar produced from rice straw to the soil resulted in a higher level of photosynthetic pigments compared to adding 1% of biochar. Therefore, the consistent findings across numerous studies suggest that the selection of biochar type and its concentration rate could have significant effects on the level of photosynthetic pigments in plants, potentially affecting the health and productivity of the plant. This information enhances our comprehension of sustainable agricultural methodologies and the role of biochar in stimulating advantageous physiological responses in plants.
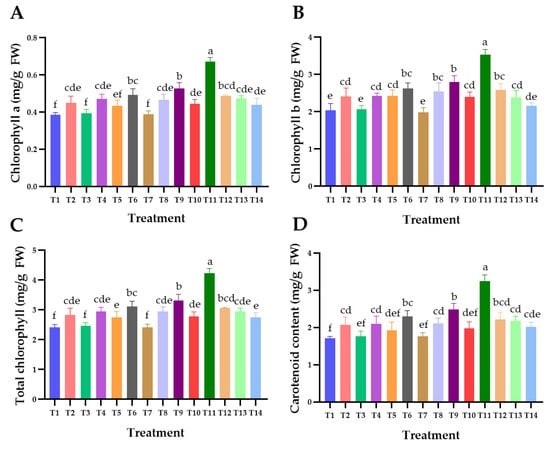
Figure 3.
Effect of each type of biochar and biochar with arbuscular mycorrhizal fungi on photosynthetic pigment content of Chinese kale. (A) Chlorophyll a, (B) chlorophyll b, (C) total chlorophyll, (D) carotenoid. The data are depicted as means, with error bars indicating ± standard deviation. Differences within the same experiment are assessed through Duncan’s multiple range test, where distinct letters denote statistical significance (p ≤ 0.05).
3.5. Total Phenolic Content
The total phenolic content in Chinese kale leaves is shown in Figure 4. The results reveal that the value of total phenolic content ranged from 0.08 ± 0.03 to 1.33 ± 0.05 mg GAE/g FW. The highest total phenolic content was obtained in Chinese kale cultivated in the soil mixed with 5% BB-char and AMF (T11), followed by Chinese kale cultivated in the soil mixed with 5% CG-char and AMF (T13). Conversely, the lowest level of total phenolic content was observed in Chinese kale cultivated in soil without any biochar or AMF (T1). Interestingly, when comparing the treatment of biochar without AMF to the treatment of biochar mixed with AMF, it was found that the biochar mixed with AMF (T9–T14) had a higher total phenolic content than the biochar without AMF (T2–T7). Our findings indicate that the synergistic effect of biochar and AMF positively influences the accumulation of total phenolic content in Chinese kale. Furthermore, the increase in phenolic compounds is probably related to the influence of AMF on nutrient absorption in the soil, as well as the influence of biochar on soil properties. The results of our findings align with the previous reports by Ma et al. [99], Upadhyay et al. [100], and Vahedi et al. [101] indicating that root exudates, such as phenolic compounds, play a crucial role in influencing the interactions between plants, soil, and microbial communities. Therefore, the combination of biochar with AMF not only enhances the content of phenolic compounds in plants but also enhances soil health and the mechanism of nutrient cycling.
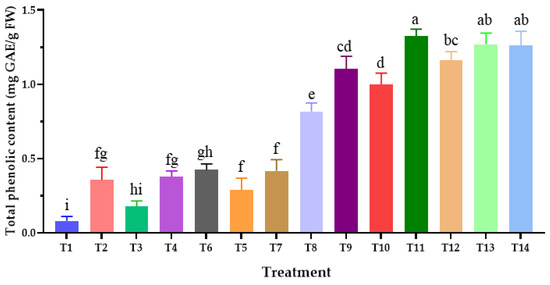
Figure 4.
Effect of each type of biochar and biochar with arbuscular mycorrhiza fungi on total phenolic content of Chinese kale. The data are presented as means with error bars indicating ± standard deviation. Differences within the same experiment are assessed using Duncan’s multiple range test, with distinct letters indicating statistical significance (p ≤ 0.05).
3.6. The Cost of Biochar Production and Its Perspective in Field Cultivation
Considering the energy cost of biochar production and its impact on field cultivation, especially when compared to traditional methods utilizing chemical fertilizers or various chemical products, is crucial for agricultural sustainability [102]. Generally, biochar pro-duction costs vary depending on factors such as raw material type, pyrolysis method, and production scale, with energy inputs mainly from heating processes ranging from 350 °C to 800 °C, resulting in production costs ranging from USD 0.67 to USD 10.0 per kilogram [103]. Although the initial energy investment for biochar may be higher, its sustainable advantages often outweigh these costs. Traditional methods relying on chemical inputs incur significant energy costs for manufacturing, transportation, and application, leading to environmental degradation, soil deterioration, and biodiversity loss and posing risks to human health and ecosystems. Integrating biochar into cultivation practices reduces reliance on chemicals, lowering overall energy consumption and environmental impact. Despite initial energy costs, biochar’s sustainable benefits in soil fertility, carbon sequestration, and crop yield make it a more environmentally friendly option compared to traditional chemical-intensive methods.
4. Conclusions
Biochar is a carbon-rich material produced from different types of biomasses by pyrolysis under oxygen-limited conditions, with significant benefits for sustainable agriculture and the environment. However, the impact of biochar depends on various factors, such as biochar type, application rate, and soil properties. Our results provide valuable insights into the potential of both biochar and biochar with AMF to enhance soil fertility and promote plant growth, as shown by its influence on Chinese kale. Furthermore, the combined effect of AMF with biochar at a 5% biochar concentration provides more effective results compared to a 10% biochar concentration. Therefore, the study suggests that biochar, especially in combination with AMF, positively affects soil properties, nutrient content, and plant growth parameters, providing potential benefits for sustainable agriculture practices. The findings demonstrate the importance of considering biochar type, concentration, and its interaction with AMF to assess its long-term impact on soil and plant health as well as ecosystem sustainability in the future. Despite biochar potentially requiring higher initial energy costs, it offers sustainable advantages compared to chemical fertilizer approaches by reducing overall energy consumption and environmental impact.
Author Contributions
Conceptualization, K.J. and S.L.; methodology, K.J., W.A., T.K., W.K. and S.L.; investigation, K.J., W.A. and S.L.; software and data curation, K.J.; validation, K.J., W.A. and S.L.; formal analysis, K.J. and W.A.; writing—original draft preparation, K.J. and W.A.; writing—review and editing, K.J., W.A., T.K., W.K. and S.L.; supervision, K.J. and S.L.; project administration, K.J.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Fund 2023 (FF66/R65EX00065), Chiang Mai University. The authors extend their gratitude to the CMU Proactive Researcher program at Chiang Mai University (grant number 827/2566), Chiang Mai, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research work was partially supported by Chiang Mai University, Chiang Mai, Thailand. The authors would like to thank the Faculty of Engineering at Chiang Mai University for providing biomasses and preparing biochar.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peng, X.; Jiang, Y.; Chen, Z.; Osman, A.I.; Farghali, M.; Rooney, D.W.; Yap, P.S. Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: A review. Environ. Chem. Lett. 2023, 21, 765–801. [Google Scholar] [CrossRef]
- Zheng, S.; Yin, K.; Yu, L. Factors influencing the farmer’s chemical fertilizer reduction behavior from the perspective of farmer differentiation. Heliyon 2022, 8, e11918. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tasnady, D. Biochar for soil carbon sequestration: Current knowledge, mechanisms, and future perspectives. C 2023, 9, 67. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Rady, M.M.; Semida, W.M.; Hemida, K.A.; Abdelhamid, M.T. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. Int. J. Recycl. Org. Waste Agric. 2016, 5, 311–321. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.V.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere 2022, 299, 134488. [Google Scholar] [CrossRef]
- Kocsis, T.; Ringer, M.; Biró, B. Characteristics and applications of biochar in soil–plant systems: A short review of benefits and potential drawbacks. Appl. Sci. 2022, 12, 4051. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management; Johannes, L., Stephen, J., Eds.; Routledge: New York, NY, USA, 2009; pp. 1–12. [Google Scholar]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. Beneficial features of biochar and arbuscular mycorrhiza for improving spinach plant growth, root morphological traits, physiological properties, and soil enzymatic activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Nepal, J.; Ahmad, W.; Munsif, F.; Khan, A.; Zou, Z. Advances and prospects of biochar in improving soil fertility, biochemical quality, and environmental applications. Front. Environ. Sci. 2023, 11, 1114752. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.H.; Kwon, E.E. Biochar as a tool for the improvement of soil and environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Sifton, M.A.; Smith, S.M.; Thomas, S.C. Biochar-biofertilizer combinations enhance growth and nutrient uptake in silver maple grown in an urban soil. PLoS ONE 2023, 18, e0288291. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Martinsen, V.; Alling, V.; Nurida, N.; Mulder, J.; Hale, S.; Ritz, C.; Rutherford, D.; Heikens, A.; Breedveld, G.D.; Cornelissen, G. pH effects of the addition of three biochars to acidic Indonesian mineral soils. Soil Sci. Plant Nutr. 2015, 61, 821–834. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Haider, G.; Raza, M.A.; Mohamed, A.K.S.; Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S. Straw-based biochar mediated potassium availability and increased growth and yield of cotton (Gossypium hirsutum L.). J. Saudi Chem. Soc. 2020, 24, 963–973. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.D.F.; Abdelaal, K.A.A.; Al Husnain, L.; AlGwaiz, H.I.M.; Hafez, Y.M.; Attia, K.A.; El-Esawi, M.A.; Ibrahim, M.F.M.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of Faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Fachini, J.; de Figueiredo, C.C.; do Vale, A.T. Assessing potassium release in natural silica sand from novel K-enriched sewage sludge biochar fertilizers. J. Environ. Manag. 2022, 314, 115080. [Google Scholar] [CrossRef]
- Saito, M.; Marumoto, T. Inoculation with arbuscular mycorrhizal fungi: The status quo in Japan and the future prospects. In Diversity and Integration in Mycorrhizas: Proceedings of the 3rd International Conference on Mycorrhizas (ICOM3) Adelaide, Australia, 8–13 July 2002; Springer: Dordrecht, The Netherlands, 2002; pp. 273–279. [Google Scholar]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.; Fu, S. Effects of arbuscular mycorrhizal fungi and biochar on growth, nutrient absorption, and physiological properties of maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef]
- Schüßler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Genera; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2010; p. 58. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhiza Symbiosis, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; p. 800. [Google Scholar]
- Prasad, R.; Bhola, D.; Akdi, K.; Cruz, C.; Sairam, K.V.S.S.; Tuteja, N.; Varma, A. Introduction to mycorrhiza: Historical development. In Mycorrhiza-Function, Diversity, State of the Art, 4th ed.; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: New York, NY, USA, 2017; pp. 1–7. [Google Scholar]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, H.; Chu, M.; Niu, X.; Bao, H.; Wang, N.; Zhan, F.; Long, X.; Yang, R.; Lin, Q.; et al. Plant microbiome and mycorrhizal fungi. In mycorrhiza-new insights. In Arbuscular Mycorrhizal Fungi in Agriculture—New Insights; de Sousa, R.N., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef] [PubMed]
- Ebbisa, A. Arbuscular mycorrhizal fungi (AMF) in optimizing nutrient bioavailability and reducing agrochemicals for maintaining sustainable agroecosystems. In Arbuscular Mycorrhizal Fungi in Agriculture-New Insights; de Sousa, R.N., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Ndiate, N.I.; Saeed, Q.; Haider, F.U.; Liqun, C.; Nkoh, J.N.; Mustafa, A. Co-application of biochar and arbuscular mycorrhizal fungi improves salinity tolerance, growth and lipid metabolism of maize (Zea mays L.) in an alkaline soil. Plants 2021, 10, 2490. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Galán, D.; Heupel, S.; Duelli, G.; Morgano, M.T.; Stapf, D.; Requena, N. Exploring the synergistic effects of biochar and arbuscular mycorrhizal fungi on phosphorus acquisition in tomato plants by using gene expression analyses. Sci. Total Environ. 2023, 884, 163506. [Google Scholar] [CrossRef] [PubMed]
- Kotby, R.A.; Mohamed, H.M.; Gomah, H.H.; Usman, A.R. Combined effects of microbial inoculation and activated carbon/biochar on the accumulation and transfer of nutrients and potentially toxic metals in maize plants grown on a contaminated soil. Soil Sediment Contam. 2023, 1–22. [Google Scholar] [CrossRef]
- Mulyadi and Jiang, L. The combined application of biochar and arbuscular mycorrhizal fungi (AMF) enhanced the physical and chemical properties of soil and rice productivity in Indonesia. Sustainability 2023, 15, 9782. [Google Scholar] [CrossRef]
- Wen, Z.; Chen, Y.; Liu, Z.; Meng, J. Biochar and arbuscular mycorrhizal fungi stimulate rice root growth strategy and soil nutrient availability. Eur. J. Soil Biol. 2022, 113, 103448. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Azimov, A.; Tyagi, S.; Pengani, K.R.; Sharma, P.; Vikram, K.V.; Poczai, P.; Nasif, O.; Ansari, M.J.; et al. Co-inoculation of biochar and arbuscular mycorrhizae for growth promotion and nutrient fortification in soybean under drought conditions. Front. Plant Sci. 2022, 13, 947547. [Google Scholar] [CrossRef]
- Chaiya, L.; Kumla, J.; Suwannarach, N.; Kiatsiriroat, T.; Lumyong, S. Isolation, characterization, and efficacy of actinobacteria associated with arbuscular mycorrhizal spores in promoting plant growth of chili (Capsicum flutescens L.). Microorganisms 2021, 9, 1274. [Google Scholar] [CrossRef]
- Gunes, H.; Demir, S.; Erdinc, C.; Furan, M.A. Effects of arbuscular mycorrhızal fungı (AMF) and bıochar on the growth of pepper (Capsicum annum L.) under salt stress. Gesunde Pflanz. 2023, 75, 2669–2681. [Google Scholar] [CrossRef]
- Kazemi, R.; Ronaghi, A.; Yasrebi, J.; Ghasemi-Fasaei, R.; Zarei, M. Effect of shrimp waste–derived biochar and arbuscular mycorrhizal fungus on yield, antioxidant enzymes, and chemical composition of corn under salinity stress. J. Soil Sci. Plant Nutr. 2019, 19, 758–770. [Google Scholar] [CrossRef]
- Sun, B.; Liu, N.; Zhao, Y.T.; Yan, H.Z.; Wang, Q.M. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Sun, B.; Yan, H.Z.; Liu, N.; Wei, J.; Wang, Q.M. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012, 131, 519–526. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of the Association of the Analytical Chemists International, 15th ed.; AOAC International: Washington, DC, USA, 1998. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 1983; pp. 403–430. [Google Scholar]
- David, D.J. The determination of exchangeable sodium, potassium, calcium and magnesium in soils by atomic-absorption spectrophotometry. Analyst 1960, 85, 495–503. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.; Jiang, L.; Hou, Y.; Yang, F.; Chen, W.; Li, X. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol. Biotechnol. Equip. 2015, 29, 646–652. [Google Scholar] [CrossRef]
- Liu, J.; Cai, G.; Qian, M.; Wang, D.; Xu, J.; Yang, J.; Zhu, Q. Effect of Cd on the growth, dry matter accumulation and grain yield of different rice cultivars. J. Sci. Food Agric. 2007, 87, 1088–1095. [Google Scholar] [CrossRef]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F. Contrasting effects of biochar on phosphorus dynamics and bioavailability in different soil types. Sci. Total Environ. 2018, 627, 963–974. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rahman, M.d.J.; Costa de Camargo, A.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and Sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Ali, L.; Manzoor, N.; Li, X.; Naveed, M.; Nadeem, S.M.; Waqas, M.R.; Khalid, M.; Abbas, A.; Ahmed, T.; Li, B.; et al. Impact of corn cob-derived biochar in altering soil quality, biochemical status and improving maize growth under drought stress. Agronomy 2021, 11, 2300. [Google Scholar] [CrossRef]
- Schneider, D.; Escala, M.; Supawittayayothin, K.; Tippayawong, N. Haracterization of biochar from hydrothermal carbonization of bamboo. J. Energy Environ. Eng. 2011, 2, 647–652. [Google Scholar]
- Jagnade, P.; Panwar, N.L.; Gupta, T.; Agrawal, C. Role of biochar in agriculture to enhance crop productivity: An overview. Biointerface Res. Appl. Chem. 2022, 13, 429. [Google Scholar]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Evol. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 2019, 10, 10. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.J.; Naidu, R.; Parikh, S.J.; Du, J.H.; Qi, F.J.; Willett, I.R. Infuences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total. Environ. 2020, 744, 140714. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.R.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Infuence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Tu, P.; Zhang, G.; Wei, G.; Li, J.; Li, Y.; Deng, L.; Yuan, H. Influence of pyrolysis temperature on the physicochemical properties of biochars obtained from herbaceous and woody plants. Bioresour. Bioprocess 2022, 9, 131. [Google Scholar] [CrossRef]
- Almutairi, A.A.; Ahmad, M.; Rafique, M.I.; Al-Wabel, M.I. Variations in composition and stability of biochars derived from different feedstock types at varying pyrolysis temperature. J. Saudi Soc. Agric. Sci. 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Zhao, J.; Luo, Z.; Tu, S.; Yin, Y. Properties of biochar obtained from pyrolysis of bamboo shoot shell. J. Anal. Appl. Pyrolysis 2015, 114, 172–178. [Google Scholar] [CrossRef]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Dey, D.; Mavi, M.S. Co-application of biochar with non-pyrolyzed organic material accelerates carbon accrual and nutrient availability in soil. Environ. Technol. Innov. 2022, 25, 102128. [Google Scholar] [CrossRef]
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Idbella, M.; Baronti, S.; Giagnoni, L.; Renella, G.; Becagli, M.; Cardelli, R.; Maienza, A.; Vaccari, F.P.; Bonanomi, G. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 2023, 195, 105217. [Google Scholar] [CrossRef]
- Qian, X.; Li, Q.; Chen, H.; Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J.; Müller, C.; Yi, Z. Enhancing soil nitrogen retention capacity by biochar incorporation in the acidic soil of pomelo orchards: The crucial role of pH. Agronomy 2023, 13, 2110. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.; Qu, Z.; Xu, X.; Huang, Q.; Huang, G. Impact of biochar addition on soil properties and water-fertilizer productivity of tomato in semi-arid region of Inner Mongolia, China. Geoderma 2018, 331, 100–108. [Google Scholar] [CrossRef]
- Mohawesh, O.; Albalasmeh, A.; Gharaibeh, M.; Deb, S.; Simpson, C.; Singh, S.; Al-Soub, B.; Hanandeh, A.E. Potential use of biochar as an amendment to improve soil fertility and tomato and bell pepper growth performance under arid conditions. J. Soil Sci. Plant Nutr. 2021, 21, 2946–2956. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Shi, L.; Li, G.; Han, J.; Pang, Z.; Liu, S.; Chen, Y.; Jia, B. Effects of biochar on soil chemical properties: A global meta-analysis of agricultural soil. Plant Soil Environ. 2022, 68, 272–289. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J. Application of two bioenergy byproducts with contrasting carbon availability to a prairie soil: Three year crop response and changes in soil biological and chemical properties. Agronomy 2016, 6, 13. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Doyle, R.; Bound, S.A.; Bowman, J.P. The effect of biochar loading rates on soil fertility, soil biomass, potential nitrification, and soil community metabolic profiles in three different soils. J. Soils Sediments 2016, 16, 2211–2222. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Han, L.; Chen, Y.; Liu, J.; Xing, B. Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol. Biochem. 2022, 169, 108657. [Google Scholar] [CrossRef]
- Novair, S.B.; Cheraghi, M.; Faramarzi, F.; Lajayer, B.A.; Senapathi, V.; Astatkie, T.; Price, G.W. Reviewing the role of biochar in paddy soils: An agricultural and environmental perspective. Ecotoxicol. Environ. Saf. 2023, 263, 115228. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Zhang, Z.; Wang, J.; Guo, S.; Li, Z.; Lin, H.; Huang, Y.; Han, Z.; Kuzyakov, Y.; Zou, J. Benefits and limitations of biochar for climate-smart agriculture: A review and case study from China. Biochar 2023, 5, 115228. [Google Scholar] [CrossRef]
- Kuryntseva, P.; Karamova, K.; Galitskaya, P.; Selivanovskaya, S.; Evtugyn, G. Biochar functions in soil depending on feedstock and pyrolyzation properties with particular emphasis on biological properties. Agriculture 2023, 13, 2003. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gu, X.; Liu, L.; Gou, J. Biochar application ameliorated the nutrient content and fungal community structure in different yellow soil depths in the karst area of Southwest China. Front. Plant Sci. 2022, 13, 1020832. [Google Scholar] [CrossRef]
- Semida, W.M.; Beheiry, H.R.; Sétamou, M.; Simpson, C.R.; Abd El-Mageed, T.A.; Rady, M.M.; Nelson, S.D. Biochar implications for sustainable agriculture and environment: A review. S. Afr. J. Bot. 2019, 127, 333–347. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Ali, M.; Zamir, M.S.I.; Qamar, R.; Kanwal, S.; Andleeb, H.; Qammar, N.; Jhangir, K.; Elkelish, A.; Mubeen, M.; et al. Biochar and arbuscular mycorrhizae fungi to improve soil organic matter and fertility. In Sustainable Agriculture Reviews 61: Biochar to Improve Crop Production and Decrease Plant Stress under a Changing Climate; Fahad, S., Danish, S., Datta, R., Saud, S., Lichtfouse, E., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 331–354. [Google Scholar]
- Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular mycorrhizal fungi symbiosis to enhance plant–soil interaction. Sustainability 2022, 14, 7840. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Bano, R.; Bharwana, S.A.; Rehman, M.Z.U.; Hussain, M.B.; Al-Wabel, M.I. Effects of biochar on growth, photosynthesis, and chromium (Cr) uptake in Brassica rapa L. under Cr stress. Arab. J. Geosci. 2018, 11, 507. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Bibi, S.; Ullah, R.; Burni, T.; Ullah, Z.; Kazi, M. Impact of resorcinol and biochar application on the growth attributes, metabolite contents, and antioxidant systems of tomato (Lycopersicon esculentum Mill.). ACS Omega 2023, 8, 45750–45762. [Google Scholar] [CrossRef] [PubMed]
- Janyasupab, P.; Brix, H.; Jampeetong, A. Effects of longan biochar as filter materials on plant responses and wastewater treatment by Typha angustifolia L. Nat. Life Sci. Commun. 2023, 22, e2023035. [Google Scholar] [CrossRef]
- Ma, W.; Tang, S.; Dengzeng, Z.; Zhang, D.; Zhang, T.; Ma, X. Root exudates contribute to belowground ecosystem hotspots: A review. Front. Microbiol. 2022, 13, 937940. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Vahedi, R.; Rasouli-Sadaghiani, M.H.; Barin, M.; Vetukuri, R.R. Effect of biochar and microbial inoculation on P, Fe, and Zn bioavailability in a calcareous soil. Processes 2022, 10, 343. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, W.; Peng, H.; Pan, B.; Xing, B. Functional biochar and its balanced design. ACS Environ. Au. 2021, 2, 115–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).