Evaluation of Biocontrol Potential of Bacillus spp. and Pseudomonas fluorescens UM270 against Postharvest Fungal Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. In Vitro Evaluation of the Antagonistic Effects of Diffusible and Volatile Compounds Produced by Bacteria
2.3. Strawberry and Grape Assay
2.4. Comparison of Secondary Metabolite Biosynthesis Gene Clusters and VOCs
2.5. Statistical Analysis
3. Results
3.1. Effect of PGPB Diffusible Compounds on Fungal Mycelial Growth
3.2. Effect of VOCs on Fungal Mycelial Growth
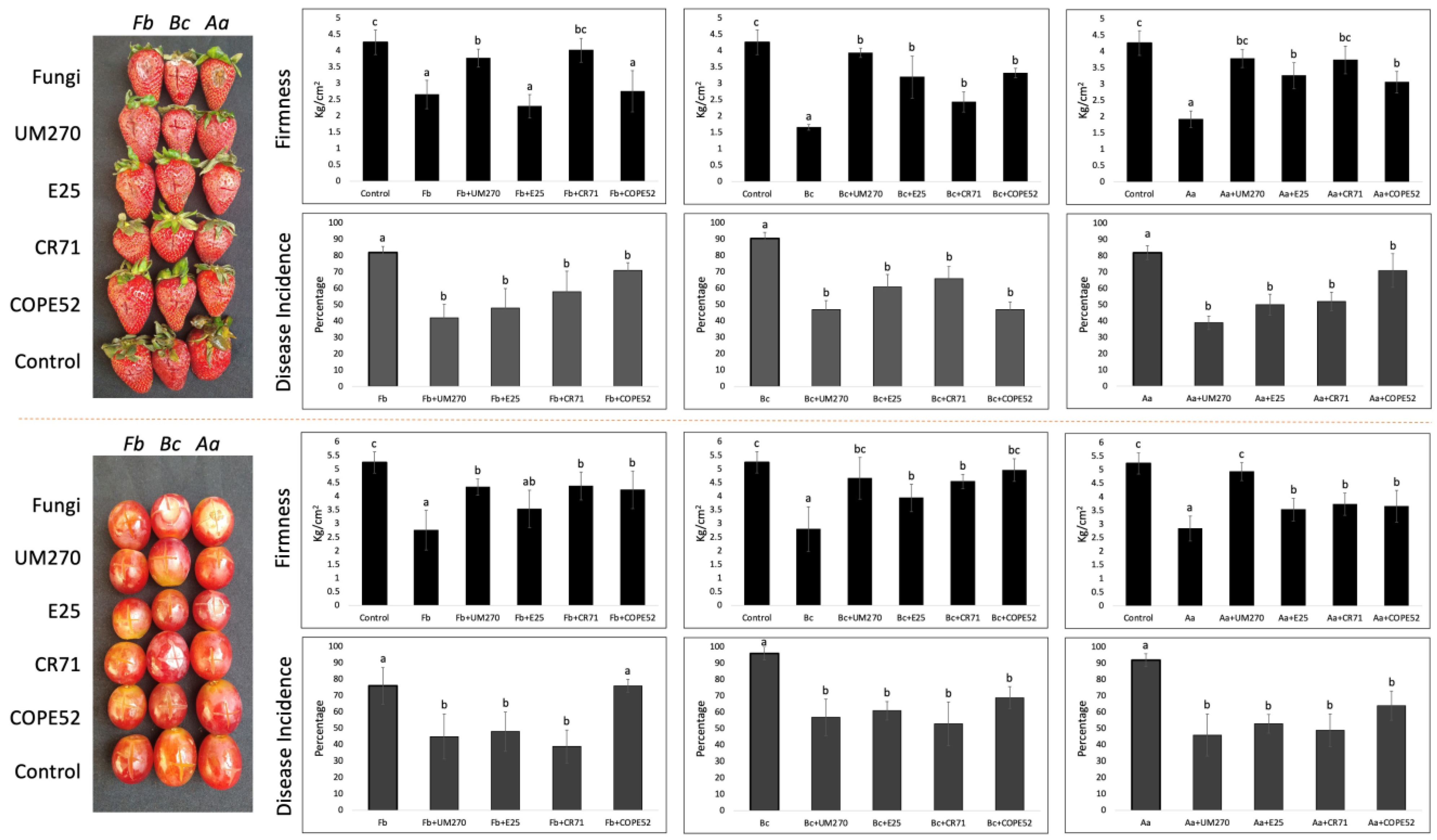
3.3. Biocontrol Assay on Strawberries and Grapes
3.4. Comparative Analysis of the Secondary Metabolite Biosynthesis Gene Clusters and Produced VOCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Shalrma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Droby, S.; Chalutz, E.; Wilson, C.L.; Wisniewski, M.E. Biological Control of Postharvest Diseases: A Promising Alternative to the Use of Synthetic Fungicides. Phytoparasitica 1992, 20, S149–S153. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil. Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Stocco, A.F.; Diaz, M.E.; Romera, M.C.R.; Mercado, L.A.; Rivero, M.L.; Ponsone, M.L. Biocontrol of postharvest Alternaria decay in table grapes from Mendoza province. Biol. Control 2019, 134, 114–122. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 96340. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Pérez-Equihua, A.; Santoyo, G.; Baltrus, D.A. Draft Genome Sequence of Bacillus sp. Strain E25, a Biocontrol and Plant Growth-Promoting Bacterial Endophyte Isolated from Mexican Husk Tomato Roots (Physalis ixocarpa Brot. Ex Horm.). Microbiol. Resour. Announc. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; del Carmen Rocha-Granados, M.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Hernández-Salmerón, J.E.; Hernández-León, R.; Del Carmen Orozco-Mosqueda, M.; Valencia-Cantero, E.; Moreno-Hagelsieb, G.; Santoyo, G. Draft Genome Sequence of the Biocontrol and Plant Growth-Promoting Rhizobacterium Pseudomonas fluorescens strain UM270. Stand. Genom. Sci. 2016, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; la Cruz, H.R.-D.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; Santoyo, G.; Flores-Cortez, I.; Alfaro-Cuevas, R.; Valencia-Cantero, E. Arthrobacter agilis UMCV2 induces iron acquisition in Medicago truncatula (strategy I plant) in vitro via dimethylhexadecylamine emission. Plant Soil. 2013, 362, 51–66. [Google Scholar] [CrossRef]
- Contreras-Pérez, M.; Hernández-Salmerón, J.; Rojas-Solís, D.; Rocha-Granados, C.; del Carmen Orozco-Mosqueda, M.; Parra-Cota, F.I.; Santos-Villalobos, S.d.L.; Santoyo, G. Draft genome analysis of the endophyte, Bacillus toyonensis COPE52, a blueberry (Vaccinium spp. var. Biloxi) growth-promoting bacterium. 3 Biotech 2019, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cedeño, L.R.; de los Santos-Villalobos, S.; Santoyo, G. Functional and Genomic Analysis of Rouxiella badensis SER3 as a Novel Biocontrol Agent of Fungal Pathogens. Front. Microbiol. 2021, 12, 709855. [Google Scholar] [CrossRef]
- Mahuku, G.S. A Simple Extraction Method Suitable for PCR-Based Analysis of Plant, Fungal, and Bacterial DNA. Plant Mol. Biol. Report. 2004, 22, 71–81. [Google Scholar] [CrossRef]
- Shi, J.F.; Sun, C.Q. Isolation, identification, and biocontrol of antagonistic bacterium against Botrytis cinerea after tomato harvest. Braz. J. Microbiol. 2017, 48, 706–714. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Thomloudi, E.-E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated Genomic and Metabolomic Analysis Illuminates Key Secreted Metabolites Produced by the Novel Endophyte Bacillus halotolerans Cal.l.30 Involved in Diverse Biological Control Activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef]
- Heo, A.Y.; Koo, Y.M.; Choi, H.W. Biological Control Activity of Plant Growth Promoting Rhizobacteria Burkholderia contaminans AY001 against Tomato Fusarium Wilt and Bacterial Speck Diseases. Biology 2022, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Diaz-Zamora, J.T.; del Carmen Orozco-Mosqueda, M.; Chávez, A.; Santos-Villalobos, S.d.L.; Valencia-Cantero, E.; Santoyo, G. Bridging genomics and field research: Draft genome sequence of Bacillus thuringiensis CR71, an endophytic bacterium that promotes plant growth and fruit yield in Cucumis sativus L. 3 Biotech 2020, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Duran, H.G.S.; de Los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. AntiSMASH 4.0—Improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Orozco-Mosqueda, M.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Bell, S.R.; Hernández Montiel, L.G.; González Estrada, R.R.; Gutiérrez Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT 2021, 149, 112046. [Google Scholar] [CrossRef]
- Mehra, L.K.; Maclean, D.D.; Savelle, A.T.; Scherm, H. Postharvest disease development on southern highbush blueberry fruit in relation to berry flesh type and harvest method. Plant Dis. 2013, 97, 213–221. [Google Scholar] [CrossRef]
- Li, C.; Krewer, G.W.; Ji, P.; Scherm, H.; Kays, S.J. Gas sensor array for blueberry fruit disease detection and classification. Postharvest Biol. Technol. 2010, 55, 144–149. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef]
- Koike, S.T.; Gordon, T.R. Management of Fusarium wilt of strawberry. Crop Prot. 2015, 73, 67–72. [Google Scholar] [CrossRef]
- Pastrana, A.M.; Kirkpatrick, S.C.; Kong, M.; Broome, J.C.; Gordon, T.R. Fusarium oxysporum f. sp. mori, a new forma specialis causing fusarium wilt of blackberry. Plant Dis. 2017, 101, 2066–2072. [Google Scholar] [CrossRef]
- Rivera, S.A.; Zoffoli, J.P.; Latorre, B.A. Infection risk and critical period for the postharvest control of gray mold (Botrytis cinerea) on blueberry in Chile. Plant Dis. 2013, 97, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide resistance profiling in Botrytis cinerea populations from blueberry in California and Washington and their impact on control of gray mold. Plant Dis. 2016, 100, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Tsalgatidou, P.C.; Thomloudi, E.-E.; Delis, C.; Nifakos, K.; Zambounis, A.; Venieraki, A.; Katinakis, P. Compatible Consortium of Endophytic Bacillus halotolerans Strains Cal.l.30 and Cal.f.4 Promotes Plant Growth and Induces Systemic Resistance against Botrytis cinerea. Biology 2023, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Hua, Z.C. Lasso Peptides: Heterologous Production and Potential Medical Application. Front. Bioeng. Biotechnol. 2020, 8, 571165. [Google Scholar] [CrossRef] [PubMed]
| Fungal Growth Inhibition by Diffusible Compounds of Bacterial Strains (%) | ||||
|---|---|---|---|---|
| Fungal Strain | Bacillus toyonensis COPE52 | Bacillus sp. E25 | B. thuringiensis CR71 | Pseudomonas fluorescens UM270 |
| Alternaria alternata 1A | 28.3 ± 4.9 b | 40.7 ± 5.8 c | 40.4 ± 11.2 c | 43.6 ± 4.8 c |
| Alternaria alternata 2Z | - | - | - | 22.4 ± 6.8 b |
| Alternaria alternata 4A | 16.4 ± 9.3 c | 27.6 ± 6.8 bc | 32.0 ± 10.9 bc | 40.9 ± 12.0 b |
| Alternaria alternata 6A | 1.9 ± 0.6 a | 5.5 ± 8.5 ab | 16.6 ± 7.0 b | 34.1 ± 7.8 c |
| Alternaria sp. 3A | 4.9 ± 11.2 a | 14.0 ± 9.6 a | 13.8 ± 10.4 a | 36.6 ± 9.8 b |
| Botryosphaeria rhodina 5A | 9.2 ± 5.7 ab | 8.4 ± 1.5 ab | 12.6 ± 3.8 b | 12.5 ± 8.9 b |
| Botrytis cinerea 62BCV | 11.5 ± 5.3 ab | 17.2 ± 7.3 ab | 35.0 ± 7.2 b | 62.6 ± 25.9 c |
| Botrytis sp. 62C | 13.6 ± 21.7 ab | 28.5 ± 9.6 bc | 48.4 ± 6.0 c | 78.6 ± 0.4 d |
| Cladosporium sp. 1BOA | 39.2 ± 11.7 c | 48.6 ± 6.2 bc | 45.5 ± 8.9 bc | 59.5 ± 5.3 b |
| Fusarium brachygibbosum 4BF | 14.0 ± 8.6 ab | 28.0 ± 7.9 bc | 38.1 ± 19.6 c | 45.1 ± 6.8 c |
| Fusarium brachygibbosum HBF | 29.5 ± 8.3 c | 34.3 ± 2.8 bc | 29.8 ± 4.8 c | 45.0 ± 12.2 b |
| Geotrichum candidum FRB | 12.2 ± 9.3 ab | 21.2 ± 9.5 ab | 26.4 ± 13.9 b | 60.7 ± 23.1 c |
| Geotrichum phurueaensis 7Z | 22.7 ± 4.1 b | 37.7 ± 7.3 d | 35.4 ± 8.8 d | 52.6 ± 8.3 c |
| Mucor circinelloides 1BF | 7.0 ± 3.0 ab | 14.6 ± 4.1 b | 14.2 ± 5.3 b | 12.1 ± 7.8 b |
| Mucor fragilis 22 | 15.8 ± 6.4 c | 18.8 ± 5.4 c | 30.7 ± 10.4 b | 12.8 ± 4.4 c |
| Mucor fragilis FRA | - | 9.1 ± 21.2 a | 8.4 ± 29.8 a | - |
| Penicillium crustosum 1F | 5.8 ± 28.2 a | 10.3 ± 14.4 a | 17.3 ± 9.2 ab | 40.8 ± 10.1 b |
| Penicillium expansum 230 | 9.7 ± 19.5 a | 6.9 ± 4.9 a | 20.8 ± 10.7 a | 45.4 ± 8.3 b |
| Penicillium expansum 5F | 13.3 ± 14.4 a | 7.0 ± 10.3 a | 24.2 ± 13.3 ab | 41.4 ± 20.7 b |
| Inhibition by Volatile Compounds of Bacterial Strains (%) | ||||
|---|---|---|---|---|
| Fungal Species/Strain | Bacillus toyonensis COPE52 | Bacillus sp. E25 | B. thuringiensis CR71 | Pseudomonas fluorescens UM270 |
| Alternaria alternata 1A | 6.4 ± 6.9 ab | 8.4 ± 8.7 ab | 7.3 ± 6.4 ab | 17.4 ± 8.1 b |
| Alternaria alternata 2Z | - | - | - | - |
| Alternaria alternata 4A | 13.6 ± 14.9 a | 11.3 ± 3.6 a | 22.0 ± 12.9 a | 27.8 ± 28.0 a |
| Alternaria alternata 6A | - | - | - | 5.4 ± 2.1 b |
| Alternaria sp. 3A | 0.5 ± 5.2 a | 2.4 ± 6.2 a | 4.1 ± 2.1 a | 4.3 ± 5.9 a |
| Botryosphaeria rhodina 5A | 3.5 ± 6.1 a | 1.2 ± 9.5 a | 2.8 ± 1.3 a | 6.1 ± 11.6 a |
| Botrytis cinerea 62BCV | 8.7 ± 6.7 a | 14.5 ± 17.2 a | 19.7 ± 14.1 a | 4.1 ± 3.5 a |
| Botrytis sp. 62C | 33.1 ± 35.6 a | 36.4 ± 27.2 a | 15.5 ± 46.5 a | 44.6 ± 37.8 a |
| Cladosporium sp. 1BOA | - | 1.3 ± 15.0 a | 5.8 ± 5.7 a | - |
| Fusarium brachygibbosum 4BF | - | 2.3 ± 2.5 a | 1.7 ± 6.7 a | - |
| Fusarium brachygibbosum HBF | - | - | - | - |
| Geotrichum candidum FRB | 0.4 ± 2.3 a | 2.8 ± 6.9 a | - | 5.8 ± 7.5 a |
| Geotrichum phurueaensis 7Z | 4.3 ± 16.2 a | 5.7 ± 19.2 a | 3.4 ± 20.3 a | - |
| Mucor circinelloides 1BF | - | - | - | - |
| Mucor fragilis 22 | - | - | - | - |
| Mucor fragilis FRA | 12.4 ± 14.4 a | 5.8 ± 10.6 a | 9.6 ± 8.3 a | - |
| Penicillium crustosum 1F | - | 2.4 ± 36.8 a | - | 6.5 ± 38.8 a |
| Penicillium expansum 230 | - | - | - | - |
| Penicillium expansum 5F | - | - | - | - |
| Gene Cluster | Bacillus sp. COPE52 | Bacillus sp. E25 | B. thuringiensis CR71 | Pseudomonas fluorescens UM270 |
|---|---|---|---|---|
| Bacitracin | 55% | - | - | - |
| Petrobactin | 100% | 100% | 100% | - |
| Bacillibactin | 46% | 46% | 46% | - |
| Fengycin | 40% | 40% | 40% | 13% |
| Molybdenum cofactor | 17% | 17% | 17% | - |
| Paeninodin | 80% | - | - | - |
| NRPS | + | + | + | + |
| LAP | + | - | + | - |
| RiPP-like | + | + | + | + |
| NRPS-like | - | + | + | - |
| Anabaenopeptin NZ857/nostamide A | - | 100% | 100% | - |
| Lassopeptide | - | + | + | - |
| transAT-PKS | - | + | + | - |
| S-layer glycan | - | 26% | 26% | - |
| Thusin | - | 100% | 100% | - |
| Serobactin C/B/A | - | - | - | 15% |
| Pyoverdin | - | - | - | 3% |
| Crochelin A | - | - | - | 7% |
| Lankacidin C | - | - | - | 13% |
| Fragin | - | - | - | 37% |
| N-acetyl glutaminylglutamine amide | - | - | - | + |
| Siderophore | - | - | - | + |
| Butyrolactone | - | - | - | + |
| 2,4-diacetylphloroglucinol | - | - | - | 100% |
| APE Vf | - | - | - | 40% |
| Volatile Compound | UM270 | E25 | CR71 | COPE52 |
|---|---|---|---|---|
| % | % | % | % | |
| Methanethiol | 15.13 | n.d. | n.d. | n.d. |
| Dimethyl sulfide | 23.4 | n.d. | n.d. | n.d. |
| 2-Butanone | n.d. | 2.32 | 2.24 | 0.99 |
| 1-Nonene | 2.02 | n.d. | n.d. | n.d. |
| Methyl thiolacetate | 1.17 | n.d. | n.d. | n.d. |
| Dimethyl disulfide | 5.62 | 2.11 | 2.65 | 2.63 |
| 1-Decene | 0.53 | n.d. | n.d. | n.d. |
| 1-Undecanol | 50.01 | n.d. | n.d. | n.d. |
| 2,4-Dithiapentane | n.d. | n.d. | n.d. | n.d. |
| 1-Dodecene | n.d. | n.d. | n.d. | n.d. |
| Dimethyl trisulfide | 0.57 | n.d. | n.d. | n.d. |
| S,S-Dimethyl dithiocarbonate | n.d. | n.d. | n.d. | n.d. |
| 2-Nonanone | n.d. | n.d. | n.d. | n.d. |
| Decyl oxirane | n.d. | n.d. | n.d. | n.d. |
| Methyl methylthiomethyl disulfide | n.d. | n.d. | n.d. | n.d. |
| 2-Amino-5-methyl benzoic acid | n.d. | n.d. | n.d. | n.d. |
| Thiazole | 0.41 | n.d. | n.d. | n.d. |
| Butylated hydroxytoluene | 0.49 | n.d. | n.d. | n.d. |
| Dimethylhexadecilamine | 0.64 | n.d. | n.d. | n.d. |
| Acetone | n.d. | 10.71 | n.d. | n.d. |
| Isopropyl alcohol | n.d. | 0.74 | n.d. | n.d. |
| Ethyl propionate | n.d. | 1.14 | 3.17 | n.d. |
| Ethyl isobutyrate | n.d. | 0.82 | 6.14 | 6.78 |
| 3-Methyl-2-pentanone | n.d. | 6.86 | n.d. | n.d. |
| Trichloromethane | n.d. | 38.85 | n.d. | n.d. |
| Ethyl-2-methylbutanoate | n.d. | n.d. | 3.49 | 6.45 |
| Ethyl isovalerate | n.d. | n.d. | 1.95 | 5.19 |
| 3-Methylbutanenitrile | n.d. | 12.93 | n.d. | n.d. |
| S-Methyl thio butyrate | n.d. | n.d. | 5.91 | 3.36 |
| 1-Butanol | n.d. | n.d. | 0.93 | n.d. |
| 1,3-Diazine | n.d. | 11.3 | 3.24 | n.d. |
| Ethyl tiglate | n.d. | 1.92 | 4.94 | 5.16 |
| Methyl pyrazine | n.d. | 1.18 | n.d. | 1.04 |
| Acetoin | n.d. | n.d. | 8.11 | 3.8 |
| Isobutyl isothiocyanate | n.d. | 10.47 | 25.86 | n.d. |
| Acetic acid | n.d. | n.d. | 5.4 | 6.03 |
| Ethyl-3-hydroxybutanoate | n.d. | 0.48 | 6.24 | n.d. |
| 2-(Methylthio)ethanol | n.d. | 2.1 | 2.74 | 2.75 |
| Propionic acid | n.d. | n.d. | 1.16 | n.d. |
| 2-Methylpropanoic acid | n.d. | n.d. | 3.72 | n.d. |
| Phenyloxirane | n.d. | 2.43 | 2.14 | 1.65 |
| Butanoic acid | n.d. | n.d. | 1.37 | 1.11 |
| 3-Methylbutanoic acid | n.d. | n.d. | 2.32 | 4.28 |
| Methyl salicylate | n.d. | n.d. | 0.29 | 0.75 |
| 2-Butenoic acid | n.d. | n.d. | 6.07 | n.d. |
| Acetamide | n.d. | 1.24 | 0.31 | n.d. |
| Benzyl alcohol | n.d. | 0.45 | 1.15 | 1.75 |
| Ethyl propanoate | n.d. | n.d. | n.d. | 1.45 |
| Ethyl butanoate | n.d. | n.d. | n.d. | 6.55 |
| Isobutane | n.d. | n.d. | n.d. | 4.6 |
| S-Methyl 3-methylbutanethioate | n.d. | n.d. | n.d. | 7.84 |
| 3-Hydroxy-2-butanone | n.d. | n.d. | n.d. | 3.49 |
| Ethyl 3-hydroxybutanoate | n.d. | n.d. | n.d. | 16.21 |
| Propanoic acid | n.d. | n.d. | n.d. | 0.97 |
| 2,3-Butanediol | n.d. | n.d. | n.d. | 2.61 |
| Menthol | n.d. | n.d. | n.d. | 0.78 |
| Ethyl phenylacetate | n.d. | n.d. | n.d. | 1.44 |
| Butyl butanoate | n.d. | n.d. | n.d. | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Cedeño, L.R.; Barajas-Barrera, I.A.; Parra-Cota, F.I.; Valenzuela-Ruiz, V.; de los Santos-Villalobos, S.; Loeza-Lara, P.D.; Herrera-Pérez, A.; del Carmen Orozco-Mosqueda, M.; Santoyo, G. Evaluation of Biocontrol Potential of Bacillus spp. and Pseudomonas fluorescens UM270 against Postharvest Fungal Pathogens. Microbiol. Res. 2023, 14, 1511-1523. https://doi.org/10.3390/microbiolres14040103
Morales-Cedeño LR, Barajas-Barrera IA, Parra-Cota FI, Valenzuela-Ruiz V, de los Santos-Villalobos S, Loeza-Lara PD, Herrera-Pérez A, del Carmen Orozco-Mosqueda M, Santoyo G. Evaluation of Biocontrol Potential of Bacillus spp. and Pseudomonas fluorescens UM270 against Postharvest Fungal Pathogens. Microbiology Research. 2023; 14(4):1511-1523. https://doi.org/10.3390/microbiolres14040103
Chicago/Turabian StyleMorales-Cedeño, Luzmaria R., Ignacio A. Barajas-Barrera, Fannie I. Parra-Cota, Valeria Valenzuela-Ruiz, Sergio de los Santos-Villalobos, Pedro D. Loeza-Lara, Alejandra Herrera-Pérez, Ma. del Carmen Orozco-Mosqueda, and Gustavo Santoyo. 2023. "Evaluation of Biocontrol Potential of Bacillus spp. and Pseudomonas fluorescens UM270 against Postharvest Fungal Pathogens" Microbiology Research 14, no. 4: 1511-1523. https://doi.org/10.3390/microbiolres14040103
APA StyleMorales-Cedeño, L. R., Barajas-Barrera, I. A., Parra-Cota, F. I., Valenzuela-Ruiz, V., de los Santos-Villalobos, S., Loeza-Lara, P. D., Herrera-Pérez, A., del Carmen Orozco-Mosqueda, M., & Santoyo, G. (2023). Evaluation of Biocontrol Potential of Bacillus spp. and Pseudomonas fluorescens UM270 against Postharvest Fungal Pathogens. Microbiology Research, 14(4), 1511-1523. https://doi.org/10.3390/microbiolres14040103









